Dysbindin-1 is encoded by the dystrobrevin-binding protein 1 gene (DTNBP1) and is located in synaptic sites throughout human and mouse brain (1). Genetic variations in DTNBP1 impact human cognitive abilities (2-3) and have been associated with risk for schizophrenia (4). Moreover, reduced dysbindin gene and protein expression have been reported in the hippocampus and prefrontal cortex (PFC) of schizophrenic patients (5-8). This latter finding suggests that a molecular phenotype associated with schizophrenia is reduced expression of dysbindin, perhaps of a specific isoform (8), but the role of secondary factors (e.g. drugs, smoking, etc.) and whether altered expression of any dysbindin transcript is the molecular mechanism of genetic risk have not been determined. These uncertainties notwithstanding, mutant mice with diminished DTNBP1 expression have become an informative animal model of reduced dysbindin protein (1, 5-8).
While the statistical evidence for association of DTNBP1 and schizophrenia across diverse population samples is variable, the molecular evidence for a role of dysbindin in dopamine and glutamate signaling, two neurotransmitters at the core of neurochemical hypotheses of psychosis, is fairly consistent. Early speculation that the molecular mechanism of dysbindin's role in psychiatric illness had to do with the dystrophin protein complex, from which its name is derived, has been eclipsed by current data that implicate a pathogenic role of dysbindin as a partner in the biogenesis of lysosome-related organelles complex (BLOC-1). Dysbindin is involved in intracellular protein trafficking involving lysosomes and related organelles and it is important for synaptic homeostasis (1, 9). A number of receptor proteins, including D2 receptors and NR2A receptor subunits, are trafficked after internalization via lysosomal-mediated degradation and dysbindin has been shown to impact these trafficking events. Mice with disrupted dysbindin show selective alterations in internal trafficking of these specific components of dopamine and glutamate signaling, and not of other receptor components that are not trafficked through the lysosomal degradation pathway (e.g. D1 receptors, NR2B components [10-12]). Thus, dysbindin reductions may represent a direct genetic bridge between these two schizophrenia related signaling systems, and the molecular mechanism of DTNBP1 as a psychosis risk gene may involve this bridge.
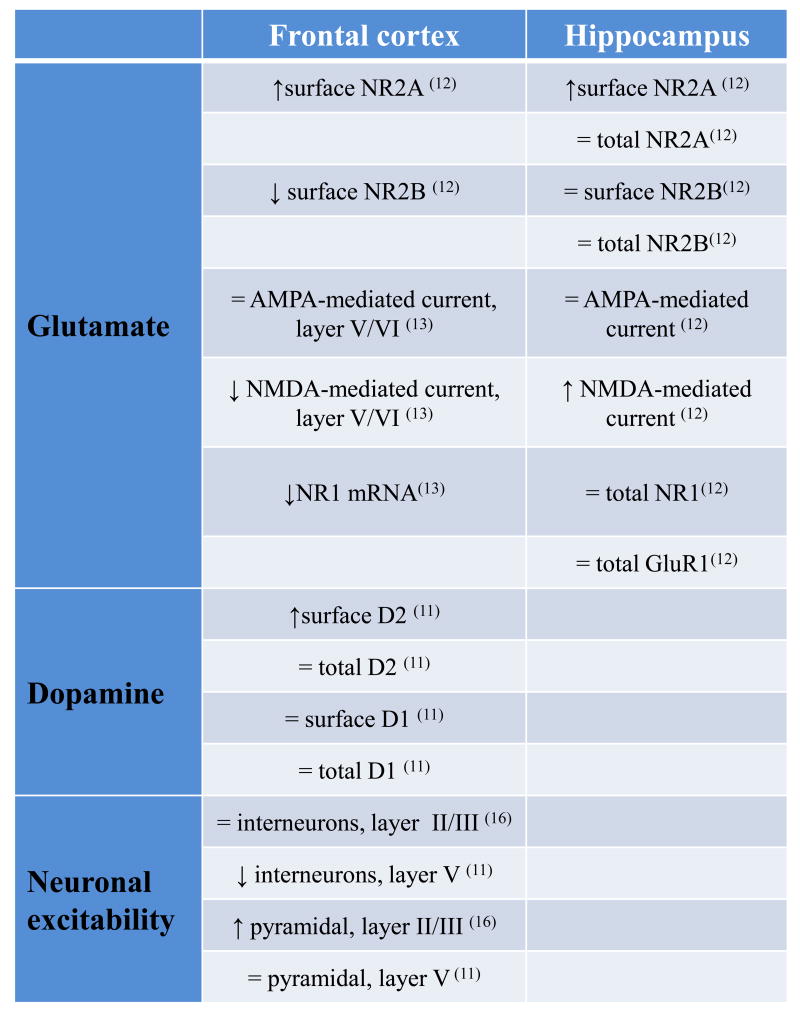
The report by Karlsgodt and colleagues in this issue of Biological Psychiatry identifies a novel effect of dysbindin reduction in mice, a decrease in NMDA excitation and NR1 mRNA expression in the PFC (13). This finding extends earlier evidence that dysbindin impacts on both pre- and post-synaptic dopamine and glutamate signaling (10-12;14-15). What are the consequences of reduced dysbindin function in the glutamate system? In the hippocampus, dysbindin reduction has been shown to result in increased neuronal surface expression of NR2A, but not NR2B; while total levels of NR2A, NR2B, NR1 and GluR1 are not altered compared to wild-type mice. These modifications result in increased NMDA- but not AMPA-mediated synaptic currents and increased LTP, but not LTD (12). In the PFC of these dysbindin mutant mice, different modifications of the glutamate system have been found, but as in the hippocampus, NR2A surface expression is increased in the PFC, consistent with a defect in BLOC-1 trafficking of internalized NR2A. However, in contrast to the hippocampus, dysbindin reduction in the PFC leads to a decrease in NR2B surface expression (12) and, as now reported by Karlsgodt et al. (13), decreased NR1 