Abstract
DHA is a long-chain fatty acid that has potent antiinflammatory properties. Whereas maternal DHA dietary supplementation has been shown to improve cognitive development in infants fed DHA-supplemented milk, the antiinflammatory effects of maternal DHA supplementation on the developing fetus and neonate have not been extensively explored. Pregnant C3H/HeN dams were fed purified control or DHA-supplemented diets (∼0.25% of total fat) at embryonic d 16 and consumed these diets throughout the study. At birth, the nursing mouse pups were placed in room air (RA; 21% O2) or >95% O2 (hyperoxia) for up to 7 d. These studies tested the hypothesis that maternal DHA supplementation would decrease inflammation and improve alveolarization in the lungs of newborn mouse pups exposed to hyperoxia. Survival, inflammatory responses, and lung growth were compared among control diet/RA, DHA/RA, control/O2, and DHA/O2 pups. There were fewer neutrophils and macrophages in lung tissues from pups nursed by DHA-supplemented dams than in those nursed by dams fed the control diet at 7 d of hyperoxia exposure (P < 0.015). Although differences due to hyperoxia exposure were observed, maternal diet did not affect keratinocyte-derived chemokine, macrophage inflammatory protein-2, IL-1β, or TNFα mRNA levels in pup tissues. Hyperoxia also induced NF-κB activity, but maternal diet did not affect NF-κB or PPARγ activities. In mice, DHA supplementation decreases leukocyte infiltration in the offspring exposed to hyperoxia, suggesting a potential role for DHA supplementation as a therapy to reduce inflammation in preterm infants.
Introduction
DHA is a long-chain (n-3) fatty acid that has recently been the focus of numerous studies both in nutrition and immunology (1, 2). DHA is essential for neurological development in mammalian species and is preferentially accreted by the fetus during the last trimester of gestation in humans (3–5). Furthermore, recent studies have indicated a vital role for long-chain fatty acids in modulation of inflammatory responses and immune function (6–8). Although the mechanisms involved are not completely understood, the antiinflammatory properties of long-chain fatty acids are thought to include changes in membrane fluidity and effects on signaling pathways such as toll-like receptor 4 (9, 10) and PPARγ (11), which result in modified gene transcription or enhancement of the production of antiinflammatory lipid mediators due to the availability of DHA as substrate (1, 6).
Transgenic mice expressing the Caenorhabditis elegans fat-1 gene encoding for an (n-3) fatty acid desaturase are able to produce (n-3) fatty acids such as DHA from (n-6) fatty acids such as arachidonic acid (12). These mice have shown remarkable decreases in inflammatory responses to acute lung inflammation (13). Other animal studies have demonstrated decreases in inflammatory responses with dietary DHA supplementation in lung injury models induced by LPS administration (14) or bacterial infection (15).
The adverse effects of DHA deficiency, specifically in Western diets, has been demonstrated and nutritional supplementation of pregnant and lactating women with long-chain fatty acids has become a widespread clinical practice (16, 17). Preterm infants are exposed to a host of inflammatory stimuli from maternal- or hospital-acquired infections and from lifesaving measures such as oxygen therapy and mechanical ventilation. These interventions damage fragile lung tissues, induce acute inflammatory responses, and contribute to arrested alveolarization and long-term impaired lung function (18, 19). In addition to missing fetal accretion of DHA, preterm infants are often maintained on parenteral nutrition for sustained periods of time or fed preterm formula or human donor milk that either does not contain or contains very low quantities of DHA (20). The developmental benefits of DHA supplementation on neurological outcomes combined with the antiinflammatory/proresolution properties of DHA could provide improved outcomes for prematurely born infants through supplementation to mothers.
The inconclusive results concerning fish oil supplementation during pregnancy and lactation led us to specifically test the differences between supplementation with preformed DHA and α-linolenic acid. These studies focused on testing the hypothesis that DHA supplementation of C3H/HeN mouse dams during pregnancy and lactation would decrease inflammatory responses and lessen hyperoxic lung injury in nursing newborn pups.
Methods
Animal models.
Animal study protocols were approved by the IACUC of The Research Institute at Nationwide Children’s Hospital (protocol no. AR07–00028). C3H/HeN male and female mice were allowed to mate and then females checked for the presence of vaginal plugs twice a day. The presence of a vaginal plug was designated as embryonic d 1. Pregnant dams were assigned to DHA-enriched or control diets and were fed the respective diets beginning at embryonic d 16 (Table 1). After birth, litters from 2 pregnant dams (dam pairs) receiving the same diets and delivering within 12 h were paired and placed in a plexiglass chamber containing either 8 L/min flow of >95% O2 or room air (RA).7 To prevent oxygen toxicity, dams were rotated every 24 h between their corresponding RA and hyperoxia litters. The pup groups were as follows: (maternal diet/exposure): control/RA, DHA/RA, control/O2, and DHA/O2.
TABLE 1.
