Abstract
Lactate is an important metabolite in normal and malignant tissues detectable by NMR spectroscopy; however, it has been difficult to clinically detect the lactate methyl resonance because it is obscured by lipid resonances. The selective homonuclear multiple quantum coherence transfer (SelMQC) technique offers a method for distinguishing lipid and lactate resonances. We implemented a 3D SelMQC version with Hadamard slice selection and 2D phase encoding (HDMD-SelMQC-CSI) on a conventional clinical MR scanner.
Hadamard slice selection is explained and demonstrated in vivo. This is followed by 1cm3 resolution lactate imaging with detection to 5 mM concentration in 20 minutes on a 3T clinical scanner. An analysis of quantum selection gradient duration and amplitude effects on lactate and lipid signal is presented. To demonstrate clinical feasibility, a 5 minute lactate scan of a patient with a non-Hodgkin's lymphoma in the superficial thigh is reported. The elevated lactate signal coincides with the T2-weighted image of this tumor. As a test of SelMQC sensitivity, a thigh tourniquet was applied to a normal volunteer and an increase in lactate was detected immediately after tourniquet flow constriction.
In conclusion, the HDMD-SelMQC-CSI sequence is demonstrated on a phantom and in two lipid-rich, clinically relevant, in vivo conditions.
Keywords: Lactate imaging, Hadamard encoding, non-Hodgkin's lymphoma, blood flow occlusion, magnetic resonance spectroscopy
Introduction
Lactate is the product of anaerobic glucose metabolism, normally only produced in small quantities in vivo in limited cell types, under certain conditions. However, in many disease states and under certain physiologic conditions, increased lactate is observed. For example, tumor cells exhibit enhanced glycolytic metabolism under aerobic or anaerobic conditions as part of the malignant phenotype, increasing local lactate levels. Due to this loss of efficient ATP production by the Krebs cycle, glucose consumption is increased, and the increased uptake is measured by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) studies of tumors (1). Therefore, imaging lactate can potentially provide the same diagnostic information as FDG-PET for tumors without the need for radioactivity or for any exogenous probes. Further, a decrease in lactate level has been shown to correlate strongly with chemotherapeutic (2–4). and radiotherapeutic (5) response in animal models. A robust technique for non-radioactive lactate imaging would, therefore, serve a dual role in cancer diagnosis and in detection of tumor therapeutic response.
Many other pathologic changes are associated with an increase in lactate. For example, in cerebral ischemia, elevated lactate is an early marker of tissue salvageable by thrombolytic therapy (6). Lactate production is enhanced in mitochondrial diseases, especially in brain and muscle (7). But even in normal muscle, in response to ischemia (8) or intense exercise (9), lactate production is enhanced following depletion of phosphocreatine and release of glycogen stores to maintain muscle energetics. In peripheral vascular disease, when blood flow is compromised to exercising muscle, the dynamics of this metabolism is altered (10), which is typically studied by 31-Phosphorus (31P) nuclear magnetic resonance spectroscopy (MRS) (11–13). Still, the relationship of lactate, the direct product of anaerobic glucose metabolism, to exercise and ischemia is unclear in vivo due to difficulties in detection of lactate in fatty tissues such as the leg.
The greatest difficulty for the in vivo NMR detection of lactate is the overlap of the −CH2-resonances of lipids with the methyl resonance of lactate at 1.3 parts per million (ppm) frequency in 1H MR spectra. As a result, the lactate resonance is often obscured by resonances of free fatty acids in the tissue of interest, in surrounding tissues, or in the pathological tissues, such as in tumors that contain lipid and lactate (14). Many techniques have been proposed for separating lactate and lipids (reviewed in (15,16)), all with drawbacks and limitations that have hindered their widespread use. One approach is provided by multiple quantum filtering, providing an acceptable compromise between final lactate signal detection, fat suppression, and motion insensitivity.
One early technique for multiple quantum filtered selective lactate spectroscopy was the selective Homonuclear Multiple Quantum Coherence Transfer (SelMQC) technique (17). This method utilized a series of pulses to select for specific quantum coherence transfer pathways for lactate versus lipid. While phase encoding localization was suggested in the original work, single voxel and slice selective localization techniques using this sequence proliferated via numerous slice selective techniques, including common techniques such as stimulated echo acquisition mode (STEAM) (18) and point-resolved spectroscopy (PRESS) (19). However, it has since been shown that achieving slice selection has a detrimental effect on the coherence transfer pathways (20). A more recent single slice implementation of SelMQC was demonstrated to detect polyunsaturated fatty acids on a wide bore research scanner (21). Still, there was additional demand for a robust sequence, demonstrated on clinical scanners, that is able to map the distribution of lactate in three dimensions within tumors with the goal of identifying tumors and tracking their response to therapy.
With those objectives in mind, we developed a 1D Hadamard encoded (HDMD), 2D phase encoded (PE) SelMQC CSI technique, termed HDMD-SelMQC-CSI, and demonstrated its utility on a whole body clinical MR scanner. The slice selection was achieved by Hadamard encoding (22), which has previously been applied with 2D PE without multiple quantum filtering (23). The Hadamard technique provides the benefits of minimal leakage of signal across slices, even in the 2 or 4 slice modes, as opposed to phase encoding with such a small number of slices. In addition, since each excitation is volume selective, each slice-encoding repetition adds to the signal-to-noise ratio (SNR) of the entire volume. Recently, this technique was implemented with a small animal scanner to examine a human tumor model in a mouse (24).
Towards the goal of clinical lactate studies, we implemented this technique on a 3T clinical scanner. First, a four slice Hadamard slice selection strategy based on frequency modulated hyperbolic secant pulses was demonstrated on a phantom. The pulse sequence was then run on a series of phantoms containing lactate and 100% oil in separate compartments in a 20 minute scan as a test of feasibility and to show that very little lipid signal remained after quantum selection. To optimize quantum selection parameters, it was shown that minimum quantum selection gradient duration and maximum amplitude provided for high lactate signal and excellent fat suppression. In human studies, the four slice Hadamard slice selection strategy has been verified to be very similar to simulations in a tumor. It was then observed that HDMD combined with CSI encoding in-plane allowed for a 3D clinical lactate scan in only 5 minutes. It was further demonstrated that the sensitivity of the multiple quantum selection technique was sufficient to detect the small amount of lactate generated in the human calf during thigh pressure cuff occlusion of blood flow. Taken together, these results provide the technical basis and proof of feasibility required for future measurements of lactate in cancer and ischemic disease.
