Abstract
Purpose
Stroke caregivers often express the need for information about stroke and assistance with stroke-related care in the early discharge period. The Telephone Assessment and Skill-Building Kit (TASK) is an 8-week program that addresses caregiver needs. This study explored the efficacy of the TASK program in improving stroke caregiver outcomes.
Method
Guided by a conceptual model, 6 outcomes (optimism, task difficulty, threat appraisal, depressive symptoms, life changes, general health perceptions) were measured in 40 caregivers randomized to the TASK (n = 21) or an attention control group (n = 19). Data were analyzed using analysis of covariance (ANCOVA), controlling for baseline scores and minutes spent with the nurse.
Results
Significant increases in optimism at 4 weeks, 8 weeks, and 12 weeks were found, with medium effect sizes for the TASK group relative to the control group (p < .05). Significant improvements in task difficulty at 4 weeks, and threat appraisal at both 8 weeks and 12 weeks were also found (p < .05).
Conclusion
Caregivers receiving the TASK intervention improved in optimism, task difficulty, and threat appraisal. Further testing of an enhanced version of the TASK program is warranted, with attention directed toward more distal stroke caregiver outcomes.
Keywords: clinical trial, depression, family caregivers, health, intervention studies, outcomes, quality of life, stroke, stress
Stroke is a leading cause of serious long-term disability.1 Because of disability, 68% to 74% of stroke survivors require the care of family members.2,3 These family caregivers need to quickly learn how to assist stroke survivors with a variety of impairments (including motor, sensory, visual, language, cognitive, and affective),1,4 while adapting to the changes in their own lives as a result of providing care.5–9 Caregivers’ needs are often not met because of inadequate training from health care providers.6,10,11 Such unmet needs can increase caregivers’ stress, strain, and risk for mortality,12 as well as impede the rehabilitation of the survivor13 and increase the survivor’s risk for costly, long-term institutionalization.13
Many authors recommend that future interventions focus on meeting caregivers’ specific needs.6,14–19 Clinically tested interventions should help caregivers gain skills that would help them avoid negative caregiver outcomes. The purpose of this study was to determine the preliminary efficacy of the Telephone Assessment and Skill-Building Kit (TASK), an 8-week intervention program that assesses caregivers’ unmet needs and helps them to obtain needed information and skills in the early discharge period.
Background
Assessment of stroke caregiver needs from the caregiver’s perspective is increasingly emphasized in the literature,6,18,20,21 as well as in current patient care guidelines for stroke rehabilitation.22,23 These guidelines22 recommend that health care providers involve family caregivers in making decisions and planning treatments for survivors, be alert to the stress and support needs of caregivers, provide information on community resources and services, and provide patient and family caregiver education about stroke and potential complications. Despite these recommendations, family caregivers are commonly neglected by health care providers in the practice setting6,10,11 and, as a result, experience a variety of unmet needs.6,20
Caregiver needs
Integrative reviews suggest that skill-building interventions are more helpful than psycho-educational support interventions.17,24–26 Stroke family caregivers have needs for building skills within a variety of areas. Caregivers desire more information about warning signs of a second stroke, risk factors, and recommended lifestyle changes.6,27 Managing emotions and behaviors in stroke survivors is among the most stressful aspects of providing care and contributes to caregiver depressive symptoms.5,21,28,29 Needs and concerns about providing physical care include such things as assisting the survivor with activities of daily living, managing symptoms and deficits, and managing medications.5,6,30 Providing instrumental care includes dealing with financial issues, providing transportation, assisting with household tasks, as well as finding someone to care for the survivor while the primary caregiver is away.5,6,21 Finally, caregivers commonly neglect their own needs, including dealing with their emotions and depressive symptoms, shouldering new responsibilities, balancing caregiving with existing responsibilities (e.g., employment), asking family and friends for help, keeping their social life going, as well as keeping their energy level up and taking care of their health.6,21
Caregiver outcomes
Caregivers with unmet needs can experience depressive symptoms, negative life changes, and poorer physical health. These depressive symptoms can be associated with added strain, which has been shown to be a risk factor for caregiver mortality.12 Estimates of the prevalence of depressive symptoms in stroke caregivers range between 30% and 52%,13,31,32 with the poorest mental health occurring when survivors are discharged home early.33 Factors associated with caregiver depressive symptoms include difficulty with caregiving tasks, high threat appraisal, and negative life changes.5–9 Tasks most difficult for caregivers include managing finances, managing the survivor’s behaviors, and providing emotional support to the survivor.5 Negative life changes include lack of time for family and friends and worsening emotional well-being, physical health, and financial well-being.8,9 The wide variety of unmet caregiver needs (e.g., obtaining information, managing emotions and behaviors, providing physical and instrumental care, dealing with personal responses) that contribute to negative caregiver outcomes (e.g., difficulty with tasks, high threat appraisal, depressive symptoms, negative life changes, poor health) underscore the need for multicomponent caregiver interventions in this population.
Stroke caregiver intervention research
Research has not produced sufficient evidence on stroke caregiver interventions that can be easily incorporated into practice.10,13,18,23,25,34 Visser-Meily and colleagues18 identified 22 studies in that area from January 1966 to March 2003; in updating their review to December 2008, five additional studies were found.35–39 Out of the total of 27 studies reviewed,18,35–39 only 13 (48%) reported positive results on one or more outcome measures. Several potential reasons for the nonsignificant findings have led to recommendations for future research.
A recent meta-analysis of four stroke caregiver intervention studies highlighted the importance of a theoretical rationale to guide such studies so that interventions can be better explained.40 This recommendation has appeared in other caregiver literature as well.24,41 Forster et al25 concluded from their review of stroke caregiver intervention studies that the provision of information alone had no effect on mood, perceived health status, or quality of life for stroke caregivers. Other interventions that combined education with problem-solving strategies were much more effective than the use of education alone.25,42,43 These studies as a whole suggest the importance of using a combination of approaches; this view is consistent with caregiver meta-analyses that reveal that multicomponent interventions using skill-building strategies are relatively more effective.17,26,44 Caregiver intervention studies that better target the needs and concerns of family caregivers, rather than the needs of stroke survivors, have been strongly recommended.18 Other studies indicate that tailoring interventions to the individual needs of caregivers is more effective than group interventions.16,17,19 These recommendations are also consistent with caregiver studies that suggest selecting caregivers who have the most needs, then tailoring interventions to address those needs.45,46
Recommendations regarding methodological issues have also been made. Lee and colleagues40 recommended that more attention be directed toward ensuring treatment fidelity of stroke caregiver interventions. Correct timing and length for interventions (7–9 sessions) is also recommended.17,18,47 In terms of outcomes measurement, quality outcome measures relevant to stroke caregivers are essential because existing research has been limited by the use of measures that lacked sensitivity to detect relevant changes.18,48
Tele-health is another area relevant to intervention design, because many stroke caregivers are unable to attend on-site sessions and face-to-face visits in the home are expensive and time-consuming.49 Although video-conferencing is gaining popularity, some caregivers have expressed discomfort with the technology, which includes concerns about privacy and home security. Other drawbacks include poor connectivity and costs related to installing equipment in the home.49 Currently, no reported stroke caregiver interventions have been delivered completely by telephone; most involve at least one face-to-face visit.50
The TASK intervention was designed based on recommendations from the literature. A conceptual model derived from Lazarus’ theory51,52 was used to evaluate the TASK intervention. TASK is an individualized, multicomponent intervention designed to promote skill building in stroke caregivers based on their needs and concerns. Delivered completely by telephone, the eight-session TASK intervention is initiated within 1 month after the stroke survivor is discharged to the home setting, a time period when caregivers have the opportunity to identify and articulate their needs. Treatment fidelity and quality outcome measures were carefully considered in the design of the study.