Composition of control and DHA diets1
| Control diet | DHA diet | |
| Flaxseed oil, g/kg | 4.5 | 0 |
| DHA (40%),2g/kg | 0 | 6.3 |
| PUFA, g/100 g | ||
| Linoleic (18:2) | 3.0 | 2.9 |
| Linolenic (18:3) | 0.7 | 0.4 |
| DHA (22:6) | 0 | 0.3 |
Diet compositions were based on the AIN-93G (45) diets with the following changes: corn starch, 391.186 g/kg; l-cysteine, 4.3 g/kg; Mineral Mix, AIN-93G-MX 42.0 g/kg; Vitamin Mix, AIN-93-VX, 14 g/kg and soybean oil, 55.5 g/kg to support reproduction and lactation.
Provided by Martek, Inc.
Twenty-four hours of hyperoxia exposure was designated as d 1. On 1, 3, 7, or 14 d of life, the pups were killed by an i.p. injection of 200 mg/kg of sodium pentobarbital and lung and liver were dissected out and were snap-frozen or formalin fixed. To fix lung tissues, tracheas were cannulated with 25-gauge silastic catheters and 10% neutral buffered formalin was instilled at 25 cm H2O pressure over 15 min of equilibration. Snap-frozen tissues were homogenized and used for analytical measurements because of the difficulty in obtaining consistent amounts of bronchoalveolar lavage fluid or blood from mouse pups at such an early age.
Diets.
The purified DHA oil was obtained as a gift from Martek Bioscience and was provided to Harlan Teklad for custom diet formulation. The compositions of the respective diets are listed in Table 1.
Morphometric and digital image analysis.
Formalin-fixed lungs were embedded in paraffin, cut perpendicularly, and stained with hematoxylin and eosin for histological assessment. Morphometric analyses were performed as described by Park et al. (21) by a person unaware of the treatment groups. The numbers of complete airspaces and the mean airspace sizes (area in μm2, perimeter in μm) were determined per high power field.
Immunohistochemistry for neutrophil and macrophage quantification.
Neutrophil and macrophage counts were performed on paraffin-embedded, antineutrophil, or antimacrophage-stained mouse tissues using digital image analysis software with settings for color and size identification (Image Pro Plus 4.0; Media Cybernetics) as previously described (22).
Fatty acid measurement.
Ten percent liver tissue homogenates (200 μL aliquot) were spiked with C23:0 (50 μL, 30 g/L) as an internal standard for extraction recovery and BHT (50 μL, 0.1%) as an antioxidant. The samples were extracted, hydrolyzed by acidification, and methylated with 10% methanolic sulfuric acid (23). FAME were measured by GC with flame ionization detection. Fatty acid levels from C8:0 to C22:6 were measured using experimentally derived standard curves.
Real-time qPCR.
RNA was isolated from lung tissues using TRIzol reagent (Invitrogen) according to the manufacturer’s published protocols. Two micrograms of RNA were reverse transcribed using a Superscript III Reverse Transcriptase kit and oligo d(T) primers (Invitrogen) according to the manufacturer’s protocols. For RT-PCR, RNA was loaded directly onto 96-well plates with each well containing primers for the individual chemokines. SYBR green/ROX master mix (1×) (Superarray Biosciences) was added to the wells and the assays were run on an Applied Biosystems 7500 system and data were analyzed according to the manufacturer’s protocol. Relative gene expression was normalized first to β-actin expression and then data from each experimental group on each day were expressed relative to data from the control/RA group on that day.
Western blots.
Cytoplasmic phospholipase A2 (PLA2) and cyclooxygenase (COX-2) protein levels were assessed by Western blot as previously described (22).
EMSA.
Lungs were harvested from d 3 and d 7 pups and immediately homogenized in sucrose buffer (in 0.25 mol/L sucrose, 10 mmol/L Tris, 1 mmol/L EDTA, pH 7.4). Homogenates were centrifuged at 4°C at 700 × g for 20 min. The supernatant was removed and discarded and the pellet was resuspended in 50 μL nuclear extract buffer [10 mmol/L HEPES, 1.5 mmol/L MgCl2, 420 mmol/L NaCl, 0.1 mmol/L EDTA, 1.5 mmol/L NaF, 10 mmol/L Na3NO4, 1 mmol/L glycerophosphate, 25% (v:v) glycerol, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, 1 mg/L pepstatin, 1 mg/L leupeptin, and 10 mg/L aprotinin at pH 7.9]. Protein concentrations of nuclear extracts were measured using the Bradford assay. Probe sequences were as follows: NF-κB forward: 5′-AGTTGAGGGGACTTTCCCAGGC-3′, reverse: 5′-GCCTGGGAAAGTCCCCTCAACT-3′; PPARγ forward: 5′-AGGTCAAAGGTC-3′ reverse: 5′-TGACCTTTGACCT-3′. The forward and reverse probes were labeled with γP32 ATP using a Rediprime II Random Prime kit (GE Healthcare). Tubes were incubated at 37°C for 30 min. The reaction mixture was purified using a Probe Quant G-50 micro column (Amersham Biosciences). Twenty micrograms of nuclear proteins were incubated with poly-dIdC binding buffer and labeled oligonucleotide probe at room temperature for 30 min. To evaluate the specificity of the oligonucleotide probe, a competition binding reaction was performed using 100× excess of unlabeled oligonucleotide probe. The reaction was separated by SDS-PAGE at 200 V for 75 min. Gels were dried and exposed in a phosphoimaging cassette. Band densities were analyzed using Image Quant software (GE Healthsciences).