Methods
Approval for all human studies was granted by the Institutional Review Board, and informed consent was obtained before all studies. A Siemens Trio 3 Tesla scanner (Erlangen, Germany) was used with a maximum gradient strength in each dimension of 26 mT/m. Gradient ramp times were fixed to 300 μs. When coil receiver arrays were used, the signal from all channels was combined by sum of squares reconstruction (25).
Pulse Sequence Overview
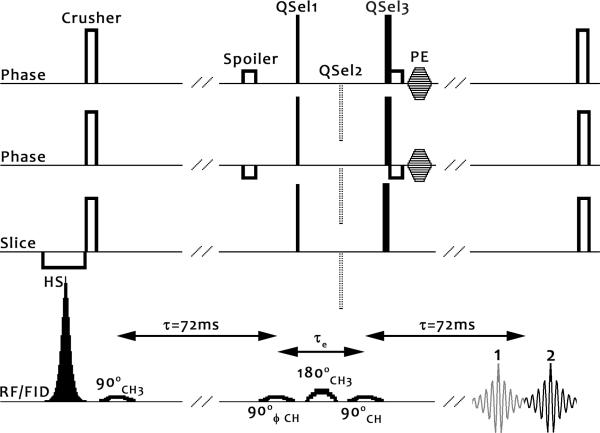
The HDMD-SelMQC-CSI sequence combines the SelMQC selection technique (17) with Hadamard slice selection (22) to create 3D lactate images. The sequence is diagrammatically displayed in Figure 1. The Gaussian pulses used for quantum selection were of 7800 μs duration, with a bandwidth of 280 Hz (2.3 ppm) at 1/e of the maximum amplitude (26).
Figure 1. The Hadamard-SelMQC pulse sequence diagram.
Frequency-modulated hyperbolic secant (HS) inversion pulses, described later in the text, are used for Hadamard style slice encoding. A crusher removes any incompletely inverted magnetization. An initial 90° 1.3ppm (CH3) frequency selective Gaussian pulse excites lipid and lactate. After an evolution period τ=1/2J of 72 ms (41), the anti-phase magnetization is split into DQ and ZQ coherences by a 90° frequency selective Gaussian applied at 4.1ppm (CH). Phase cycling was best accomplished in our experience by cycling this RF pulse, and it has been designated with a ϕ symbol.
Choice of quantum selection gradients (QSel) during the evolution period, τe, determines the final observed quantum coherence pathway. Minimization of this evolution time reduces J-coupling modulation and diffusion based signal loss. The QSel gradient moment ratios (1:0:2) in the solid lines correspond to selection of one double quantum coherence pathway (termed DQ→ZQ), observed as echo #2, and this is the echo pathway we have chosen in this study. An alternate choice is the other double quantum coherence pathway (ZQ→DQ, QSel moments of 0:−1:2) which generates echo #1. We chose DQ→ZQ because the entire echo is typically included in the receiver time window, though both pathways were observed to provide almost identical sensitivity. Both options provide very high levels of fat suppression, as the lipid experiences a spin-echo sequence with unbalanced gradients.
Another choice is to refocus all coherences (DQ→DQ, QSel moments 1:−1:2), and obtain both echo #1 and #2. In this case a non-selective 180 hard pulse should be employed to minimize the evolution time. However, both lipid and lactate signals are obtained since the gradients are no longer unbalanced for lipid, leading to full lipid signal in a single acquisition. Still, if the ϕ pulse is cycled in combination with the receiver, averaging of two cycled acquisitions eliminates lipid signal, leaving lactate. Given the large lipid-to-lactate ratios in lipid-rich areas, this technique is highly susceptible to motion effects and very minor scanner instabilities. It also doubles the required acquisition time.
Hadamard Slice Selection
Hadamard slice selection was performed by a series of hyperbolic secant (HS) inversion pulses (27) of the form,
| Equation 1 |
where β is 1.978, t goes from −π to +π, i is sqrt(−1), μ is proportional to the width of the inversion, and pulses may be convolved with multiple frequencies, f. The frequencies for the slice selection in this work are defined in Table 1. HS pulses produce selective slice inversions, and the subsequent Hadamard transformation of the output data yields the corrected slice data shown in Equation 2 for four slices.
| Equation 2 |
In Equation 2, Hadi refers to the ith Hadamard pulse pre-encoded data and Slicei is the resulting slice. For the 4 slice experiments, all 8 pulses were used. For the 2 slice experiments, pulses #1, 3, 5, and 7 were used.
Table l.
Pulse parameters used for Hadamard Slice Selection. The parameters β, μ, f1, f2 are used in Equation 1. The profile specifies the band of inversion where + refers to an uninverted band and − refers to an inverted band.
| β | μ | f1 | f2 | Profile | |
|---|---|---|---|---|---|
| Pulse 1 | 1.978 | 20 | − − − − | ||
| Pulse 2 | 1.978 | 10 | +−−+ | ||
| Pulse 3 | 1.978 | 10 | 19.75 | ++−− | |
| Pulse 4 | 1.978 | 5 | −29.62 | 9.87 | −+−+ |
| Pulse 5 | 0 | ++++ | |||
| Pulse 6 | 1.978 | 5 | −29.62 | 29.62 | −++− |
| Pulse 7 | 1.978 | 10 | −19.75 | −−++ | |
| Pulse 8 | 1.978 | 5 | 29.62 | −9.87 | +−+− |
To test the slice profiles generated by the HS pulses, each pulse was added at the beginning of each TR to a spoiled gradient recalled echo (GRE) readout sequence with the slice selection gradient in the readout frequency encoding direction and with phase image reconstruction. This was performed on a 10cm diameter phantom placed into a human head tranceiver birdcage coil (Bruker Biospin MRI, Billerica, MA). To further investigate the suitability of the proposed methods, we applied the same pulses and readout to the 4 × 4 × 4 cm core of a non-Hodgkins lymphoma (NHL) in the thigh of a 63 year old male patient. A 5 cm, 2 channel receive-only surface coil array was placed over the tumor. Transmission was performed with the scanner body coil. A phase image of the profile of each HS pulse was acquired. Localized shimming was performed to reduce the water linewidth to less than 30Hz full width at half maximum within the entire volume of the tumor. From tests with the body coil, we found that an HS pulse duration of 20ms and peak power of 2.2 kHz provided robust spin inversion in all samples and subjects reported in this manuscript. Additional parameters for HS testing used for data shown in Figure 2 and Figure 5A were: TR 500 ms, TE 3.67 ms, FOV 100 × 100 mm, Matrix 64 × 64, bandwidth 260 Hz/pixel, α=30°.