Conceptual Model
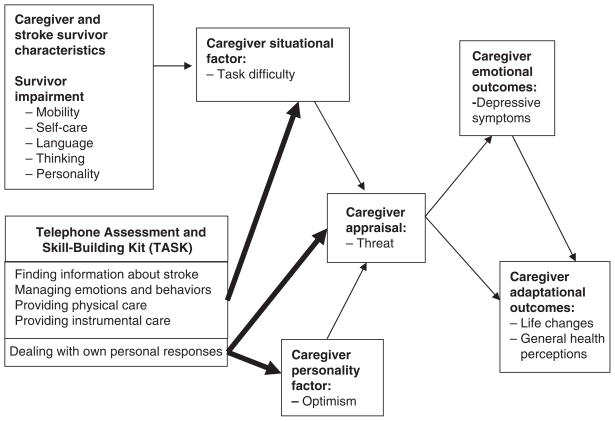
The conceptual model for the study (Figure 1) was derived from Lazarus’s transactional theory of stress.51,52 Lazarus posits that when individuals encounter a stressful situation, personality and situational factors are mediated by appraisal, resulting in emotional and adaptational outcomes.51,52 In this study, caregiver optimism represents a personality factor, task difficulty represents a situational factor, threat appraisal represents a mediator, depressive symptoms represent an emotional outcome, and life changes and general health perceptions represent adaptational outcomes. Threat appraisal is defined as the anticipation of harm or loss associated with providing care.7–9,51,52 Caregiver life changes refer to changes in social functioning, subjective well-being, and physical health specifically as a result of providing care,7–9 consistent with Lazarus’s definition of adaptational outcomes.51,52
Figure 1.
Conceptual model.
The TASK intervention was designed to reduce caregiver task difficulty by addressing caregivers’ needs in four main areas: (a) finding information about stroke, (b) managing the survivor’s emotions and behaviors, (c) providing personal care, and (d) providing instrumental care. It was also designed to increase caregiver optimism and reduce threat appraisal by helping caregivers deal with their personal responses to providing care. Reducing task difficulty and threat appraisal and improving optimism were expected to result in better emotional and adaptational outcomes for the caregiver. The conceptual model derived from Lazarus’s theory has been supported in prior studies.7–9 For example, model constructs including task difficulty, threat appraisal, and depressive symptoms explained a significant amount of variance in life changes.7–9 Caregiver threat appraisal was also found to be an independent predictor of caregiver emotional distress and general health perceptions.7
Method
Design
A randomized controlled clinical trial design was used to test the efficacy of the TASK intervention. Guided by the conceptual model in Figure 1, six theoretically based outcomes were measured at baseline, 4 weeks (half-way though intervention), 8 weeks (end of intervention), and 12 weeks (4 weeks after end of intervention). Caregiver and stroke survivor characteristics were also measured at baseline.
TASK intervention
Written tip sheets were developed for each of the 32 items in the Caregiver Needs and Concerns Checklist (CNCC)6 addressing the five areas of skill-building needs: (a) finding information about stroke, (b) managing the survivor’s emotions and behaviors,(c)providing physical care,(d)providing instrumental care, and (e) dealing with personal responses to providing care. In addition, five process tip sheets provided skill-building strategies such as strengthening existing skills, screening for depressive symptoms, maintaining realistic expectations, problem solving, and communicating with health professionals. A workbook detailing stress management strategies53–55 for the survivor and the caregiver was also developed.
The tip sheets and workbook were revised based on evaluations by 10 experts including 4 nurses, 1 project manager involved in the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) studies,56 1 neuropsychologist, and 4 experienced stroke caregivers. The final TASK notebook contained 38 written tip sheets, a stress management workbook, and a brochure on family caregiving from the American Stroke Association (ASA). After baseline, the notebook was mailed to caregivers randomized to the TASK intervention. Caregivers in the TASK intervention group received eight weekly calls by a nurse who facilitated caregivers’ weekly assessment of skill needs using the CNCC,6 followed by individualized interventions that addressed priority skill needs identified by the caregiver. The individualized interventions based on each caregiver’s priority skill needs enabled the caregivers to build their skills in the areas most pertinent to them during the early discharge period.
Attention control
The attention control group received a brochure on family caregiving from the ASA and eight weekly calls from a nurse. During the calls, nurses only provided active listening and paraphrasing; they provided no advice or information to the caregivers other than telling them to contact their health care provider or to contact the ASA for additional materials.
Treatment fidelity
Special effort was made to enhance, maintain, and track treatment fidelity of both the TASK intervention and attention control conditions during the pilot. The Treatment Fidelity Checklist (TFC) published by Borelli and colleagues41 was used as a guide. The TFC includes topics related to design, training, delivery, receipt, and enactment of interventions.41 Design involved monitoring treatment dose for both intervention and control groups. Two caregivers in the TASK group and two in the control group missed one out of the eight weekly nurse calls. The remaining 36 caregivers completed all eight calls. Number of minutes of telephone contact was monitored as well. The design was also enhanced by basing the TASK intervention on a conceptual model that allowed for tailoring of interventions to meet individual caregiver needs. Training was provided for the nurses using standardized protocols and treatment manuals for both intervention and control groups. Prior to interacting with caregivers, nurses were required to “pass” role-playing sessions using structured checklists. Nurses were also required to have a current registered nurse license. Delivery was monitored through audio-taping of calls and evaluation of adherence to protocols using checklists. Individualized retraining was provided based on evaluations. Receipt was assessed by asking caregivers to rate the amount of information they received each week (too little, just right, too much). Enactment was assessed by asking caregivers to rate (a) how much they used the skill-building strategies from the prior week (not used, used a little, some, a lot); (b) how helpful each of the TASK tip sheets were (not helpful, little, moderate, very, extremely); and (c) how their problems were resolved (not resolved, making progress, fully resolved, resolved on its own).
Sample and procedures
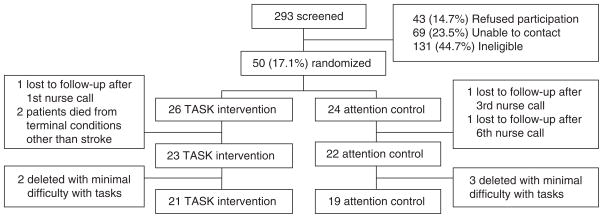
Caregivers were recruited from a local rehabilitation hospital and three local acute care hospitals. The participant flow diagram is provided in Figure 2. A total of 293 caregivers were screened from March 2005 through June 2006. Of those, 69 (23.5%) could not be contacted, 43 (14.7%) refused participation, and 131 (44.7%) were ineligible. Of the 131 ineligible caregivers, 47 considered the stroke survivors to be the same as before the stroke or had fewer than two caregiver tasks; 35 reported that the survivor was in long-term care; 16 reported that the survivor had died; 10 caregivers were unable to hear or read or were not fluent in the English language; 10 reported that the survivor had a history of alcohol or drug abuse or severe mental illness; 8 reported that the survivor did not have a stroke; 4 caregivers were outside the enrollment window; and 1 caregiver was pregnant.
Figure 2.
Participant flow diagram.