Statistical methods.
Survival data were analyzed using Cox regression with a shared γ frailty for pups from the same dam pair. Survival curves were generated using the mean frailty value of 1. Weight data were analyzed using a linear mixed model containing random effects for dam and dam pair and fixed effects for diet, exposure, day, and their 2-way and 3-way interactions and reported as estimated growth curves. Correlations between residual errors from the same pup were modeled using an autoregressive order 1 structure. The Aikaike Information Criterion was used to choose between linear, quadratic, and cubic trends in time. Most of the remaining outcomes, presented as mean ± SEM, were analyzed using a linear mixed model containing random effects for dam and dam pair and fixed effects for diet, exposure, d (3 or 7), and their 2-way and 3-way interactions. However, in some instances, the variances of one or more random effects were zero, which required us to use different approaches. NF-κB, macrophage inflammatory protein-2 (MIP-2), COX-2, and cPLA2 data were first analyzed using a model containing only a random effect for dam pair and then again using a model containing only a random effect for dam and we reported the results from the most conservative model (i.e. the model that gave the largest P-value). Because both the variance of the random dam and dam pair effects equaled zero for IL-1β, we analyzed these data using generalized estimating equations and the MBN method for correcting for small sample bias. Once again, we ran the analysis for IL-1β twice, once ignoring within-dam correlations and once ignoring within-pair correlations, and reported the most conservative results. A different problem arose in our analysis of NF-κB and PPARγ; only 1 dam pair was fed the control diet at each time point. Hence, for these outcomes, the diet-time and diet-exposure-time interactions were confounded by pair and we had to assume that there were no interactions with time to analyze the data. If a significant exposure-diet interaction was identified, we compared diet groups at each level of exposure using a Bonferroni-corrected α of 0.025. Survival analyses were performed using Intercooled Stata version 11 (StataCorp). Mixed model and GEE analyses were performed using SAS version 9.2.
Results
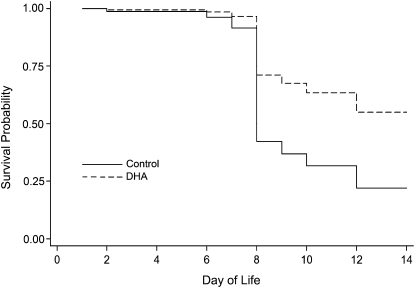
Over the first 7 d of life, survival was similar across maternal diet groups regardless of exposure. Among pups exposed to hyperoxia, an equal percentage of control and DHA diet pups died prior to killing (4 pups or 3%/group) and the distribution of the death times was similar across groups. However, among mice exposed to hyperoxia, the survival curves diverged around d 8. Based on our survival model, a pup nursed by dams fed control diet had a 40% chance of surviving to d 8 or later, whereas the chances of survival were 72% among pups nursed by dams fed DHA diet (Fig. 1). Because of the high mortality at d 8, the studies described in this report were terminated at d 7, which is earlier than morphometric differences in lung growth are consistently detectable (22). Only 1 pup (0.4%) exposed to RA died prior to killing; this pup was in the maternal DHA diet group and died at d 2. All other pups exposed to RA survived until the time of killing regardless of maternal diet group.
FIGURE 1.
Survival of pups nursed by dams fed control or DHA diets and exposed to RA or hyperoxia (<95% O2). Pups were placed in hyperoxia immediately after birth and survival was monitored for 14 d. Despite differences in estimated survival, the survival curves did not significantly differ (likelihood ratio test P = 0.08).
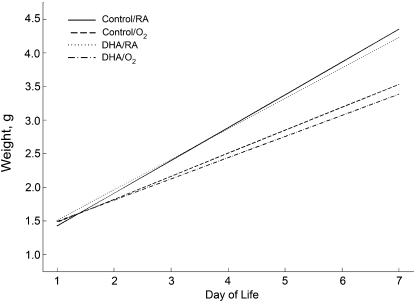
Estimated growth curves were generated at each level of diet and exposure (Fig. 2). Hyperoxia exposure decreased growth over d 1–7. Body weight increased 0.33 ± 0.01 g/d in the hyperoxia-exposed pups and 0.47 ± 0.01 g/d in the RA-exposed pups. Maternal diet did not affect pup weight.
FIGURE 2.
Growth curves of newborn mouse pups nursed by dams fed control or DHA diets and exposed to RA or hyperoxia (<95% O2). Exposure to hyperoxia decreased overall growth, P < 0.001.
Pups in the maternal DHA diet groups had higher hepatic DHA levels than those in the maternal control diet groups regardless of exposure and day of killing (Table 2). These data confirm that DHA tissue contents were altered in the pups by DHA supplementation to the dams.
TABLE 2.