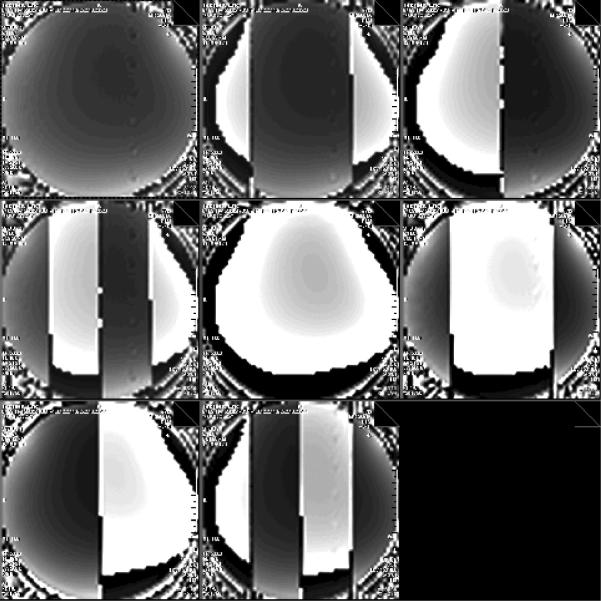
Figure 2. Eight hyperbolic secants used for Hadamard slice selection.
To generate each image, one of the eight frequency modulated HS inversion pulses described in Table 1 was transmitted at the beginning of each TR before a spoiled gradient echo sequence. The HS inversion pulse was applied in this case with a gradient in the readout axis of the GRE sequence for visualization of the HS pulse. Displayed here is a scanner screenshot capturing all eight HS pulse results with phase reconstruction. Pulses are ordered from left to right, top to bottom (#1 top left, #8 bottom center).
Phantom Experiments
Lactic acid (Sigma-Aldrich #35202, St. Louis, USA) samples at various concentrations were prepared in a series of 15 mL plastic conical tubes with addition of 0.01% sodium azide to prevent spoilage. An additional tube was loaded with 100% safflower seed oil (Sigma-Aldrich #58281, St. Louis, USA). These tubes were then submerged in distilled water in a 10 cm glass jar. This phantom was placed into a circularly polarized, vendor supplied, transceiver head coil. The four slice selected (eight HS pulses) HDMD-SelMQC-CSI sequence was run with TR 1.5 s, FOV 10 × 10 cm, Matrix 10 × 10 with elliptical sampling, 4 kHz bandwidth, 2048 sampling points, 2 averages with phase cycling, quantum selection (QSel) gradient strength of 26 mT/m, 300 μs duration for the single strength gradient, 600μs for the double. A small amount of water signal (1000 × suppressed) was observable without additional water suppression with these quantum selection gradient durations and amplitudes. To generate the demonstration phantom data, the addition of pre-pulses (28) before the HS inversion completely removed the water signal without any deleterious effects on the lipid and lactate resonances.
For the analysis of gradient strengths and durations, two 50 mL conical tubes were filled with either 100 mM lactate or 100% safflower seed oil. These were affixed to the bottom of a distilled water bath separated by several centimeters. The bath was placed into an eight-channel vendor supplied receive-only head coil; body coil excitation was employed. The SelMQC sequence was run with 2 slice Hadamard selection, one slice wholly selecting lactate and no oil, and the other slice wholly selecting oil and no lactate. No phase encoding was used. The QSel amplitude was incremented to 0, 2, 5, 10, 15, 20, and 26 mT/m with a fixed duration of 300μs. Then the QSel amplitude was fixed at 26 mT/m and the duration was incremented to 0.5, 1, 2, 3, 4, 5, and 10 msec (+200 μs maximum duration after ramp up, +700 μs, +1700 μs, etc). Other parameters were 4 preparation scans, a TR of 1.5s, 1024 points, 4 kHz bandwidth, and a single average.
Non-Hodgkin's Lymphoma Patient
A 5 cm 2 channel receive coil was placed over the thigh of a 34 year old female patient, who had a diffuse large B-cell non-Hodgkins lymphoma. A multi-slice axial T2-weighted image series was obtained for localization of the tumor with the following parameters: FOV 250 mm × 250 mm, thickness 5 mm, TR 5860 ms, TE 109 ms. Using this localization, the HDMD-SelMQC-CSI sequence was implemented with the following parameters: FOV 250 mm × 250 mm, Matrix 10 × 10 (elliptically sampled), slice thickness 25 mm, TR 1500 ms, acquisition time 5 min, 2 slice Hadamard encoding (4 pulses). The output was Fourier transformed and reconstructed as a 16 × 16 matrix, resulting in a voxel resolution of 15.6 × 15.6 × 25 mm3 (6.1 cm3).
Thigh Cuff Experiment in the Normal Volunteer
A healthy male volunteer, 67 years of age, was positioned with his calf in an 8 channel receive-only knee coil in the center of the scanner. Non-magnetic sand bags were used to hold the calf in place inside the coil. A non-magnetic thigh tourniquet system (Zimmer 1000, Warsaw, IN) was used to inflate and later deflate a cuff with a pressure of 250 mmHg. This system has previously been shown to highly restrict flow in the calf muscles of volunteers (29,30).
A series of unlocalized, phase cycled, Double Quantum to Zero Quantum (DQ→ZQ) pathway signals were acquired during the experiment. The parameters for these acquisitions were: 300μs quantum selection gradient duration (1 ×, 600 μs for 2 ×), 26 mT/m amplitude, DQ→ZQ selection, TR 3 s, 256 repetitions, and water suppression pre-pulses on. The data are shown in the final figure as the average of the two phase cycles for each point (6 seconds/point).
Results
Figure 2 displays the GRE phase outputs of all eight HS pulses from the parameters used in Table 1 for slice encoding. This demonstrates that the 8 pulse, 4 slice version of the sequence is feasible and allows for pulse calibration and testing.