Following baseline data collection, a total of 50 caregivers were randomized to the TASK intervention (n = 26) or attention control group (n = 24) using a block randomization procedure in blocks of six to promote equal sample sizes. Randomization assignments were created a priori by the statistician, with outcome data collectors being blinded to randomized group allocation throughout the study period. Five caregivers were removed from the analysis because of minimal difficulty with tasks. This exclusion criterion will be used in future studies to conserve resources and to target the intervention to caregivers most in need, as recommended in other caregiver research.45,46 There was very low attrition over the course of the study. One survivor died from pancreatic cancer and one died from chronic liver failure. Screening procedures will be revised in the future to avoid enrolling caregivers managing end of life issues. Only one caregiver in the TASK group and two caregivers in the attention control group were lost to follow-up. The final sample for data analysis included 40 caregivers (TASK, n = 21; attention control, n = 19). The study was approved by the university institutional review board, and caregivers gave informed consent prior to data collection.
Instruments
A demographic data form was used to collect information regarding caregiver and stroke survivor characteristics. Stroke survivor impairment was measured using the Stroke Specific Quality of Life Scale Proxy (SSQOL-Pr), a 49-item tool measuring quality of life in stroke survivors from the perspective of the caregiver.57,58 SSQOL-Pr subscales represent quality of life domains that are specifically affected by stroke. These domains represent common areas of stroke impairment from the perspective of the caregiver. Five subscales were selected for this study consistent with the conceptual model (mobility, self-care, language, thinking, and personality). Possible subscale scores range from 1 to 5, with lower scores meaning more impairment. The SSQOL-Pr was necessary because some survivors had aphasia and/or cognitive impairment and because stroke survivors were not enrolled into the study. Both the SSQOL and the SSQOL-Pr have acceptable evidence of internal consistency reliability, construct validity, and responsiveness to change,57,58 although the SSQOL-Pr scores have yielded lower scores for quality of life perceptions than SSQOL scores for patients.57 Cronbach alphas for the SSQOL-Pr have ranged from .83 to .92.9 In this study, Cronbach alphas were acceptable (mobility .93, self-care .83, language .96, thinking .74, personality .91).
The Revised Life Orientation Test (LOT-R) was used to measure caregiver optimism as an antecedent personality factor. The LOT-R consists of six items rated on a Likert-type scale, with higher scores indicating greater optimism. The LOT-R has documented evidence of reliability and validity59 and has been used in studies with stroke caregivers9,60,61 as well as cancer caregivers.62 Cronbach’s alpha for this study was .73.
Perceived difficulty with tasks was measured by the Oberst Caregiving Burden Scale Difficulty Subscale (OCBS). The OCBS measures the perceived difficulty associated with 15 different types of caregiving tasks, with scores ranging from 15 to 75.5 Item responses range from not difficult =1 to extremely difficult = 5. Acceptable evidence of both content and construct validity has been reported in cancer caregivers.63 Evidence of construct validity, including unidimensionality, has been reported in stroke caregivers.5 Internal consistency reliability estimates in stroke caregivers have ranged from .84 to .94.5,7–9 Cronbach’s alpha for this sample was .85.
Defined as the anticipation of harm or loss associated with providing care, threat appraisal was measured by the Appraisal of Caregiving Threat Subscale (ACS). The ACS measures the degree to which caregivers’ tasks, relationships, interpersonal support, lifestyle, emotional and physical health, and the overall personal impact of caregiving are appraised as threatening.64 Scores range from 12 to 60, and items are rated on a 5-point Likert-type scale ranging from strongly disagree to strongly agree. Evidence of internal consistency reliability, content, and construct validity has been reported in cancer caregivers.64 Internal consistency reliability estimates in stroke caregivers have ranged between .86 and .92.7–9 Cronbach’s alpha for this sample was .92.
The Patient Health Questionnaire Depression Scale (PHQ-9) measuring caregiver depressive symptoms is based on the nine DSM-IV criteria for depressive disorders. Items are rated on a 4-point scale ranging from not at all to nearly every day, with total scores ranging from 0 to 27. Evidence of internal consistency reliability, validity, specificity, and sensitivity has been reported in primary care populations65 as well as in stroke survivors.66 In stroke caregivers, internal consistency estimates have ranged from .80 to .86.9 The advantages of using the PHQ-9 are its brevity as well as its clinically meaningful cutoff points for no (0–4), mild (5–9), moderate (10–14), moderately severe (15–19), and severe depression (20–27) that are easily interpreted by health care providers.65,66 Cronbach’s alpha for this sample was .84.
The 15-item Bakas Caregiving Outcomes Scale (BCOS) measures caregiver life changes specifically as a result of providing care.8,9 Items address changes in social functioning, subjective well-being, and physical health consistent with Lazarus’s51,52 definition of adaptational outcomes. Items are rated on a 7-point scale ranging from changed for the worst to changed for the best. Total scores range from 15 to 105, with lower scores indicating more negative life changes. Evidence of content validity, internal consistency reliability, test-retest reliability, construct validity, criterion-related validity, and unidimensionality for the total scale has been reported in three different samples of stroke caregivers.8,9 Internal consistency reliability estimates have ranged from .77 to .90.8,9 Cronbach’s alpha for this sample was .92.
The SF-36 Health Survey General Health Subscale (SF-36GH) is a widely used measure with established psychometric properties.67 The SF-36GH consists of five items rated on a Likert-type scale that are scored to range from 0 to 100, with higher scores indicating better general health perceptions. The SF-36GH has been used in research with stroke caregivers with acceptable documented reliability.7–9 Cronbach’s alpha for this sample was .87.
Data analysis
Data were screened and double-checked for errors in data entry. One-sample Kolmogovov-Smirnov Z tests were used to assess normality, and Cronbach’s alpha was used to estimate internal consistency reliability. No significant non-normality was detected, and Cronbach’s alphas ranged from .73 to .96 for all of the scales. Descriptive statistics, independent samples t tests, and chi-square tests were used to describe caregiver and survivor characteristics and to assess for baseline group differences. There was a significant difference in the total number of minutes spent on the telephone with the nurse for the TASK group (mean =236.8 minutes) relative to the attention control group (128.8 minutes), t =4.252, p < .001. This difference indicated the need for adjustment of the findings to account for dose-response effects.68 Total number of minutes of telephone contact with the nurse was therefore used as a covariate in assessing preliminary intervention effects for each of the outcomes. For each time point, data were analyzed using univariate analysis of covariance (ANCOVA) for each outcome, controlling for baseline scores and number of minutes spent with the nurse. The study was not designed to have sufficient power to detect treatment by group interactions, because it was a pilot; therefore separate univariate models were tested for each time point separately. This was a pilot study, so no adjustments were made to the significance level because of multiple testing. Partial η2 was used to estimate effect sizes using SPSS for ANCOVA (small, ≤.08; medium, .09–.24; large, ≥.25).69,70 Partial η2 is used in ANCOVA to represent the percent variance in the dependent variable explained by the independent variable (i.e., treatment group) while controlling for covariates.69,70 It is a comparable estimate to r2.69,70 Outcomes included caregiver optimism, task difficulty, threat appraisal, depressive symptoms, life changes, and general health perceptions. Alpha of p < .05 for a two-tailed test was used to interpret the findings.
Results
Caregiver and stroke survivor characteristics for each group, including survivor impairment, are provided in Table 1. Similar to other caregiver studies,8,9,46,71,72 the mean age of caregivers was 57 years, with 73% of the caregivers being female, 58% spouses, 25% adult children, and 17% representing other types of relationships. In terms of race, 25% were African American, 73% Caucasian, and 2% other, which is reflective of the region where recruitment sites were located. There were significant differences in terms of survivor gender, with more male survivors in the attention control group (χ2 = 5.11, p = .02). Adjusting for this variable did not influence the findings. There were no other significant baseline group differences in caregiver and survivor characteristics. Survivor impairment scores were moderate across all five subscales on a 1 to 5 scale, with 5 meaning low impairment.