DHA levels in liver of pups nursed by dams fed the control or DHA supplemented diets and exposed to RA or O2 at d 3 or 71
| d 3 |
d 7 |
|||
| Diet/exposure | μmol/g liver | % Total fatty acids | μmol/g liver | % Total fatty acids |
| Cont/RA | 6.68 ± 1.01 | 0.14 ± 0.01 | 7.65 ± 1.04 | 0.13 ± 0.01 |
| DHA/RA | 10.4 ± 0.98* | 0.19 ± 0.01# | 10.2 ± 0.85* | 0.19 ± 0.01# |
| Cont/O2 | 7.24 ± 1.20 | 0.15 ± 0.01‡ | 8.43 ± 1.01 | 0.15 ± 0.0.01‡ |
| DHA/O2 | 11.4 ± 1.08* | 0.19 ± 0.01#‡ | 10.9 ± 0.96* | 0.19 ± 0.01#‡ |
Data are means ± SEM, = 6–13 pups (3–6 litter pairs, 4–8 litters). *Effect of diet, P = 0.004; #effect of diet, P < 0.001; ‡effect of exposure, = 0.009.
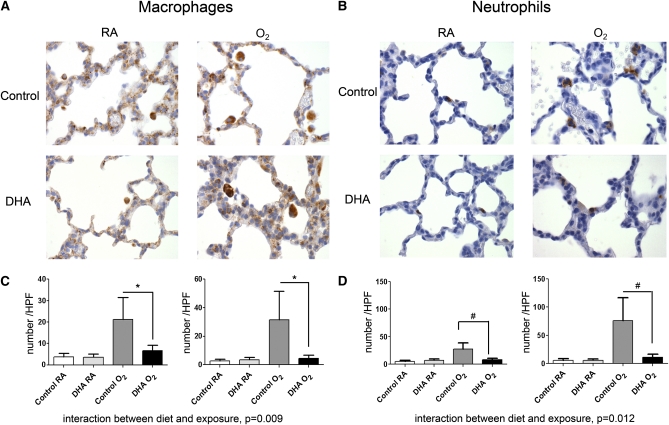
Neutrophil and macrophage numbers were assessed by immunohistochemistry and were low in pups exposed to RA regardless of their diet (Fig. 3A,B). Pups exposed to DHA/O2 had neutrophil and macrophage levels that were similar to both groups of pups exposed to RA (Fig. 3A,B). Macrophage (Fig. 3C) and neutrophil (Fig. 3D) numbers were dramatically elevated in the lung tissues of control/O2 pups as early as d 3 (Fig. 3D, left) and persisted through d 7 (Fig. 3D, right); there was no effect of day. DHA supplementation of the dam diminished hyperoxia-induced neutrophil and macrophage influx in the lungs of the pups.
FIGURE 3.
Immunohistochemistry (A,B) and morphometric analysis (C,D) of macrophages (A,C) and neutrophils (B,D) in pup lung tissues at d 3 (left) or d 7 (right). Photos were taken at 400x magnification. Data were collected by assessing 1 histological section per mouse, counting 5 independent fields per section and using the mean of the 5 fields as a single data point. Data were ln-transformed prior to analysis and back-transformed for the figure. Data are as means ± SEM, n = 5–8 pups (2–4 litter pairs, 2–5 litters). Symbols indicate that the groups differ: *P = 0.004, #P = 0.001.
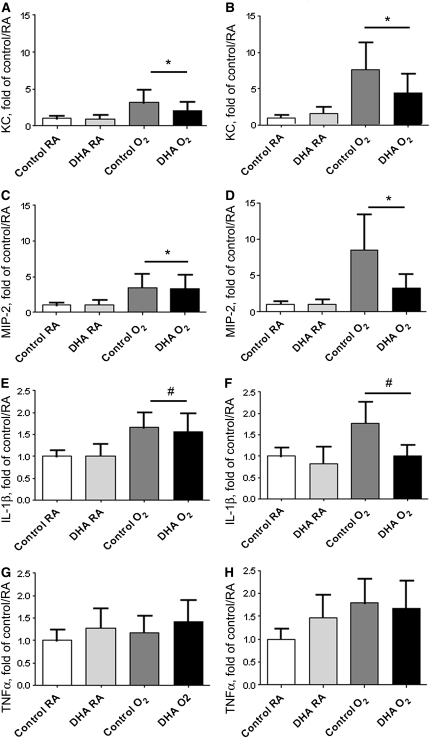
The mRNA levels of the chemokines keratinocyte-derived chemokine (KC) (Fig. 4A,B) and MIP-2 (Fig. 4C,D), responsible for attracting neutrophils and macrophages, were greater in the lungs of the pups exposed to hyperoxia at both d 3 (Fig. 4A,C) and d 7 (Fig. 4B,D). Hyperoxia exposure also increased cytokine mRNA levels in lung tissue homogenates in both control and DHA-supplemented groups; the increase in IL-1β at d 3 (Fig. 4E) and d 7 (Fig. 4F) was significant (P = 0.009), whereas the increase in TNFα at d 3 (Fig. 4G) and d 7 (Fig. 4H) was marginally significant (P = 0.064). Chemokine and cytokine levels were not effected by diet of the dams.
FIGURE 4.