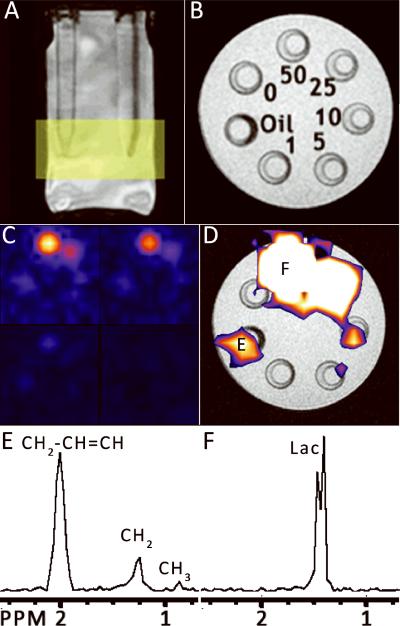
Figure 3 demonstrates HDMD-SelMQC-CSI imaging on a series of lactate phantoms. This reveals that 5–10 mM of lactate, a physiologic level, can be imaged at 1 cm3 resolution in a 20 minute scan. The intensity of the 50 mM lactate peak at 1.3 ppm is about 5 times greater than the intensity of the 1.3 ppm peak of 100% safflower seed oil, indicating that 100% oil will appear as about 10 mM lactate with these techniques. A peak at 2.0 ppm is also seen from the lipid phantom after quantum selection. This is a double quantum selected unsaturated lipid resonance and will be explained later in the discussion section.
Figure 3. HDMD-SelMQC-CSI images of lactate phantoms.
(A) A localizer image shows the 15 mL conical phantoms containing lactate submerged in water. The yellow rectangle shows the slab position for the Hadamard slice selection. (B) A cross section of the phantoms from the localizer. The numbers indicate the concentration of lactate in each phantom or the 100% safflower seed oil phantom. In (C), the results of the lactate imaging are shown as the magnitude integral of the area from 1.1 to 1.5 ppm in each of the 4 slices. These slices begin at the top slice in the top left and proceed downwards in clockwise order. The bottom slice is entirely out of the tubes and shows only noise. (D) Overlaid upon the localizer image from (B) is the integrated signal from the top slice (upper left corner of (C)), shown with threshholding to remove the noise signal. The letters marked E and F correspond to the spectra (E) and (F), where E is the oil phantom and F is the 50 mM lactate phantom. These are magnitude spectra zoomed into the area of interest (0.75 – 2.5 ppm).
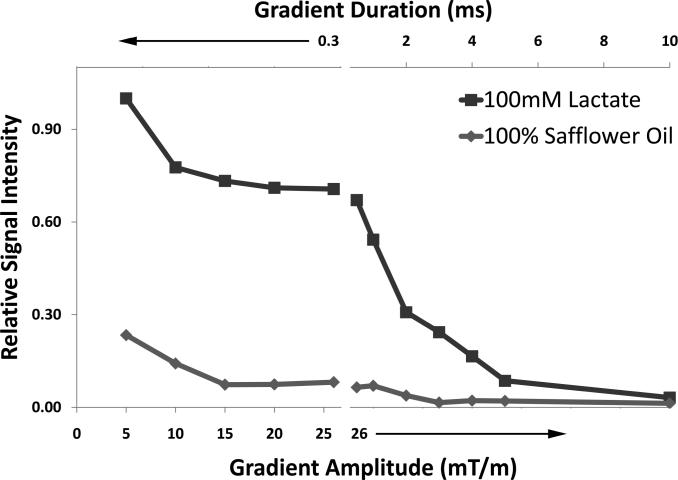
Figure 4 analyzes of the effects of quantum selection gradient parameters on signal obtained from 100 mM lactate and 100% oil phantoms. Gradient amplitudes from 15–26 mT/m are shown to be equivalent for separation of fat and lactate at 1.3 ppm. The data point for 26 mT/m strength and 300 μs duration indicates that 100% safflower oil will appear at the same intensity as 11.6 mM lactate. As predicted previously (17), the shortest possible duration gradients (and selective 180° pulse) are desired to maximize lactate signal. This was confirmed by increasing the duration of the gradients with 26 mT/m fixed gradients.
Figure 4. Analysis of QSel gradient amplitude and duration.
A 100 mM lactate phantom and a 100% safflower seed oil phantom were excited simultaneously and separated by 2 slice Hadamard selection. The quantum selection gradient durations were first fixed at 0.3 ms, and amplitude was increased from 0 mT/m to 26 mT/m. The relative signal intensities are not plotted at 0 mT/m and 2 mT/m QSel amplitude as the fat signal is several times greater than the lactate signal at those amplitudes. After the break in the x-axis, the gradients are fixed at 26 mT/m and the duration is stepped from 0.5 ms to 10 ms. Each point represents the integral of the spectral peak centered at 1.3 ppm for either the oil or lactate phantom.
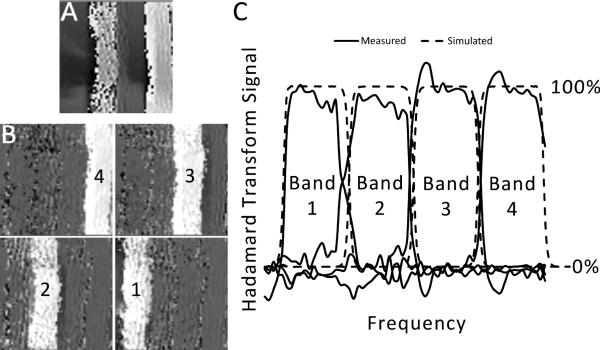
Figure 5A shows the phase-reconstructed GRE readout of one of the HS pulses analogous to Figure 2 but this time applied to a human NHL tumor in vivo. All eight HS profiles were acquired and the Hadamard transformation (Equation 2) applied to the phase images to create the image in part B. The results of this test are concordant with predictions by Hadamard transformation of the pulse profiles.
Figure 5. In vivo four slice hadamard selection.
(A) Shown is the result of an in vivo phase reconstructed GRE readout, where the 8th Hadamard pulse is applied in the frequency encoding direction before each TR. The two white bands correspond to the inverted portions. (B) The result of the four slice Hadamard transformation (Equation 2), which is based on the eight images obtained from all eight pulses, is displayed. The bright regions are the areas of net signal, where each number corresponds to the “Bands” in (C). In (C), the average profile of each of these selected slices is plotted. For comparison, the simulated Hadamard transformation profile for these 8 pulses is shown. This simulation is based on Fourier Transformation of the pulse profiles given by Equation 1 and Table 1 and Hadamard Transformation of those results by Equation 2. An excellent agreement is seen between the expected and achieved profiles.