Table 1.
Caregiver and stroke survivor characteristics (N = 40)
| Caregiver characteristics | Intervention (n =21) |
Control (n =19) |
Statistic | p |
|---|---|---|---|---|
| Mean (SD) or frequency (%) | Mean (SD) or frequency (%) | |||
| Age | 56.43 (9.61) | 57.84 (11.8) | t = −.42 | .68 |
| Gender | ||||
| Male | 8 (38%) | 3 (16%) | χ2 = 2.49 | .12 |
| Female | 13 (62%) | 16 (84%) | ||
| Race | ||||
| African American | 5 (24%) | 5 (26%) | χ2 = .01 | .93 |
| Caucasian | 15 (71%) | 14 (74%) | ||
| Other (coded missing for race) | 1 (5%) | |||
| Relationship to stroke survivor | ||||
| Spouse | 11 (53%) | 12 (63%) | χ2 = 1.69 | .43 |
| Adult child/adult child in law | 7 (33%) | 3 (16%) | ||
| Other | 3 (14%) | 4 (21%) | ||
| Education in years | 14.38 (2.65) | 13.89 (3.18) | t = .53 | .60 |
| Income | ||||
| More than enough to make ends meet | 9 (43%) | 7 (37%) | χ2 = .33 | .85 |
| Enough to make ends meet | 7 (33%) | 8 (42%) | ||
| Not enough to make ends meet | 5 (24%) | 4 (21%) | ||
| Stroke survivor characteristics | ||||
| Age | 67.14 (16.01) | 62.47 (14.29) | t = .97 | .34 |
| Gender | ||||
| Male | 8 (38%) | 14 (74%) | χ2 = 5.11 | .02*a |
| Female | 13 (62%) | 5 (26%) | ||
| Impairment | ||||
| Mobility | 3.52 (1.12) | 3.56 (1.13) | t = −.14 | .89 |
| Self-care | 3.78 (.98) | 3.74 (.89) | t = .11 | .91 |
| Language | 3.90 (1.19) | 3.76 (1.28) | t = .38 | .71 |
| Thinking | 2.86 (1.03) | 2.98 (1.33) | t = −.33 | .74 |
| Personality | 3.00 (1.24) | 2.58 (1.20) | t = 1.09 | .28 |
Controlling for stroke survivor gender did not change the findings of the outcome analyses.
p < .05.
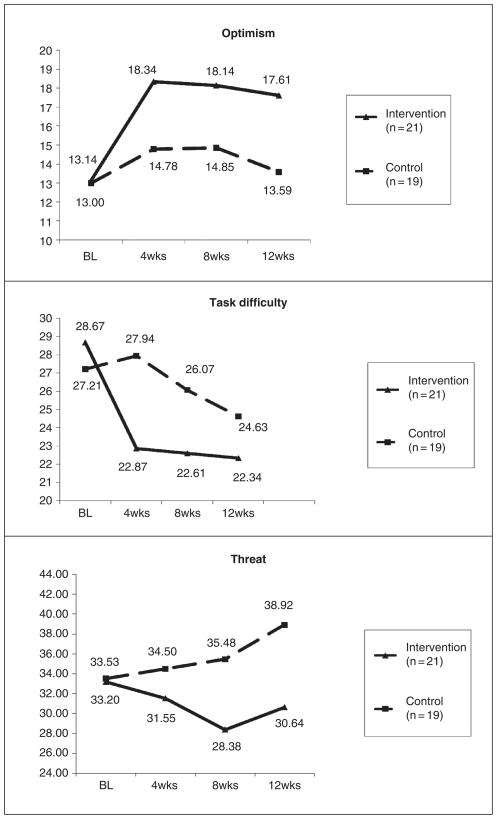
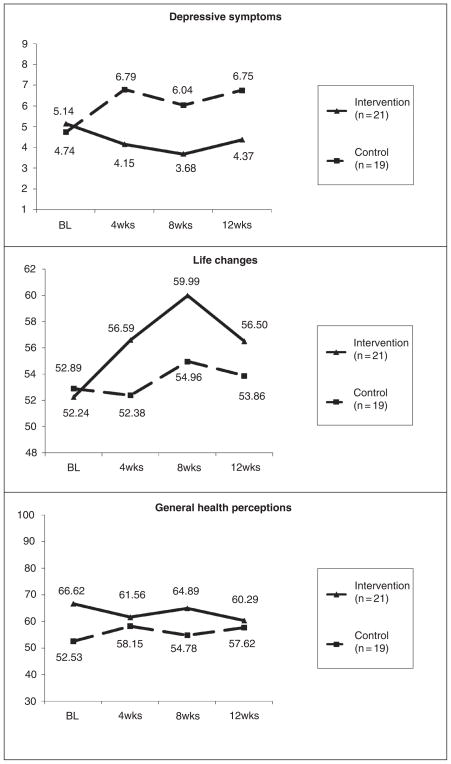
Table 2 provides data regarding the preliminary efficacy of the TASK program in relation to the attention control group. Significant increases in caregiver optimism at 4 weeks, F(1, 36) = 5.95, p = .02, η2 = .14, 8 weeks, F(1, 36) = 6.13, p = .02, η2 = .15, and 12 weeks, F(1, 36) = 6.40, p = .02, η2 = .15, were found, with medium effect sizes for the TASK intervention group relative to the control group. Significant decreases in task difficulty at 4 weeks, F(1, 36) = 5.30, p = .03, η2 = .13, were found; however, findings were not significant at 8 and 12 weeks, most likely because of the low scores at these time points for the intervention group. There were no significant improvements in threat appraisal at 4 weeks; however, threat appraisal at both 8 weeks, F(1, 36) = 5.67, p = .02, η2 = .14, and 12 weeks, F(1, 36) = 8.50, p = .01, η2 = .19, was significantly decreased, with medium effect sizes. Although not significant in this small sample, moderate decreases were found for depressive symptoms at 4 weeks, F(1, 36) = 3.35, p = .08, η2 = .09; however, the effect sizes became small at 8 and 12 weeks. Small, nonsignificant improvements were noted in life changes, and general health perceptions remained fairly stable. Table 3 shows the adjusted means and standard error estimates for each of the outcomes at each time point. Figures 3 and 4 provide visual graphs of the data based on the adjusted means.
Table 2.
Group differences using ANCOVA with baseline values and number of minutes as covariates for each time point
| 4 weeks F (1, 36) |
8 weeks F (1, 36) |
12 weeks F (1, 36) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | η2a | Ob | R2 | F | p | η2a | Ob | R2 | F | p | η2a | Ob | R2 | |
| Optimism | 5.95 | .02* | .14 | .66 | .19 | 6.13 | .02* | .15 | .67 | .25 | 6.40 | .02* | .15 | .69 | .21 |
| Task difficulty | 5.30 | .03* | .13 | .61 | .61 | 2.42 | .13 | .06 | .33 | .50 | 1.04 | .31 | .03 | .17 | .55 |
| Threat | 1.02 | .32 | .03 | .17 | .33 | 5.67 | .02* | .14 | .64 | .28 | 8.50 | .01* | .19 | .81 | .42 |
| Depressive symptoms | 3.35 | .08 | .09 | .43 | .40 | 2.67 | .11 | .07 | .36 | .36 | 1.95 | .17 | .05 | .28 | .42 |
| Life changes | 2.14 | .15 | .06 | .30 | .57 | 1.87 | .18 | .05 | .27 | .48 | .67 | .42 | .02 | .13 | .51 |
| General health | .44 | .51 | .01 | .10 | .74 | 3.30 | .08 | .08 | .42 | .73 | .26 | .61 | .01 | .08 | .73 |
Table 3.