KC (A,B), MIP-2 (C,D) and cytokine (E–H) mRNA levels in lungs of pups nursed by dams fed control or DHA diets and exposed to RA or hyperoxia (<95% O2) at d 3 (left) or d 7 (right). Relative expression was measured using qRT-PCR with cycle numbers normalized to β-actin as a loading control. Data were normalized to the control/RA means on each day. Statistical analyses were performed on the ΔCT values. Data are means ± SEM, n = 5–6 pups (2–4 litter pairs, 3–5 litters). Symbols indicate the effect of exposure, *P < 0.001, #P = 0.009.
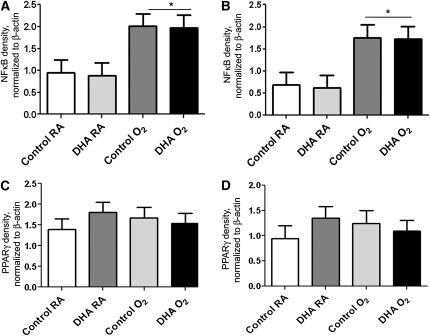
DNA binding activities of NF-κB (Fig. 5A,B) and PPARγ (Fig. 5C,D) were assessed by EMSA. NF-κB activity was increased by hyperoxia exposure in both control and DHA-supplemented groups at d 3 (Fig. 5A) and d 7 (Fig. 5B). However, limitations in the number of samples per analyses prevented the comparisons across days. PPARγ functions as a receptor for DHA and can propagate signaling. There was no effect of diet or hyperoxia exposure on PPARγ activity at d 3 (Fig. 5C) or d 7 (Fig. 5D). As in our analysis of NF-κB, no comparisons across days were performed.
FIGURE 5.
NF-κB (A,B) and PPARγ (C,D) DNA binding activities in lungs of pups nursed by dams fed control or DHA diets and exposed to RA or hyperoxia (<95% O2) at d 3 (A,C) or d 7 (B,D). Data are means ± SEM, n = 3 pups (1–3 litter pairs, 2–3 litters). *Effect of exposure, P < 0.001.
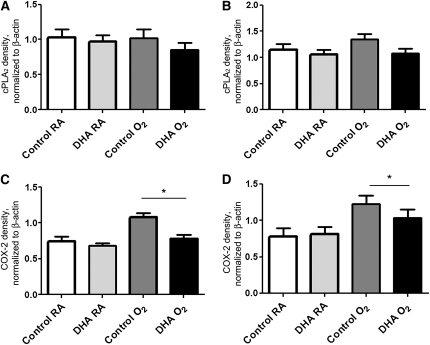
The effect of maternal DHA supplementation on the expression of lipid inflammatory mediators was investigated using Western-blot analysis for cPLA2 and COX-2. In the mouse pups, cPLA2 protein expression was not affected by diet or exposure at either d 3 (Fig. 6A) or d 7 (Fig. 6B). However, COX-2 expression was higher among mice exposed to hyperoxia than in those exposed to RA on both days (Fig. 6C,D). On d 3 (Fig. 6C), COX-2 expression was elevated in the control/O2 pups compared with all other groups, whereas at d 7 (Fig. 6D), expression of COX-2 was greater in both hyperoxia-exposed groups than in the corresponding RA-exposed groups. The increases in COX-2 at d 3 coincided temporally with the observed increases in neutrophil and macrophage infiltrates in the control/O2 group.
FIGURE 6.
cPLA2 (A,B) and COX-2 (C,D) protein levels in lungs of pups nursed by dams fed control or DHA diets and exposed to RA or hyperoxia (<95% O2) at d 3 (A,C) and d 7 (B,D). An adult lung control sample was loaded on each gel to facilitate gel to gel comparisons. Data are means ± SEM, n = 5–13 pups (2–7 litter pairs, 2–7 litters), *Effect of exposure, P = 0.004.
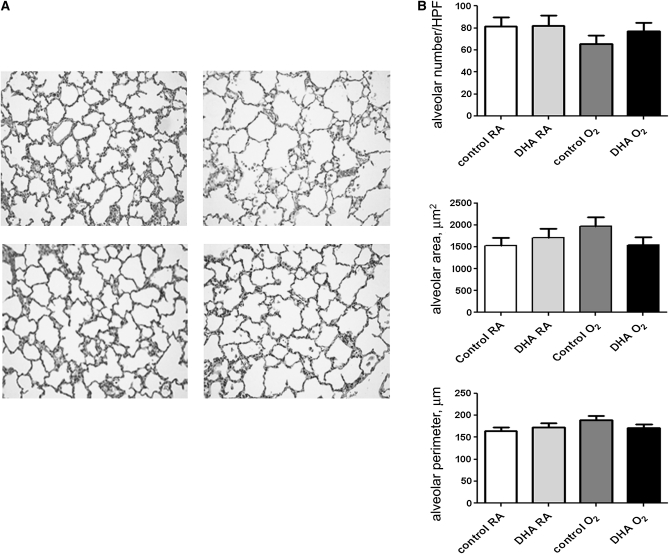
Lung alveolarization, as assessed by morphometric analyses, did not differ among the groups on d 3, but on d 7, lung tissue sections obtained from control/O2 pups tended to have fewer alveoli and greater alveolar area (P = 0.087) than all other groups (Fig. 7).