Figure 6 demonstrates the HDMD-SelMQC-CSI for the detection of lactate in an NHL patient. The map of lactate was acquired in only 5 minutes, demonstrating the feasibility of the clinical use of this technique.
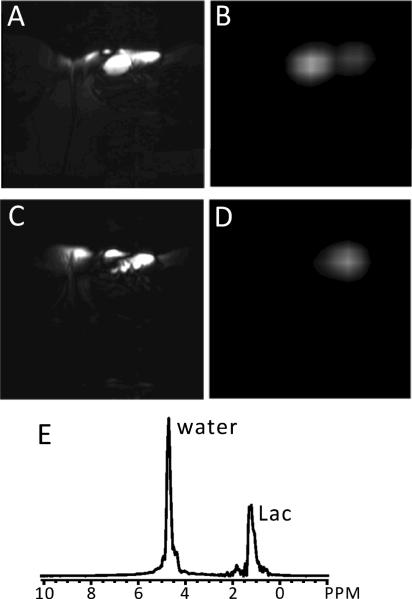
Figure 6. HDMD-SelMQC-CSI lactate images from an NHL patient.
(A) and (C) show T2-weighted images and (B) and (D) are the corresponding HDMD-SelMQC-CSI lactate images from a 34 yr old female diffuse large B-cell non-Hodgkin's lymphoma patient having a tumor in the inguinal node of the upper right thigh. In the T2-weighted axial images (A and C) two nodes of 25 mm × 25 mm × 40 mm and 20 mm × 15 mm × 35 mm were observed. The two slice lactate images (B and D) match well with the slices from T2-weighted images. Part (E) shows a magnitude spectrum from a voxel of tumor in (B). The peak centered at 1.3 ppm is considered to be lactate (Lac, with minimal lipid contamination as per the discussion) with residual water (4.7 ppm) included. This water can be completely removed by adding water suppression pre-pulses, but has not been large enough to cause errors due to receiver saturation.
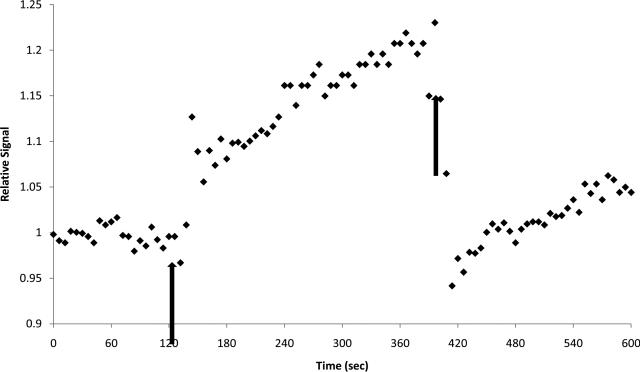
In Figure 7, dynamic information was obtained by the HDMD-SelMQC-CSI sequence. The SelMQC portion of the sequence without localization was run repeatedly on a volunteer before, during, and after blood flow restriction by thigh cuff occlusion. An immediate rise in lactate was observed with occlusion, and then fast washout of lactate was detected after release of occlusion.
Figure 7. The SelMQC obtained 1.3ppm peak changes in the calf during thigh cuff occlusion.
The magnitude integral of the 1.1 – 1.5 ppm region is plotted over time during thigh cuff occlusion of downstream blood flow in the leg of a volunteer. Each diamond represents a single 6 second measurement. The arrows correspond to the start and stop of the tourniquet pressure cuff at 250 mm Hg pressure. The integral of the area steps up quickly after application of the tourniquet and continues to increase with the approximately 5 minutes of thigh cuff occlusion. After blood flow is restored, the integral quickly falls to its baseline level.
Discussion
Lactate and lipid contributions to the 1.3ppm resonance after MQC filtering
The signal from the 1.3 ppm region is, when not quantum coherence selected, a combination of lactate and lipid. We tested the lactate and lipid contribution in the SelMQC sequence with phantoms as follows. By high resolution spectroscopy with a known concentration standard, we measured the concentration of 1.3 ppm protons in the 100% safflower seed oil to be 46 M in a separate experiment. From Figure 3, the 100% oil had similar signal intensity to the signal of 10 mM lactate (30 mM lactate CH3 protons) after SelMQC. This was also confirmed by the comparison of signals obtained from a 100 mM lactate phantom and a 100% oil phantom separated by Hadamard excitation as shown in Figure 4. The lactate phantom in that case had 9.04 times higher signal than the 100% oil phantoms with the parameters used in the human experiments. Taking into account the ratio of the fat and lactate signals and their 1.3 ppm spin densities yields an approximate 1500 fold fat suppression versus lactate. Thus, in spectra in vivo, the possible contamination from lipid depends on the initial content of the lipid in the tissue of interest.
For physiologic concentrations of lipid, lipid contamination is unlikely to contribute much signal with the sequence parameters used in this study. Tumor models of NHL have a lactate level of ~10 mM as measured in tumor extract experiments (2) and from in vivo experiments with a 10 mM lactate external standard placed adjacent to a human lymphoma xenograft (24). The lipid signal in the tumor after SelMQC filtering can be at the level of 10 mM lactate only when the tumor free lipid concentration is the same as 100% safflower seed oil, an unrealistic condition. To evaluate the physiologic levels of lipid signal, and thus the potential level of lipid contamination of SelMQC spectra, we used 135ms PRESS spectra from tumors of three NHL patients (data not shown). We first assumed that the tumor is 80% water, and then normalized the signal from the 1.3ppm peak against the water peak and T2 corrected the two signals. This yielded an average of about 1 M 1.3 ppm lipid protons within these three tumors. As 46 M lipid protons were equivalent in signal to 10 mM lactate after MQC filtering, 1 M lipid protons would only contribute 2% contamination to the 10 mM lactate signal in a tumor with the parameters used in this study. Future work will include development of a model-fitting based approach for achieving relative or absolute quantification of lactate.
In the case of the thigh cuff experiment, a baseline signal is observed from the lipid throughout the entire calf. It is very difficult to quantify the lipid and lactate sources of that signal. It would be impossible without painful muscle biopsy to determine the amount of lactate seen at baseline and then during flow occlusion. Given that the patient is at rest during the occlusion, it is likely that a very small amount of lactate is being observed. To our knowledge, these changes have not previously been reported with such temporal resolution. It is exciting to see that there is enough sensitivity to see this change with high temporal resolution for future functional and metabolic studies involving muscle exercise.