Adjusted means for each group, controlling for baseline values and number of minutes as covariates for each time point
| Group | Baselinea | 4 weeks | 8 weeks | 12 weeks | |
|---|---|---|---|---|---|
| M (SE) | M (SE) | M (SE) | M (SE) | ||
| Optimism | Intervention | 13.14 (.61) | 18.34 (.92) | 18.14 (.84) | 17.61 (1.00) |
| Control | 13.00 (.48) | 14.78 (.97) | 14.85 (.89) | 13.59 (1.06) | |
| Task difficulty | Intervention | 28.67 (2.25) | 22.87 (1.38) | 22.61 (1.39) | 22.34 (1.41) |
| Control | 27.21 (1.86) | 27.94 (1.47) | 26.07 (1.48) | 24.63 (1.49) | |
| Threat | Intervention | 33.20 (2.16) | 31.55 (1.83) | 28.38 (1.86) | 30.64 (2.84) |
| Control | 33.53 (1.59) | 34.50 (1.94) | 35.48 (1.98) | 38.92 (1.87) | |
| Depressive symptoms | Intervention | 5.14 (1.23) | 4.15 (.91) | 3.68 (.91) | 4.37 (1.07) |
| Control | 4.74 (1.11) | 6.79 (.96) | 6.04 (.96) | 6.75 (1.13) | |
| Life changes | Intervention | 52.24 (3.18) | 56.59 (1.80) | 59.99 (2.31) | 56.50 (2.02) |
| Control | 52.89 (2.47) | 52.38 (1.91) | 54.96 (2.45) | 53.86 (2.14) | |
| General health | Intervention | 66.62 (5.20) | 61.56 (3.12) | 64.89 (3.35) | 60.29 (3.13) |
| Control | 52.53 (5.59) | 58.15 (3.32) | 54.78 (3.57) | 57.62 (3.34) |
Note: N = 40; intervention, n = 21; control, n = 19.
Baseline group differences were not significant: optimism, t = .18, p = .86; task difficulty, t = .49, p = .63; threat, t = −.12, p = .91; depressive symptoms, t = .24, p = .81; life changes, t = −.16, p = .87; general health, t = 1.85; p = .07.
Figure 3.
Stroke caregiver optimism, task difficulty, and threat appraisal. BL = baseline.
Figure 4.
Stroke caregiver depressive symptoms, life changes, and perceived health. BL=baseline.
Discussion
By addressing individual needs and concerns through skill-building interventions, the TASK program was hypothesized to improve stroke caregiver outcomes based on a conceptual model derived from Lazarus’s theory.51,52 The TASK intervention was found to be most efficacious at improving caregiver optimism. This finding may be surprising because optimism is typically regarded as a relatively stable personality trait that influences how one appraises stress.51,59 Bakas and colleagues9 found that caregiver optimism was moderately negatively correlated with threat appraisal and depressive symptoms, consistent with previous stroke caregiver research.61 In cancer caregivers, optimism was found to be an important predictor of caregiver depression, as well as an influence on their health and daily schedule.62 Optimism may be regarded as an important trait to identify caregivers at risk for negative outcomes such as depression62; findings from this study suggest that the TASK intervention improves optimism. The TASK program includes stress management strategies that help caregivers deal with their personal responses, such as recognizing automatic negative thoughts and replacing them with more positive, realistic thoughts.53–55 These findings suggest that interventions designed to enhance optimism through stress management techniques might have the potential to reduce threat appraisal and decrease caregiver depressive symptoms. The TASK program was shown to be efficacious in reducing caregiver threat appraisal at both 8 and 12 weeks. Although not statistically significant in this small sample, the TASK program did reveal a medium effect size for decreasing caregiver depressive symptoms at 4 weeks in comparison with the attention control group. Specifically, there was a 2.64-point difference in PHQ-9 scores between the groups. The findings regarding the efficacy of the TASK intervention in increasing optimism and reducing threat appraisal and a trend toward reducing depressive symptoms provide support for the conceptual model.
Further support for the conceptual model was the finding that the TASK intervention was efficacious at reducing task difficulty at 4 weeks relative to the control group. The TASK intervention included strategies to help caregivers find information about stroke, manage the survivor’s emotions and behaviors, and provide physical and instrumental care based on the caregivers’ self-identified needs. Task difficulty in the TASK group dropped rapidly from baseline to 4 weeks and then remained consistently low at 8 and 12 weeks. Caregivers in the control group showed a slight increase in task difficulty at 4 weeks and then dropped gradually at 8 and 12 weeks, never reaching the low levels attained by the TASK group. In other words, the caregiving needs were met much earlier in the intervention group than in the control group. This finding has important clinical implications, particularly for stroke caregivers during the first few weeks of providing care after discharge when their needs are the greatest.6,10,20,21,23
Although TASK effects on more distal outcomes of depressive symptoms, life changes, and general health perceptions were not significant, data trends in Figure 4 showed fewer depressive symptoms and more positive caregiver life changes in the TASK group relative to the control group. These findings suggest the need to improve the TASK intervention not only to influence these outcomes indirectly through the proximal outcomes of optimism, threat appraisal, and task difficulty but also to influence depressive symptoms and life changes directly. For example, in addition to using skill-building strategies to reduce caregiver depressive symptoms in caregivers who identified managing their own emotional responses as a need, the TASK intervention could be enhanced by actively screening all caregivers for depressive symptoms using the PHQ-9, then applying a care-managed approach for treating depressive symptoms in those caregivers who screen positive for depression. Such approaches involving antidepressants and care management by a nurse have been successfully used in studies with stroke survivors.73,74 The AIM intervention73 involved “activating” the stroke patient to recognize their own depressive symptoms, “initiating” the use of antidepressant medications, and “monitoring” and adjusting treatment by a nurse. Mitchell and colleagues74 developed a brief psychosocial/behavioral intervention in conjunction with antidepressant therapy to reduce depressive symptoms in stroke patients. These types of interventions could be incorporated into the TASK program to better meet the needs of caregivers who screen positive for depression. The TASK program incorporated the use of stress management strategies derived from cognitive behavioral therapy53–55 to address the self-identified emotional needs in caregivers, which likely contributed to improved optimism and reduced threat appraisal in this study. Strengthening the use of these stress management strategies in caregivers who screen positive for depression, along with initiating and monitoring antidepressant therapy, would help to better treat depressed caregivers and would extend the theoretical basis of the intervention to target those most in need.
To directly affect their life changes, caregivers could be screened for deterioration in aspects of their lives using the BCOS,8,9 with individualized interventions targeted toward these areas. In this study, caregivers tended to identify needs related to the care of the stroke survivor before identifying needs regarding their personal responses to providing care. Rather than waiting for caregivers to begin to self-identify their needs, the TASK intervention could be enhanced by actively screening for life changes earlier in the intervention process to allow more time to focus on the caregiver’s personal responses to providing care. In other words, while addressing caregiver self-identified needs regarding the care of the stroke survivor, the TASK program could also provide individualized interventions based on responses to the BCOS to enhance life changes earlier in the intervention process.