FIGURE 7.
Histology (A) and morphometric analyses (B) of lung tissues from pups nursed by dams fed control or DHA diets and exposed to RA or O2 on d 7. (A) Histological sections revealed lung growth deficits due to hyperoxia exposure. Photos were taken at 100× magnification. Alveolar number data were natural ln-transformed prior to analysis and back transformed for the figure. Data are means ± SEM, n = 4–7 pups (2–4 litter pairs, 2–4 litters).
Discussion
Survival in hyperoxia was different between C3H/HeN mouse pups from control and DHA diet-fed dams (Fig. 1), which necessitated concluding the current studies at d 7. The occurrence of hyperoxia-induced death prior to 14 d in newborn C3H/HeN mice from control diet-fed dams was surprising and differs from previous reports from our laboratory (24, 25). The causes of these differences in mortality are not readily apparent but may be explained by the contents of the purified diets, which lack natural ingredients found in standard rodent nonpurified diet (Table 1). Our choice to use purified diets in our studies was intended to isolate the effects of preformed DHA supplementation from those of the precursor α-linolenic acid (Table 1).
Elevated hepatic DHA concentrations at d 3 and 7 in pups nursed by DHA-supplemented dams indicates that DHA from the dams’ diet was transferred to the pups (Table 2). These data agree with data from studies of maternal fatty acid supplementation in human infants (3, 26, 27) and support maternal supplementation as a safe and effective method for increasing DHA levels in the offspring. Body growth rates in the pups were not affected by maternal supplementation but were affected by hyperoxia exposure, as we have previously reported (Fig. 2) (22).
One of the more striking findings in these studies was the inhibition of both neutrophil and macrophage accumulation in the lungs of the hyperoxia-exposed newborns by DHA administration to the dams (Fig. 3). Similarly, investigations using other adult models of lung injury have demonstrated suppression of proinflammatory cytokines and inflammatory infiltrates with dietary long-chain fatty acid supplementation (15, 28), but to our knowledge we are the first to demonstrate this in hyperoxia-exposed newborn mice. Seminal reports describing the sequence of events in hyperoxic lung injury identified endothelial disruption and loss of endothelial integrity as the earliest physiologically detectable effect (29–31). Endothelial disruption includes increased affinity of adhesion molecules for leukocytes and extravasation of neutrophils and macrophages from the circulation into the alveolar interstitium or alveolar space. Several more recent studies in atherosclerosis models have reported suppression of adhesion molecule expression as a mechanism for the antiinflammatory properties of DHA (32–35). Stabilization of the endothelium and decreased adhesion molecule expression could explain the observed decreases in neutrophil and macrophage numbers in the lungs of DHA/O2 pups.
Lung epithelial cells secrete chemotactic signals that further induce the localization and activity of leukocytes. The chemokines KC and MIP-2 and the cytokines IL-1β and TNFα increase during acute lung injury. In our model, the temporal relationship between inflammatory cell infiltration and expression of chemokines and cytokines was confirmed by the increases in KC, MIP-2, and IL-1β expression at 3 d and 7 d in O2 pups (Fig. 4); however, there was no effect of diet. These data suggest that the DHA-mediated dampening of macrophage and neutrophil infiltration into the lungs of hyperoxia-exposed pups is not simply through suppression of chemotaxis.
Inflammatory responses are mediated through common pathways and often include activation of NF-κB (36). Our initial hypothesis was that activation of NF-κB by hyperoxia was in part responsible for early influx of inflammatory cells into the lung and that activation of NF-κB would be lessened by DHA supplementation. NF-κB was indeed activated by hyperoxia exposure, but no effect of diet was observed. Our data suggest that the mechanism by which DHA inhibits inflammatory cell recruitment does not involve direct inhibition of NF-κB–mediated pathways (Fig. 5). PPARγ is a receptor that directly binds DHA and mediates its antiinflammatory, proresolution activities (11, 37). We found no differences in PPARγ DNA binding activity among the groups (Fig. 5), which suggests that DHA decreases inflammatory responses in this lung injury model by a mechanism unrelated to PPARγ signaling.