Considerations for HDMD-SelMQC-CSI on clinical scanners
It is impossible on human scanners to reach the gradient amplitudes generated on small animal scanners, and this partially decreases the quantum selection ability of the sequence on human scanners. Without increasing the length of the gradients, impacting the lactate signal, a small amount of fat and water signal cannot be completely eliminated at high concentration. Still, as we discussed, the fat signal is not intense enough to impede the use of the sequence at least in applications to NHL. The residual water signal can be removed by water suppresion pulses before excitation (pre-pulses), but even without these pulses the water signal is easily separated based on chemical shift and has not been large enough to cause receiver gain difficulties.
When considering whether to scan with ZQ→DQ (Zero Quantum→Double Quantum), DQ→ZQ, or DQ→DQ (Double Quantum→Double Quantum) selection gradients, several observations were made (see ref (17) for more detail about pathway definitions and selection). In our observations, the ZQ→DQ selection produced an echo that was sometimes shifted such that the beginning was obscured before the receiver start. In theory and in practice (when shims were better and the echo was not so far shifted), the DQ→ZQ produced similar signal, and that was our selection choice. Refocusing both quantum pathways in the DQ→DQ pathway with phase cycling led to excellent results in phantoms (31), but in vivo fat suppression based on that technique requires two averages and depends on the volunteer not moving at all. This is unrealistic given the several minute scan times required here. Given the relative insensitivity to motion of the DQ→ZQ sequence and the faster single averaging requirement, we felt this was the superior choice for practical implementation.
As shown by He et al. (17), short duration pulses are desired to minimize the evolution time, and thus maximize lactate sensitivity reduced over the evolution time by J-coupling modulation, diffusion, and relaxation. Yet, the pulses must still be frequency selective enough to not inadvertently excite the J-coupled resonance at the improper time. As a compromise between these two extremes, the pulse shape and duration (Gaussian 7800μs) used here results in an approximately 2ppm FWHM frequency excitation. This is broad enough to excite and detect a peak at 2.0ppm (seen in Figure 3); probably the 2.0ppm −CH2−CH=CH- resonance seen in lipids, which is J-coupled to a 5.38ppm resonance (32). To investigate the possibility that the observed 2.0ppm peak is a J-coupled peak, two SelMQC FIDs were acquired from the 100% safflower seed oil phantom with QSel amplitudes 13mT/m and 26mT/m. The amplitude of the 2.0ppm peak was unaffected by this change (in contrast to the 1.3ppm lipid peak), suggesting the proposed resonance assignment. Given that the 2.0ppm peak is well separated by chemical shift from the 1.3ppm lactate resonance, it is easy to separate the two.
Peak transmitter powers are the primary limit for the Hadamard Slice Selection HS pulses. The 3T whole body scanner typically shims to 10–50Hz FWHM for in vivo conditions on tumors, and this leads to a relatively high pulse power and duration to retain adiabaticity for whole body transmission. Empirically on humans and phantoms, we determined that a duration of 20ms and amplitude of 2.2kHz provided robust inversion. Yet, the specific absorption rate (SAR) by the HDMD-SelMQC-CSI sequences has been measured always to be less than 20% of maximum by the scanner power monitor, leading to excellent flexibility. For example, a 3D Hadamard selected version of this sequence could be used for 4×4×4 matrix spectroscopy or other localizations such as Image Selected In vivo Spectroscopy (ISIS, (33)) could be employed based on these pulses.
Implications for non-Hodgkin's lymphomas
Predicting or determining NHL response to therapy is a goal for imaging, but current techniques for this purpose are limited. Pretreatment 31P MRS has been proposed for its ability to predict the treatment response of NHL patients (34,35). However, the pretreatment 31P NMR only acts as a predictor of treatment failure. Furthermore, because of the low NMR sensitivity of the 31P nucleus compared to 1H, 31P NMR has been limited to large (≥27 cm3) superficial tumors (34). In contrast, 1H MRS is able to detect signal from much smaller tumors. Indeed, in the current study we detected lactate signal from a voxel in the tumor as small as 6.1 cm3. Because of the relatively high SNR of the lactate signal seen in the presented spectrum, it is expected that lactate from even smaller voxels in the tumors might be detected in a reasonable acquisition time.
It should also be noted that FDG-PET is often employed in NHL patients before and after treatment for the purpose of predicting and detecting tumor treatment response (36). The post-treatment PET scan is typically performed either after 2–4 cycles or 6 cycles of treatment. Yet, lactate changes detected by 1H MRS represent an even earlier therapeutic response in an animal model of lymphoma (2). Further, MRS lactate measurements do not involve radioactivity. As such, lactate measurements in lymphoma patients may provide a very valuable tool for detecting the treatment response of individual patients.
Possibilities for muscle ischemia imaging
A one minute time course change in muscle metabolites in the human calf with similar resting ischemia has been observed previously by 31P NMR (37). Since 31P NMR has measured phosophocreatine (PCr) and pH changes in resting ischemia, it is plausible that lactic acid is produced and that the pH change may in part be due to lactic acid formation. A dual-tuned coil approach (such as (38,39)) could compare lactate results to phosphorus spectroscopy to further elucidate this relationship. This would investigate the interaction of lactate, pH, PCr, and other metabolites, as suggested previously by experiments with frog muscle (40). Performing these measurements on humans using clinical scanners will allow for the investigation of muscle energetics in exercise, peripheral vascular disease, muscle ischemia, and other phenomena.
Conclusion
Our goal was the implementation of a robust lactate imaging sequence on a clinical scanner with scan times that would be feasible within a clinical scan. Here we showed the considerations for this implementation and demonstrated its utility in patient tumors. Our future goal is the tracking of lactate response to treatment. This is based on a recent work that showed lactate is a very early and sensitive indicator of response to chemotherapy in human diffuse large B-cell xenografts (2). Studies are underway to correlate lactate with response to therapy in NHL patients using this sequence.
We have also shown that sensitivity is high enough to detect functional lactate changes in the calf muscle. Another future application of this work is investigation of muscle lactate changes with exercise in healthy volunteers and patients with peripheral vascular disease.