Findings on general health perceptions were inconclusive, suggesting that the measure may have been too global to detect any effects from the TASK intervention. Future research should include more specific health measures, such as the self-care and healthy behaviors measure used in the Resources for Enhancing Alzheimer’s Caregiver Health study (REACH II).75 The self-care and healthy behaviors measure consists of 11 questions that address such areas as getting enough rest, getting routine checkups, and adhering to a medication schedule.75 The TASK intervention could be further enhanced by screening for self-care and healthy behaviors and delivering individualized interventions based on the results.75 The research agenda for stroke caregiving must move beyond psychological distress and depression to address the health of family caregivers.10 In one landmark prospective study, family caregivers experiencing strain had a 63% higher risk of mortality compared with non-caregiving controls.12 Other studies found that stroke caregiver perceptions of their own health worsened over the course of providing care.8,9 Because insufficient attention has been given to the health status and health promotion activities of family caregivers,10,13,18,76,77 further enhancement of the TASK intervention to address these areas in stroke caregivers is recommended.
Limitations
Because this was the first pilot study testing the efficacy of the TASK intervention, the sample size was small. A larger, more adequately powered randomized controlled clinical trial is recommended to test the revised TASK intervention using an intent-to-treat design.
Another limitation of the study was the potential for treatment diffusion because the same nurse made calls to caregivers in both TASK intervention and attention control groups. Despite detailed training and monitoring of adherence to TASK intervention and attention control protocols, the possibility of treatment diffusion could have affected the findings. Using separate nurse interveners for the TASK intervention and attention control groups is strongly recommended for future studies.
Lastly, generalizability of the findings from this study is limited to stroke family caregivers who reflect the sample characteristics shown in Table 1. Hispanic caregivers and those from minority groups other than African American were poorly represented. Multisite studies would be needed to recruit a more ethnically diverse sample for future testing of the TASK intervention.
Clinical implications
The TASK intervention exhibited evidence of efficacy in increasing caregiver optimism and in reducing task difficulty and threat appraisal. These findings provided support for the conceptual model derived from Lazarus’s theory.51,52 Further methodological refinements in the TASK intervention are recommended to better screen for and treat caregiver depressive symptoms using a care-managed approach.73,74 Screening for negative life changes using the BCOS8,9 to further individualize interventions earlier in the intervention process for caregivers is also recommended. The TASK intervention could also include screening for self-care and healthy behaviors to better individualize interventions to improve caregiver health.75 Findings from this study support the use of the CNCC to allow caregivers to self-identify their needs, from which individualized skill-building interventions can be delivered. The point at which caregivers should also be screened for depressive symptoms, life changes, and healthy behaviors to further individualize skill-building interventions to address more distal stroke caregiver outcomes requires further study. Too many interventions delivered at once early in the intervention process may overwhelm the caregiver, however waiting too long to focus on more distal stroke caregiver outcomes may not allow enough time for interventions to be efficacious. Finding the trigger point when caregivers are ready to focus on their personal responses to providing care is important and may be the optimal time to provide the additional screening for depressive symptoms, life changes, and healthy behaviors. The length of the TASK program may also need to be extended for those who screen positive for depression and for those who require more skill-building interventions to improve life changes and healthy behaviors. These are factors that need to be considered in revising the TASK intervention to address more distal stroke caregiver outcomes. Once the TASK intervention has been revised, it should be tested for efficacy in a larger randomized clinical trial using an intent-to-treat design. Efforts to recruit a more ethnically diverse sample should be considered in future studies as well.
Conclusion
Despite recent efforts toward establishing stroke systems of care, stroke survivors and family caregivers do not consistently receive the training they need for transitioning to the home environment.20,23,78 Based on recommendations from the literature, the TASK program was designed to be a multicomponent program to promote skill building based on the needs and concerns of caregivers during the first few months after survivors are discharged home. Delivered completely by telephone, the TASK program offers an inexpensive way to provide stroke caregivers with information about stroke, assistance with stroke-related care, and follow-up after discharge. The TASK program not only addresses caregiver needs regarding the care of the survivor but also addresses caregiver needs related to their personal responses to providing care. In this small pilot study, the TASK program showed improvements in caregiver optimism, task difficulty, and threat appraisal consistent with the conceptual model derived from Lazarus’s theory.51,52 Further testing of an enhanced version of the TASK program in a larger randomized controlled clinical trial is warranted, with attention in subsequent studies directed toward more distal caregiver depressive symptoms, life changes, and health outcomes.
Acknowledgments
Funding for this study was provided by the National Institute for Nursing Research (NINR) NIH # K01 NR008712 (PI Tamilyn Bakas). We thank Joan S. Grant, DSN, CS, Professor, University of Alabama School of Nursing; Kurt Kroenke MD, Professor, Indiana University School of Medicine; and Merle Mishel, PhD, FAAN, Professor, University of North Carolina at Chapel Hill School of Nursing for their training and consultation on this project and Phyllis Dexter, PhD, RN, Assistant Scientist and Editor, Indiana University School of Nursing Center for Nursing Research, for her editorial assistance. We also thank the research staff (Kelly Gannon, Ann Harlow, Samantha Jessup, Charles Kain, Janet Kain, Ann Larner, Marjorie Pike, Barbara Poole, Sherry Riggio) and the many content reviewers for the TASK program (Nancy Chernott, Joan Grant, Ann Harlow, Marjorie Kurt, Judith Matthews, Karen May, Laurie Plue, Jean Renken, Ken Rueth, Michael Shain) for their expertise. Thanks also to the family caregivers who participated in this project.
Contributor Information
Tamilyn Bakas, Indiana University School of Nursing, Indianapolis, Indiana.
Carol J. Farran, Rush University College of Nursing, Chicago, Illinois.
Joan K. Austin, Indiana University School of Nursing, Indianapolis, Indiana.
Barbara A. Given, Michigan State University College of Nursing, East Lansing, Michigan.
Elizabeth A. Johnson, Ball State University School of Nursing, Muncie, Indiana.
Linda S. Williams, Richard L. Roudebush Veterans Administration Medical Center, and Associate Professor, Indiana University School of Medicine, Indianapolis, Indiana.