mRNA. Moreover, in contrast to that previously found in the hippocampus (12), Karlsgodt and colleagues further report reduced NMDA-mediated current in the PFC of dysbindin mutant mice. (See table 1 for a summary of differential dysbindin effects in the glutamate and dopamine systems between the hippocampus and the PFC). Thus, it seems that dysbindin reductions might differentially affect the glutamate system in the PFC compared with the hippocampus, but it is unclear how dysbindin's predictable role in the BLOC-1 system would have differential ramifications in these cortical regions. It is tempting to speculate that the consistent effect of dysbindin on NR2A trafficking impacts differently on these cortical regions due to their differing connectivity and developmental trajectories.
What are the consequences of reduced dysbindin function in the dopamine system? Quite analogous to its impact on lysosomal trafficking of NR2A but not NR2B subunits, dysbindin reduction increases expression of surface D2 receptors, but not D1, on cortical neurons (10-11). This is due to an enhanced reinsertion of D2 to the neuronal membrane, presumably because the lysosomal trafficking pathway is altered (11). It is interesting to note that the total levels of D2 and D1 receptors are not changed in the cortex of dysbindin mutant mice compared to wild-type mice. Only the levels of D2 receptors on the cell surface are increased (11). The increase in surface D2 receptors results in enhanced sensitivity to D2 agonists, both behaviorally and in terms of the excitability of layer V interneurons and layers II/III pyramidal neurons (11, 16). This may have important consequences in the clinical setting because genetic variations that result in decreased dysbindin functioning might predict important differences in the efficacy and dosing of D2-related drugs (e.g. several antipsychotics).
The effect of increased D2 receptors on the cell surface of dysbindin disrupted mice has rather specific physiologic effects, both regional and cellular. In layer V of the PFC, there is reduced excitability of interneurons but not of pyramidal neurons (11). In contrast, in the more superficial layers II/III of the PFC, dysbindin disruption increases neuron excitability in pyramidal neurons but not interneurons (16). These results implicate potential microcircuit pathophysiological alterations in the PFC following dysbindin reduction. Thus, dysbindin reduction results in a D2-dominated state and alters the pattern of excitability in PFC microcircuits, both mechanisms implicated in schizophrenia and especially in its cognitive deficits (17).
These molecular and physiologic effects of reduced dysbindin on cortical glutamate and dopamine systems directly and the GABA system indirectly would be expected to impact on cortically mediated cognition. Indeed, there are several studies showing an effect of genetic variation in DTNBP1 on human cognition (2-3) and dysbindin altered mice also show abnormalities in cognitive performance. As reported by us (16) and by Karlsgodt et al.(13), dysbindin reductions in C57BL/6J (B6) background mice produce spatial working memory deficits. These working memory deficits seem to depend on the experimental condition and are possibly dependent on altered dopamine/D2 pathways (16), glutamate/NR1 pathways (13) or most likely both. Interestingly, dysbindin seems to have a stronger effect on prefrontally-relevant cognition (e.g. working memory) in comparison to more hippocampal-related cognitive functions (e.g. reference memory) (16). However, further investigation of other executive functions and hippocampal-dependent tasks is required to fully address this question.