Acknowledgements
The authors wish to acknowledge our many colleagues that form the “Cooperative Group of MRS Applications to Cancer”, who have participated with us in the cooperative U-01 grant “Predicting Tumor Response by 31P MRS”, and have provided us with a wide variety of assistance as this research has been conducted. This work has been supported by NIH grants R01CA118559 (T.R. Brown), R01CA101700 (JDG), R01-CA102756 (HP), RR02305 (RR), 2U24CA083105 (JDG and L. Chodosh) and F30NS059116 (EAM). The authors also wish to acknowledge the assistance of Gamaliel Isaac in the development of the pulse sequence.
References
- 1.Czernin J, Phelps ME. Positron emission tomography scanning: current and future applications. Annu Rev Med. 2002;53:89–112. doi: 10.1146/annurev.med.53.082901.104028. [DOI] [PubMed] [Google Scholar]
- 2.Lee SC, Huang MQ, Nelson DS, Pickup S, Wehrli S, Adegbola O, Poptani H, Delikatny EJ, Glickson JD. In vivo MRS markers of response to CHOP chemotherapy in the WSU-DLCL2 human diffuse large B-cell lymphoma xenograft. NMR Biomed. 2008;21(7):723–733. doi: 10.1002/nbm.1250. [DOI] [PubMed] [Google Scholar]
- 3.Poptani H, Bansal N, Graham RA, Mancuso A, Nelson DS, Glickson JD. Detecting early response to cyclophosphamide treatment of RIF-1 tumors using selective multiple quantum spectroscopy (SelMQC) and dynamic contrast enhanced imaging. NMR Biomed. 2003;16(2):102–111. doi: 10.1002/nbm.816. [DOI] [PubMed] [Google Scholar]
- 4.Lee SC, Delikatny EJ, Poptani H, Pickup S, Glickson JD. In vivo (1)H MRS of WSU-DLCL2 human non-Hodgkin's lymphoma xenografts: response to rituximab and rituximab plus CHOP. NMR Biomed. 2009;22(3):259–265. doi: 10.1002/nbm.1316. [DOI] [PubMed] [Google Scholar]
- 5.Bhujwalla ZM, Glickson JD. Detection of tumor response to radiation therapy by in vivo proton MR spectroscopy. Int J Radiat Oncol Biol Phys. 1996;36(3):635–639. doi: 10.1016/s0360-3016(96)00371-9. [DOI] [PubMed] [Google Scholar]
- 6.Liu YJ, Chen CY, Chung HW, Huang IJ, Lee CS, Chin SC, Liou M. Neuronal damage after ischemic injury in the middle cerebral arterial territory: deep watershed versus territorial infarction at MR perfusion and spectroscopic imaging. Radiology. 2003;229(2):366–374. doi: 10.1148/radiol.2292020639. [DOI] [PubMed] [Google Scholar]
- 7.Moller HE, Kurlemann G, Putzler M, Wiedermann D, Hilbich T, Fiedler B. Magnetic resonance spectroscopy in patients with MELAS. J Neurol Sci. 2005;229–230:131–139. doi: 10.1016/j.jns.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Harris K, Walker PM, Mickle DA, Harding R, Gatley R, Wilson GJ, Kuzon B, McKee N, Romaschin AD. Metabolic response of skeletal muscle to ischemia. Am J Physiol. 1986;250(2 Pt 2):H213–220. doi: 10.1152/ajpheart.1986.250.2.H213. [DOI] [PubMed] [Google Scholar]
- 9.Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R502–516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- 10.Keller U, Oberhansli R, Huber P, Widmer LK, Aue WP, Hassink RI, Muller S, Seelig J. Phosphocreatine content and intracellular pH of calf muscle measured by phosphorus NMR spectroscopy in occlusive arterial disease of the legs. Eur J Clin Invest. 1985;15(6):382–388. doi: 10.1111/j.1365-2362.1985.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 11.Greiner A, Esterhammer R, Messner H, Biebl M, Muhlthaler H, Fraedrich G, Jaschke WR, Schocke MF. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J Vasc Surg. 2006;43(5):978–986. doi: 10.1016/j.jvs.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Williams DM, Fencil L, Chenevert TL. Peripheral arterial occlusive disease: P-31 MR spectroscopy of calf muscle. Radiology. 1990;175(2):381–385. doi: 10.1148/radiology.175.2.2326464. [DOI] [PubMed] [Google Scholar]
- 13.Schunk K, Romaneehsen B, Mildenberger P, Kersjes W, Schadmand-Fischer S, Thelen M. Dynamic phosphorus-31 magnetic resonance spectroscopy in arterial occlusive disease. Correlation with clinical and angiographic findings and comparison with healthy volunteers. Invest Radiol. 1997;32(11):651–659. doi: 10.1097/00004424-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Vigneron DB, Cha S, Graves EE, Crawford F, Chang SM, Nelson SJ. Relationship of MR-derived lactate, mobile lipids, and relative blood volume for gliomas in vivo. AJNR Am J Neuroradiol. 2005;26(4):760–769. [PMC free article] [PubMed] [Google Scholar]
- 15.Allen PS, Thompson RB, Wilman AH. Metabolite-specific NMR spectroscopy in vivo. NMR Biomed. 1997;10(8):435–444. doi: 10.1002/(sici)1099-1492(199712)10:8<435::aid-nbm480>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.van Dijk JE, Mehlkopf AF, Bovee WM. Comparison of double and zero quantum NMR editing techniques for in vivo use. NMR Biomed. 1992;5(2):75–86. doi: 10.1002/nbm.1940050206. [DOI] [PubMed] [Google Scholar]
- 17.He Q, Shungu DC, van Zijl PC, Bhujwalla ZM, Glickson JD. Single-scan in vivo lactate editing with complete lipid and water suppression by selective multiple-quantum-coherence transfer (Sel-MQC) with application to tumors. J Magn Reson B. 1995;106(3):203–211. doi: 10.1006/jmrb.1995.1035. [DOI] [PubMed] [Google Scholar]
- 18.Sotak CH, Freeman DM. A Method for Volume-Localized Lactate Editing Using Zero-Quantum Coherence Created in a Stimulated-Echo Pulse Sequence. J Magn Reson. 1988;77(2):382–388. doi: 10.1002/mrm.1910070315. [DOI] [PubMed] [Google Scholar]