References
- 1.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics – 2006 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;13(6):e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Dewey HhM, Thrift AG, Mihalopoulos C, et al. Informal care for stroke survivors: results from the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2002;33:1028–1033. doi: 10.1161/01.str.0000013067.24300.b0. [DOI] [PubMed] [Google Scholar]
- 3.Dorsey MK, Vaca KJ. The stroke patient and assessment of caregiver needs. J Vasc Nurs. 1998;16:62–67. doi: 10.1016/s1062-0303(98)90003-6. [DOI] [PubMed] [Google Scholar]
- 4.Kelly-Hayes M, Robertson JT, Broderick JP, et al. The American Heart Association Stroke Outcome Classification. Stroke. 1998;29:1274–1280. doi: 10.1161/01.str.29.6.1274. [DOI] [PubMed] [Google Scholar]
- 5.Bakas T, Austin JK, Jessup SL, Williams LS, Oberst MT. Time and difficulty of tasks provided by family caregivers of stroke survivors. J Neurosci Nurs. 2004;36(2):95–106. doi: 10.1097/01376517-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bakas T, Austin JK, Okonkwo KF, Lewis RR, Chadwick LC. Needs, concerns, strategies, and advice of stroke caregivers the first 6 months after discharge. J Neurosci Nurs. 2002;34:242–251. doi: 10.1097/01376517-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bakas T, Burgener SC. Predictors of emotional distress, general health, and caregiving outcomes in family caregivers of stroke survivors. Top Stroke Rehabil. 2002;9(1):34–45. doi: 10.1310/GN0J-EXVX-KX0B-8X43. [DOI] [PubMed] [Google Scholar]
- 8.Bakas T, Champion V. Development and psychometric testing of the Bakas Caregiving Outcomes Scale. Nurs Res. 1999;48:250–259. doi: 10.1097/00006199-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bakas T, Champion V, Perkins SM, Farran CJ, Williams LS. Psychometric testing of the Revised 15-item Bakas Caregiving Outcomes Scale. Nurs Res. 2006;55:346–355. doi: 10.1097/00006199-200609000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Low JTS, Payne S, Roderick P. The impact of stroke on informal carers: a literature review. Social Sci Med. 1999;49:711–725. doi: 10.1016/s0277-9536(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 11.Wright LK, Hickey JV, Buckwalter KC, Clipp EC. Human development in the context of aging and chronic illness: the role of attachment in Alzheimer’s disease and stroke. Int J Aging Human Dev. 1995;41(2):133–150. doi: 10.2190/WK4B-PGEH-84QP-JUP7. [DOI] [PubMed] [Google Scholar]
- 12.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. J Am Med Assoc. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 13.Han B, Haley WE. Family caregiving for patients with stroke: review and analysis. Stroke. 1999;30:1478–1485. doi: 10.1161/01.str.30.7.1478. [DOI] [PubMed] [Google Scholar]
- 14.Family Caregiver Alliance. Caregiver Assessment: Principles, Guidelines and Strategies for Change. Report from a National Consensus Development Conference; San Francisco: Author; 2006. [Google Scholar]
- 15.Family Caregiver Alliance. Caregiver Assessment: Voices and Views from the Field; Report from a National Consensus Development Conference; San Francisco: Author; 2006. [Google Scholar]
- 16.Knight BG, Lutzky SM, Macofsky-Urban F. A meta-analytic review of interventions for caregiver distress: recommendations for future research. Gerontologist. 1993;33(2):240–248. doi: 10.1093/geront/33.2.240. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42(3):356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- 18.Visser-Meily A, van Heugten C, Post M, Schepers V, Lindeman E. Intervention studies for caregivers of stroke survivors: a critical review. Patient Educ Counsel. 2005;56:257–267. doi: 10.1016/j.pec.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Yin T, Zhou Q, Bashford C. Burden on family members. Nurs Res. 2002;51(3):199–208. doi: 10.1097/00006199-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Ski C, O’Connell B. Stroke: the increasing complexity of carer needs. J Neurosci Nurs. 2007;39(3):172–179. [PubMed] [Google Scholar]
- 21.King RB, Semik PE. Stroke caregiving: difficult times, resource use, and needs during the first 2 years. J Gerontol Nurs. 2006;32:37–44. doi: 10.3928/00989134-20060401-07. [DOI] [PubMed] [Google Scholar]
- 22.Duncan PW, Zorowitz R, Bates B, et al. Management of adult stroke rehabilitation care. Stroke. 2005;36:e100–e143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 23.Van Heugten C, Visser-Meily A, Post M, Lindeman E. Care for carers of stroke patients: evidence-based clinical practice guidelines. J Rehabil Med. 2006;38:153–158. doi: 10.1080/16501970500441898. [DOI] [PubMed] [Google Scholar]
- 24.Bourgeois MS, Schulz R, Burgio L. Interventions for caregivers of patients with Alzheimer’s disease: a review and analysis of content, process, and outcomes. Int J Aging Human Dev. 1996;43(1):35–92. doi: 10.2190/AN6L-6QBQ-76G0-0N9A. [DOI] [PubMed] [Google Scholar]
- 25.Forster A, Smith J, Young J, Knapp P, House A, Wright J. Information provision for stroke patients and their caregivers. Cochrane Library; 2004. p. 3. [Google Scholar]
- 26.Pinquart M, Sorensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: a meta-analysis. J Gerontol Psychol Sci. 2003;58B(2):112–128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- 27.Hanger HC, Walker G, Paterson LA, McBride S, Sainsbury R. What do patients and their carers want to know about stroke? A two-year follow-up study. Clin Rehabil. 1998;12:45–52. doi: 10.1191/026921598668677675. [DOI] [PubMed] [Google Scholar]
- 28.Cameron JI, Cheung AM, Streiner DL, Coyte PC, Stewart DE. Stroke survivors’ behavioral and psychologic symptoms are associated with informal caregivers’ experiences of depression. Arch Phys Med Rehabil. 2006;87(2):177–183. doi: 10.1016/j.apmr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Clark PC, Dunbar SB, Aycock DM, Courtney E, Wolf SL. Caregiver perspectives of memory and behavior changes in stroke survivors. Rehabil Nurs. 2006;31(1):26–32. doi: 10.1002/j.2048-7940.2006.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 30.Ostwald SK, Wasserman J, Davis S. Medications, co-morbidities, and medical complications in stroke survivors: the CAReS Study. Rehabil Nurs. 2006;31:10–14. doi: 10.1002/j.2048-7940.2006.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson CS, Linto J, Stewart-Wynne EG. A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke. 1995;26:843–849. doi: 10.1161/01.str.26.5.843. [DOI] [PubMed] [Google Scholar]
- 32.Berg A, Psych L, Palomaki H, Lonnqvist J, Lehtihalmes M, Phil L, Kaste M. Depression among caregivers of stroke survivors. Stroke. 2005;36:639–643. doi: 10.1161/01.STR.0000155690.04697.c0. [DOI] [PubMed] [Google Scholar]
- 33.Anderson C, Rubenach S, Mhurchu CN, Clark M, Spencer C, Windsor A. Home or hospital for stroke rehabilitation? Results of a randomized controlled trial. I: Health outcomes at 6 months. Stroke. 2000;31:1024–1031. doi: 10.1161/01.str.31.5.1024. [DOI] [PubMed] [Google Scholar]
- 34.Knapp P, Young J, House A, Forster A. Non-drug strategies to resolve psychosocial difficulties after stroke. Age Ageing. 2000;29:23–30. doi: 10.1093/ageing/29.1.23. [DOI] [PubMed] [Google Scholar]
- 35.Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, Donaldson N. Training care givers of stroke patients: randomized controlled trail. Br Med J. 2004;328(7448):1099–1103. [Google Scholar]
- 36.King RB, Hartke RJ, Denby F. Problem-solving early intervention: a pilot study of stroke caregivers. Rehabil Nurs. 2007;32(2):68–76. doi: 10.1002/j.2048-7940.2007.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 37.Mant J, Winner S, Roche J, Wade DT. Family support for stroke: one year follow up of a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2005;76:1006–1008. doi: 10.1136/jnnp.2004.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith J, Forster A, Young J. A randomized trial to evaluate an education programme for patients and carers after stroke. Clin Rehabil. 2004;18:726–736. doi: 10.1191/0269215504cr790oa. [DOI] [PubMed] [Google Scholar]