Lipid metabolites can be a substantial source of inflammatory mediators possessing both pro- and antiinflammatory properties (38, 39). The enzymes cPLA2 and COX-2 are induced by stress and are upstream of many bioactive lipid metabolites. Changes in the activity of either or both enzymes could directly influence leukocyte chemotaxis. In this study, we observed no differences in cPLA2 protein levels at either time point (Fig. 6 A,B). These findings are contrary to our earlier report that exposure to hyperoxia increased cPLA2 levels at d 7 and 14 in pups nursed by dams fed a standard nonpurified diet (22). These differences may be related to the purified diet used in the present studies or may be due to minor temporal shifts in expression that would have become evident by d 8. Our previous studies have demonstrated that COX-1 levels in the lungs of newborn mice were not altered by hyperoxia exposure (L. K. Rogers and T. E. Tipple, unpublished data). Consequently, COX-1 levels were not measured in the present study. However, COX-2 protein levels increased at d 3 in the control/O2 pups, which is earlier than we previously reported (22). Furthermore, the change temporally coincided with the observed increase in macrophages and neutrophils in the lungs of control/O2 pups. The increase in COX-2 protein expression persisted through d 7 and may be an important component of the mechanism(s) by which DHA inhibits hyperoxia-induced inflammatory processes. Lipid products of DHA, such as resolvins and protectins, have been shown to decrease inflammatory responses in many disease models (40–42). Alternatively, Chapkin et al. (36) recently reported that DHA alters membrane structure, causing increased fluidity with subsequent effects on lipid raft formation and protein signaling complexes. Alterations in signaling pathways other than those investigated in the present study may provide an explanation for the decreases in leukocyte infiltration in DHA supplemented mouse pups
Hyperoxic exposure during the neonatal period inhibits lung alveolarization in newborn mice (43, 44). Although inflammation plays a major role, the mechanisms responsible for the effects of hyperoxia in lung development have not been clearly defined. At d 7, the lungs of mouse pups are still in the canulicular stage and extensive alveolarization has not yet taken place. Although we did not observe dramatic differences in morphometric analyses of lung tissues from DHA/O2 compared with control/O2 pups at d 7, there was a trend toward improved lung development. Obviously, later time points using lower oxygen concentrations that are associated with improved survival are essential to adequately assess the effects of DHA supplementation on hyperoxia-induced alterations in lung growth (Fig. 7).
In summary, our studies indicate that DHA supplementation of pregnant and lactating dams lessens inflammatory responses to hyperoxia in the lungs of nursing newborn mice. These data support the hypothesis that DHA-mediated suppression of chemotaxis and proinflammatory responses associated with neutrophil and macrophage activation positively affects outcomes. However, discernable improvements in lung growth and development will require studies using lower oxygen concentrations to improve survival. Clinically, maternal DHA supplementation is a promising and low-cost therapy for preterm infants that has the potential not only to improve neurological development but also to lessen inflammatory responses and improve outcomes.
Acknowledgments
We thank Xiaomei Meng and Molly Augustine for their exceptional technical assistance and Martek, Inc. for providing the purified DHA oil. L.K.R., C.J.V., S.E.W., and T.E.T. designed research; M.V., R.D.B., K.D., and X.Z. conducted research; M.P. analyzed data and wrote the statistical portions of the paper; L.K.R. and T.E.T. wrote the paper; and L.K.R. primarily responsible for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the American Thoracic Society (L.K.R.), The Research Institute at Nationwide Children’s Hospital (C.J.V., S.E.W., and T.E.T.), and the NIH (K12 HD043372 to T.E.T).
Abbreviations used: COX, cyclooxygenase; KC, keratinocyte-derived chemokine; MIP-2, macrophage inflammatory protein-2; O2, >95% oxygen; PLA2, phospholipase A2; RA, room air. The 4 pup groups were control diet, room air (control/RA); DHA-supplemented diet, room air (DHA/RA); control diet, >95% O2 (control/O2); and DHA-supplemented diet, >95% O2 (DHA/O2).
Literature Cited
- 1.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–35 [DOI] [PubMed] [Google Scholar]
- 2.Yavin E. Versatile roles of docosahexaenoic acid in the prenatal brain: from pro- and anti-oxidant features to regulation of gene expression. Prostaglandins Leukot Essent Fatty Acids. 2006;75:203–11 [DOI] [PubMed] [Google Scholar]
- 3.Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr. 2009;89:S678–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6:437–49 [DOI] [PubMed] [Google Scholar]
- 5.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;(26 Suppl A):S70–5 [DOI] [PubMed] [Google Scholar]
- 6.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–8 [DOI] [PubMed] [Google Scholar]
- 7.Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J Ren Nutr. 2007;17:296–304 [DOI] [PubMed] [Google Scholar]
- 8.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505 [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–86 [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–51 [DOI] [PubMed] [Google Scholar]
- 11.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67:867–74 [DOI] [PubMed] [Google Scholar]