- 19.Jouvensal L, Carlier PG, Bloch G. Practical implementation of single-voxel double-quantum editing on a whole-body NMR spectrometer: localized monitoring of lactate in the human leg during and after exercise. Magn Reson Med. 1996;36(3):487–490. doi: 10.1002/mrm.1910360325. [DOI] [PubMed] [Google Scholar]
- 20.Lei H, Dunn J. The effects of slice-selective excitation/refocusing in localized spectral editing with gradient-selected double-quantum coherence transfer. J Magn Reson. 2001;150(1):17–25. doi: 10.1006/jmre.2001.2304. [DOI] [PubMed] [Google Scholar]
- 21.He Q, Shkarin P, Hooley RJ, Lannin DR, Weinreb JC, Bossuyt VI. In vivo MR spectroscopic imaging of polyunsaturated fatty acids (PUFA) in healthy and cancerous breast tissues by selective multiple-quantum coherence transfer (Sel-MQC): a preliminary study. Magn Reson Med. 2007;58(6):1079–1085. doi: 10.1002/mrm.21335. [DOI] [PubMed] [Google Scholar]
- 22.Souza SP, Szumowski J, Dumoulin CL, Plewes DP, Glover G. SIMA: simultaneous multislice acquisition of MR images by Hadamard-encoded excitation. J Comput Assist Tomogr. 1988;12(6):1026–1030. [PubMed] [Google Scholar]
- 23.Gonen O, Arias-Mendoza F, Goelman G. 3D localized in vivo 1H spectroscopy of human brain by using a hybrid of 1D-Hadamard with 2D-chemical shift imaging. Magn Reson Med. 1997;37(5):644–650. doi: 10.1002/mrm.1910370503. [DOI] [PubMed] [Google Scholar]
- 24.Pickup S, Lee SC, Mancuso A, Glickson JD. Lactate imaging with Hadamard-encoded slice-selective multiple quantum coherence chemical-shift imaging. Magn Reson Med. 2008;60(2):299–305. doi: 10.1002/mrm.21659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16(2):192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- 26.Callaghan PT. Principles of Nuclear Magnetic Resonance Microscopy. Clarendon; Oxford: 1994. p. 516. [Google Scholar]
- 27.Goelman G, Leigh JS. Multiband Adiabatic Inversion Pulses. J Magn Reson A. 1993;101(2):136–146. [Google Scholar]
- 28.Ogg RJ, Kingsley PB, Taylor JS. WET, a T1-and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104(1):1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- 29.Wu W, Wang J, Detre J, Wehrli F, Mohler E, 3rd, Ratcliffe S, Floyd T. Hyperemic flow heterogeneity within the calf, foot, and forearm measured with continuous arterial spin labeling MRI. Am J Physiol-Heart C. 2008;294(5):H2129. doi: 10.1152/ajpheart.01399.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu W, Wang J, Detre J, Ratcliffe S, Floyd T. Transit delay and flow quantification in muscle with continuous arterial spin labeling perfusion-MRI. J Magn Reson Imaging. 2008;28(2):445–452. doi: 10.1002/jmri.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellon EA, Pickup S, Isaac G, Lee SC, Delikatny EJ, Reddy R, Glickson JD. Imaging of physiologic lactate concentrations by SelMQC spectroscopy with Hadamard slice selection on a clinical scanner. 16th Scientific Meeting, International Society for Magnetic Resonance in Medicine; Toronto. 2008. p. 1577. [Google Scholar]
- 32.Singer S, Sivaraja M, Souza K, Millis K, Corson JM. 1H-NMR detectable fatty acyl chain unsaturation in excised leiomyosarcoma correlate with grade and mitotic activity. J Clin Invest. 1996;98(2):244–250. doi: 10.1172/JCI118785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ordidge RJ, Connelly A, Lohman JAB. Image-selected in vivo spectroscopy(ISIS). A new technique for spatially selective NMR spectroscopy. J Magn Reson. 1986;66(2):283–294. [Google Scholar]
- 34.Arias-Mendoza F, Smith MR, Brown TR. Predicting treatment response in non-Hodgkin's lymphoma from the pretreatment tumor content of phosphoethanolamine plus phosphocholine. Acad Radiol. 2004;11(4):368–376. doi: 10.1016/s1076-6332(03)00721-9. [DOI] [PubMed] [Google Scholar]
- 35.Arias-Mendoza F, Payne GS, Zakian K, Stubbs M, Cruz-Lobo JG, Schwarz AJ, Dave A, Howe F, Maisey NR, Cunningham D, Poptani H, Smith MR, O'Connor OA, Pettengell R, Leach MO, Koutcher JA, Griffiths JR, Heerschap AH, Glickson JD. Treatment Response Predictor Using 31P MRS for CHOP and R-CHOP Therapy in Diffuse Large B-Cell Lymphoma. 16th Scientific Meeting, International Society for Magnetic Resonance in Medicine; Toronto. 2008. p. 768. [Google Scholar]
- 36.Kumar R, Maillard I, Schuster SJ, Alavi A. Utility of fluorodeoxyglucose-PET imaging in the management of patients with Hodgkin's and non-Hodgkin's lymphomas. Radiol Clin North Am. 2004;42(6):1083–1100. doi: 10.1016/j.rcl.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J. 1993;291(Pt 3):681–686. doi: 10.1042/bj2910681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boska MD, Nelson JA, Sripathi N, Pipinos II, Shepard AD, Welch KM. 31P MRS studies of exercising human muscle at high temporal resolution. Magn Reson Med. 1999;41(6):1145–1151. doi: 10.1002/(sici)1522-2594(199906)41:6<1145::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Pipinos II, Shepard AD, Anagnostopoulos PV, Katsamouris A, Boska MD. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J Vasc Surg. 2000;31(5):944–952. doi: 10.1067/mva.2000.106421. [DOI] [PubMed] [Google Scholar]
- 40.Vezzoli A, Gussoni M, Greco F, Zetta L. Effects of temperature and extracellular pH on metabolites: kinetics of anaerobic metabolism in resting muscle by 31P- and 1H-NMR spectroscopy. J Exp Biol. 2003;206(Pt 17):3043–3052. doi: 10.1242/jeb.00521. [DOI] [PubMed] [Google Scholar]
- 41.van der Grond J, Braun KPJ. Incorrect Echo Times yield a 10% reduction in the Lactate Intensityand a 9% reduction in the T2 of Lactate. 8th Scientific Meeting, International Society for Magnetic Resonance in Medicine; Denver. 2000. p. 1952. [Google Scholar]