- 39.Shyu YL, Chen M, Chen S, Wang H, Shao J. A family caregiver-oriented discharge planning program for older stroke patients and their family caregivers. J Clin Nurs. 2008;17:2497–2508. doi: 10.1111/j.1365-2702.2008.02450.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Soeken K, Picot SJ. A meta-analysis of interventions for informal stroke caregivers. West J Nurs Res. 2007;29(3):344–356. doi: 10.1177/0193945906296564. [DOI] [PubMed] [Google Scholar]
- 41.Borrelli B, Sepinwall D, Ernst D, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol. 2005;73(5):852–860. doi: 10.1037/0022-006X.73.5.852. [DOI] [PubMed] [Google Scholar]
- 42.ven den Heuvel ETP, de Witte LP, Nooyen-Haazen I, Sanderman R, Meyboom-de Jong B. Short-term effects of a group support program and an individual support program for caregivers of stroke patients. Patient Educ Counsel. 2000;40:109–120. doi: 10.1016/s0738-3991(99)00066-x. [DOI] [PubMed] [Google Scholar]
- 43.Grant JS, Elliott TR, Weaver M, Bartolucci AA, Giger JN. Telephone intervention with family caregivers of stroke survivors after rehabilitation. Stroke. 2002;33:2060–2065. doi: 10.1161/01.str.0000020711.38824.e3. [DOI] [PubMed] [Google Scholar]
- 44.Burgio L, Corcoran M, Lichstein KL, et al. for the REACH Investigators. Judging outcomes in psychosocial interventions for dementia caregivers: the problem of treatment implementation. Gerontologist. 2001;41(4):481–489. doi: 10.1093/geront/41.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gitlin LN, Belle SH, Burgio LD, et al. for the REACH investigators. Effect of multicomponent interventions on caregiver burden and depression: the REACH multisite initiative at 6-month follow-up. Psychol Aging. 2003;18(3):361–374. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wisniewski SR, Belle SH, Coon DW, et al. for the REACH Investigators. The Resources for Enhancing Alzheimer’s Caregiver Health (REACH): project design and baseline characteristics. Psychol Aging. 2003;18(3):375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron JI, Gignac MA. “Timing it right”: a conceptual framework for addressing family caregivers’ support needs from the hospital to the home. Patient Educ Counsel. 2008;70:305–314. doi: 10.1016/j.pec.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Visser-Meily JMA, Post MWM, Riphagen II, Lindeman E. Measures used to assess burden among caregivers of stroke patients: a review. Clin Rehabil. 2004;18:601–623. doi: 10.1191/0269215504cr776oa. [DOI] [PubMed] [Google Scholar]
- 49.Buckley KM, Tran BQ, Prandoni CM. Receptiveness, use and acceptance of telehealth by caregivers of stroke patients in the home. Online J Issues Nurs. 2004;9(3):51–65. [PubMed] [Google Scholar]
- 50.Davis LL. Telephone-based interventions with family caregivers: a feasibility study. J Family Nurs. 1998;4(3):255–270. [Google Scholar]
- 51.Lazarus RS. Emotion and Adaptation. New York: Oxford University Press; 1991. [Google Scholar]
- 52.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- 53.Beck AT, Rush AJ, Shaw BE, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- 54.Burns DD. The Feeling Good Handbook. New York: Plume; 1999. [Google Scholar]
- 55.Greenbarger D, Padesky CA. Mind Over Mood: Change How You Feel by Changing the Way You Think. New York: Guilford Press; 1995. [Google Scholar]
- 56.Schulz R, Belle SH, Czaja SJ, Gitlin LN, Wisniewski SR, Ory MG for the REACH investigators. Introduction to the special session on Resources for Enhancing Alzheimer’s Caregiver Health (REACH) Psychol Aging. 2003;18(3):357–360. doi: 10.1037/0882-7974.18.3.357. [DOI] [PubMed] [Google Scholar]
- 57.Williams LS, Bakas T, Plue L, Brizendine EJ, Tu W, Hendrie H, Kroenke K. How valid are family proxy assessments of stroke patients’ health-related quality of life? Stroke. 2006;37:2081–2085. doi: 10.1161/01.STR.0000230583.10311.9f. [DOI] [PubMed] [Google Scholar]
- 58.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 59.Scheier MM, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Personality Social Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 60.Schulz R, Tompkins CA, Rau MT. A longitudinal study of the psychosocial impact of stroke on primary support persons. Psychol Aging. 1988;3:131–141. doi: 10.1037//0882-7974.3.2.131. [DOI] [PubMed] [Google Scholar]
- 61.Tompkins CA, Schulz R, Rau MT. Post-stroke depression in primary support persons: predicting those at risk. J Consul Clin Psychol. 1988;56:502–508. doi: 10.1037//0022-006x.56.4.502. [DOI] [PubMed] [Google Scholar]
- 62.Given CW, Stommel M, Given B, Osuch J, Kurtz ME, Kurtz JC. The influence of cancer patients’ symptoms and functional states on patients’ depression and family caregivers’ reaction and depression. Health Psychol. 1993;12(4):277–285. doi: 10.1037//0278-6133.12.4.277. [DOI] [PubMed] [Google Scholar]
- 63.Oberst MT. Unpublished manuscript. University of Wisconsin-Madison; 1990. Caregiving Burden Scale. [Google Scholar]
- 64.Oberst MT. Unpublished manuscript. University of Wisconson-Madison; 1991. Appraisal of Caregiving Scale. [Google Scholar]
- 65.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Internal Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams LS, Brizendine EJ, Plue L, Tu W, Bakas T, Hendrie H, Kroenke K. Performance of the PHQ-9 as a screening tool for post-stroke depression. Stroke. 2005;36:635–638. doi: 10.1161/01.STR.0000155688.18207.33. [DOI] [PubMed] [Google Scholar]
- 67.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Davis LL, Weaver M, Habermann B, Buckwalter K. Participant-centered adaptations in caregiver trials: strategies for managing confounds. Nurs Outlook. 2005;53:73–78. doi: 10.1016/j.outlook.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale NJ: Erlbaum; 1988. [Google Scholar]
- 70.Warner RM. Applied Statistics: From Bivariate Through Multivariate Techniques. Thousand Oaks, CA: Sage; 2008. [Google Scholar]
- 71.Nelson MM, Smith MA, Martinson BC, Kind A, Luepker RV. Declining patient functioning and caregiver burden/health: The Minnesota Stroke Survey – quality of life after stroke study. Gerontologist. 2008;48(5):573–592. doi: 10.1093/geront/48.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parag V, Hackett ML, Yapa CM, Kerse N, McNaughton H, Feigin VL, Anderson CS. The impact of stroke on unpaid caregivers: results from the Auckland Regional Community Stroke Study, 2002–2003. Cerebrovasc Dis. 2008;25:548–554. doi: 10.1159/000131673. [DOI] [PubMed] [Google Scholar]
- 73.Williams LS, Kroenke K, Bakas T, Plue LD, Brizendine E, Tu W, Hendrie H. Care management of poststroke depression: a randomized, controlled trial. Stroke. 2007;38:998–1003. doi: 10.1161/01.STR.0000257319.14023.61. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell PH, Teri L, Veith R, Buzaitis A, Tirschwell D, Becker K, et al. Living well with stroke: design and methods for a randomized controlled trial of a psychosocial behavioral intervention for poststroke depression. J Stroke Cerebrovasc Dis. 2008;17(3):109–115. doi: 10.1016/j.jstrokecerebrovasdis.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson DG, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups. Ann Internal Med. 2006;145(10):727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farran CJ, Loukissa D, Hauser PM, McCann JJ, Swanson B, Zeller JM. Psychoneuroimmunological outcomes in dementia caregiver intervention studies: an idea whose time has come? Online J Knowledge Synthesis Nurs. 2001:8. Document number 6. [PubMed] [Google Scholar]
- 77.Given BA, Given CW. Health promotion for family caregivers of chronically ill elders. Ann Rev Nurs Res. 1998;16:197–217. [PubMed] [Google Scholar]
- 78.Cameron JI, Tsoi C, Marsella A. Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637–2643. doi: 10.1161/STROKEAHA.107.501064. [DOI] [PubMed] [Google Scholar]