In the interpretation of the present and future investigations, it is important to note that dysbindin genetic variations likely interact with the genetic background. In particular, the dysbindin genetic mutation in mice originally occurred spontaneously in the inbred DBA/2J strain (the so called “sandy mice”). On this genetic background, dysbindin reduction produces dramatic locomotor and coordination deficits which might make cognitive investigation more difficult. Moreover, DBA/2J mice per se are impaired in theta burst long-term potentiation, in aspects of learning and memory, and are homozygous for other mutations related to cognitive behavior, and have higher dopaminergic activity in the forebrain compared to the B6 background. This has encouraged backcrossing of the “sandy mouse” with a B6 background strain to limit these confounders (16, 18, and 13), though it is not clear in the Karlsgodt et al., study to what degree the B6 backcrossing has been carried out. Furthermore, a direct comparison of working memory performance between the dysbindin mutation on different genetic backgrounds between (19) and the present work by Karlsgodt et al. (13), is problematic because the test parameters have not been uniformly analyzed.
Dysbindin has substantial value as a genetic link to neurochemical systems strongly implicated in psychosis, particularly dopamine, glutamate and GABA. It illustrates, at least in principle, a mechanism for genetic susceptibility that makes intuitive sense in terms of what we already suspect is important in the pathophysiology of schizophrenia. The debate will surely continue about whether the statistical evidence for genetic association is strong enough and whether altered DTNBP1 expression in schizophrenic brain is a primary phenomenon driven by the clinical risk SNPs. Nevertheless, the compelling biological evidence for a role of dysbindin in modulating neuronal signaling implicated in psychosis should encourage research to follow dysbindin in the search for strategies to individualize outcome with available therapeutic agents and to find new ones.
Figure 1. Modifications in dysbindin knockout mice versus wild-type.
↑: increased; ↓: decreased; =: no change.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health, National Institute of Health.
Footnotes
Financial Disclosures: The authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Talbot K, Ong WY, Blake DJ, Tang J, Louneva N, Carlson GC, et al. Dysbindin-1 and Its Protein Family. In: Javitt DC, Kantrowitz J, editors. Handbook of Neurochemistry and Molecular Neurobiology. 3rd. New York: Springer Science; 2009. pp. 107–241. [Google Scholar]
- 2.Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, et al. Genetic variation in DTNBP1 influences general cognitive ability. Human molecular genetics. 2006;15:1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- 3.Fallgatter AJ, Herrmann MJ, Hohoff C, Ehlis AC, Jarczok TA, Freitag CM, et al. DTNBP1 (dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology. 2006;31:2002–2010. doi: 10.1038/sj.npp.1301003. [DOI] [PubMed] [Google Scholar]
- 4.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. American journal of human genetics. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. The Journal of clinical investigation. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophrenia research. 2008;98:105–110. doi: 10.1016/j.schres.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Archives of general psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG, et al. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Human molecular genetics. 2009;18:3851–3863. doi: 10.1093/hmg/ddp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iizuka Y, Sei Y, Weinberger DR, Straub RE. Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci. 2007;27:12390–12395. doi: 10.1523/JNEUROSCI.1689-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, et al. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0904289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang TT, Yang F, Chen BS, Lu Y, Ji Y, Roche KW, et al. Dysbindin regulates hippocampal LTP by controlling NMDA receptor surface expression. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0910499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A, Jentsch JD. Biol Psychiatry. 2010;69:xxx–xxx. doi: 10.1016/j.biopsych.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagai T, Kitahara Y, Shiraki A, Hikita T, Taya S, Kaibuchi K, et al. Dysfunction of dopamine release in the prefrontal cortex of dysbindin deficient sandy mice: an in vivo microdialysis study. Neuroscience letters. 2010;470:134–138. doi: 10.1016/j.neulet.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 15.Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Human molecular genetics. 2004;13:2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- 16.Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN, et al. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in neurosciences. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L, et al. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav. 2009;8:390–397. doi: 10.1111/j.1601-183X.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology. 2009;34:2601–2608. doi: 10.1038/npp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]