- 12.Kang JX. Fat-1 transgenic mice: a new model for omega-3 research. Prostaglandins Leukot Essent Fatty Acids. 2007;77:263–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer K, Kiessling A, Ott J, Schaefer MB, Hecker M, Henneke I, Schulz R, Guenther A, Wang J, et al. Acute lung injury is reduced in fat-1 mice endogenously synthesizing n-3 fatty acids. AM J Respir Crit Care Med. 2009;179:474–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman SD, Weinstein D, Blanco PG, Martinez-Clark P, Urman S, Zaman M, Morrow JD, Alvarez JG. Characterization of LPS-induced lung inflammation in cftr−/− mice and the effect of docosahexaenoic acid. J Appl Physiol. 2002;92:2169–76 [DOI] [PubMed] [Google Scholar]
- 15.Tiesset H, Pierre M, Desseyn JL, Guery B, Beermann C, Galabert C, Gottrand F, Husson MO. Dietary (n-3) polyunsaturated fatty acids affect the kinetics of pro- and antiinflammatory responses in mice with Pseudomonas aeruginosa lung infection. J Nutr. 2009;139:82–9 [DOI] [PubMed] [Google Scholar]
- 16.Koletzko B, Agostoni C, Carlson SE, Clandinin T, Hornstra G, Neuringer M, Uauy R, Yamashiro Y, Willatts P. Long chain polyunsaturated fatty acids (LC-PUFA) and perinatal development. Acta Paediatr. 2001;90:460–4 [PubMed] [Google Scholar]
- 17.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–64 [DOI] [PubMed] [Google Scholar]
- 18.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998;53:81–94 [DOI] [PubMed] [Google Scholar]
- 19.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006;11:354–62 [DOI] [PubMed] [Google Scholar]
- 20.Valentine CJ, Morrow G, Fernandez S, Gulati P, Bartholomew D, Long D, Welty SE, Morrow AL, Rogers LK. Docosahexaenoic acid and amino acid content in pastuerized donor milk are low for preterm infants. J Pediatr. 2010;157:906–10 [DOI] [PubMed] [Google Scholar]
- 21.Park MS, Rieger-Fackeldey E, Schanbacher BL, Cook AC, Bauer JA, Rogers LK, Hansen TN, Welty SE, Smith CV. Altered expressions of fibroblast growth factor receptors and alveolarization in neonatal mice exposed to 85% oxygen. Pediatr Res. 2007;62:652–7 [DOI] [PubMed] [Google Scholar]
- 22.Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res. 2009;65:33–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl. 1995;671:113–31 [DOI] [PubMed] [Google Scholar]
- 24.Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res. 2009;65:33–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers LK, Tipple TE, Britt RD, Welty SE. Hyperoxia exposure alters hepatic eicosanoid metabolism in newborn mice. Pediatr Res. 2010;67:144–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helland IB, Saugstad OD, Saarem K, Van Houwelingen AC, Nylander G, Drevon CA. Supplementation of n-3 fatty acids during pregnancy and lactation reduces maternal plasma lipid levels and provides DHA to the infants. J Matern Fetal Neonatal Med. 2006;19:397–406 [DOI] [PubMed] [Google Scholar]
- 27.van Goor SA, Dijck-Brouwer DA, Hadders-Algra M, Doornbos B, Erwich JJ, Schaafsma A, Muskiet FA. Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins Leukot Essent Fatty Acids. 2009;80:65–9 [DOI] [PubMed] [Google Scholar]
- 28.Kinniry P, Amrani Y, Vachani A, Solomides CC, Arguiri E, Workman A, Carter J, Christofidou-Solomidou M. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr. 2006;136:1545–51 [DOI] [PubMed] [Google Scholar]
- 29.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–31 [DOI] [PubMed] [Google Scholar]
- 30.Crapo JD, Barry BE, Foscue H, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Res Dis. 1980;122:123–43 [DOI] [PubMed] [Google Scholar]
- 31.Crapo JD, Freeman BA, Barry BE, Turrens JF, Young SL. Mechanisms of hyperoxic injury to the pulmonary microcirculation. Physiologist. 1983;26:170–6 [PubMed] [Google Scholar]
- 32.Mayer K, Merfels M, Muhly-Reinholz M, Gokorsch S, Rosseau S, Lohmeyer J, Schwarzer N, Krull M, Suttorp N, et al. Omega-3 fatty acids suppress monocyte adhesion to human endothelial cells: role of endothelial PAF generation. Am J Physiol Heart Circ Physiol. 2002;283:H811–8 [DOI] [PubMed] [Google Scholar]
- 33.Goua M, Mulgrew S, Frank J, Rees D, Sneddon AA, Wahle KW. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: involvement of the transcription factor NF-kappaB? Prostaglandins Leukot Essent Fatty Acids. 2008;78:33–43 [DOI] [PubMed] [Google Scholar]
- 34.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71:S213–23 [DOI] [PubMed] [Google Scholar]
- 35.De Caterina R, Spiecker M, Solaini G, Basta G, Bosetti F, Libby P, Liao J. The inhibition of endothelial activation by unsaturated fatty acids. Lipids. 1999;34 Suppl:S191–4 [DOI] [PubMed] [Google Scholar]
- 36.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bannenberg G, Arita M, Serhan CN. Endogenous receptor agonists: resolving inflammation. ScientificWorldJournal. 2007;7:1440–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serhan CN. Novel omega–3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther. 2005;105:7–21 [DOI] [PubMed] [Google Scholar]
- 39.Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8:115–21 [DOI] [PubMed] [Google Scholar]
- 40.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–37 [DOI] [PubMed] [Google Scholar]
- 41.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153 Suppl 1:S200–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonnans C, Levy BD. Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am J Respir Cell Mol Biol. 2007;36:201–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitiello PF, Staversky RJ, Gehen SC, Johnston CJ, Finkelstein JN, Wright TW, O'Reilly MA. p21Cip1 protection against hyperoxia requires Bcl-XL and is uncoupled from its ability to suppress growth. Am J Pathol. 2006;168:1838–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1101–11 [DOI] [PubMed] [Google Scholar]
- 45.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]