Abstract
Proper understanding of processes underlying visual perception requires information on the activation order of distinct brain areas. We measured dynamics of cortical signals with magnetoencephalography while human subjects viewed stimuli at four visual quadrants. The signals were analyzed with minimum current estimates at the individual and group level. Activation emerged 55–70 ms after stimulus onset both in the primary posterior visual areas and in the anteromedial part of the cuneus. Other cortical areas were active after this initial dual activation. Comparison of data between species suggests that the anteromedial cuneus either comprises a homologue of the monkey area V6 or is an area unique to humans. Our results show that visual stimuli activate two cortical areas right from the beginning of the cortical response. The anteromedial cuneus has the temporal position needed to interact with the primary visual cortex V1 and thereby to modify information transferred via V1 to extrastriate cortices.
Anatomy of connections between primate visual cortices suggests a hierarchical organization of signal processing (1). However, the order of processes at different functional areas cannot be directly deduced from the anatomical hierarchy without relevant timing information. In monkeys, several areas become active immediately after the primary visual cortex (V1), while another set of areas is active at clearly longer latencies (2, 3). The areas showing early responses, such as V5/middle temporal area (MT), medial superior temporal area, and frontal eye field, are specialized in analyzing dynamical visual information and in visuomotor transformation (for reviews, see refs. 4 and 5). The areas with longer latencies, such as V4, are sensitive to object form and color and participate in object recognition (6, 7). The dissimilarities in activation latencies and functional properties of these areas suggest diversity in the type and amount of necessary input and preprocessing before activation. Given the differences between monkey and human visual cortices (for a review, see ref. 8), we aimed to explore the dynamics and distribution of early cortical activation in humans. Neuromagnetic signals were recorded while the subjects viewed pattern reversal or luminance stimuli at the four visual quadrants. The signals were analyzed with minimum current estimates (MCEs), which require minimal human intervention for determining the location and orientation of the cortical currents (9). The individual three-dimensional estimates were aligned with a nonlinear transformation according to individual brain shapes (10, 11), and both the individual and group average MCEs were compared with the existing maps of human visual cortices (12–16).
Materials and Methods
Subjects and Stimuli.
We studied five female and five male subjects (mean age 27 years, range 20–42 years). The stimuli were generated with a Macintosh computer and presented with a dataprojector (VistaPro, Electrohome Ltd., Ontario, Canada) on a back projection screen, with viewing distance of 84 cm. The subjects fixated a small cross on a gray (30 cd/m2) background. Black (4 cd/m2) and white (BW; 61 cd/m2) or red and green (RG; red luminance 29.5, x = 0.539, y = 0.408 in CIE space, green mean values luminance 31.4, x = 0.331 and y = 0.559) checkerboard patterns with reversing contrasts or luminance stimuli were presented to the four visual field quadrants (4–12° from fixation, the peripheral stimuli covered a 45° sector, and were symmetrically off the horizontal and vertical meridians; the check size of the pattern stimuli increased from 0.8 to 1.9° with eccentricity). The luminance stimulus comprised of increase of luminance from the gray background (30 cd/m2) to the maximum luminance (61 cd/m2) for duration of 470 ms. The three stimulus types covered the same visual field locations. The individual equiluminant values for the RG stimulus were determined with flicker photometry; the red was invariable but the green was varied. The upper and lower visual field stimuli were delivered in separate sessions. The left and right quadrants were stimulated in random order within the same session, with random 1–1.5 s interstimulus intervals. During the pattern reversal sessions, the pattern was continuously on, displaying the two upper or the two lower quadrant patterns, and the stimulation comprised of reversals of the contrast in one quadrant at a time.
Signal Recording and Analysis.
Signals were recorded with a 306-channel Vectorview neuromagnetometer (Neuromag Ltd., Helsinki, Finland), filtered (passband 0.1–200 Hz), sampled at 600 Hz, and averaged time locked to the pattern reversal or to the onset of the luminance stimulus. Vertical and horizontal electro-oculograms were recorded and epochs contaminated with eye movements or blinks were rejected online.
Environmental noise was attenuated in the measured signals by extracting signal-space vectors with strongest noise fields. The signals were then preprocessed by omitting noisy channels, applying a 200-ms prestimulus baseline and a 40-Hz low-pass filter. The analysis applied MCE (9), an implementation of the minimum L1-norm estimate (17); MCE explains the measured signals with a current distribution that has the smallest sum of current amplitudes. Estimates of individual signals were calculated separately for each time point. For the group average, the individual estimates were aligned on a standard brain (18) and then averaged. The alignment phase applies first a 12-parameter affine transformation (11), followed by a refinement with an elastic nonlinear transformation (10). The match is based on the comparison of gray-scale values of the individual and the standard brain MR images. As a result, major sulci and other important brain structures are aligned.
For more detailed analysis, regions of interest (ROIs) were selected to cover steadily active areas. The center and extent of each ROI was automatically adjusted to fit the estimated activity. The activity within a ROI was calculated as a weighted average of the estimate: the maximum weight was in the center of the ROI and the weight extended to the neighboring locations with the form of a three-dimensional generalized normal distribution. The reported radius of ROI is the distance at which the weighting factor is 60% of that at the center. A ROI was accepted for further study if (i) the peak source activity exceeded 2 nA⋅m and was at least twice the peak activity during prestimulus baseline; (ii) the activation duration exceeded 10 ms; and (iii) the location was stable for over 10 ms. An active brain area could remain undetected in our recordings and analysis because of a too short distance between two active areas, because of cancellation of the magnetic signal attributable to source current configuration, or because of masking of a deeper brain activity by simultaneous strong activation close to the sensors. Only BW pattern reversal data were used for source localization in individual subjects.
Determination of Cortical Areas.
The sources were first located from group average data to find common features of activation, and then the mean coordinates across subjects were compared with earlier imaging and histology studies (12–16) for determination of the functional areas. Because our coordinate space corresponded to the standard brain coordinates of the European Computerized Human Brain Database,‡ the Talairach coordinates (20) in the earlier studies had to be transformed to the same coordinate system for comparison and display purposes. In addition, our source coordinates were transformed into Talairach space; the rightward shift of the midline at the posterior parts of cerebrum (14) was taken into account by aligning the midsagittal plane, in addition to anterior and posterior commisures, with the hemispheric midline at the posterior cortex.
Results and Discussion
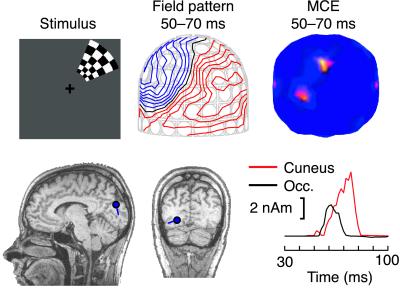
Fig. 1 presents the estimated brain activity in Subject 1 after right upper visual field BW pattern reversal stimulation. The magnetic field pattern between 50 and 70 ms cannot be explained with only one active area. Accordingly, the MCE display shows two maxima, one located in the anteromedial cuneus, abutting the parieto-occipital sulcus, and the other in the more inferior left occipital lobe. The rising edge of the response is 3–4 ms (about two samples) earlier at the left occipital cortex than at the cuneus, suggesting that these sources reflect two separate brain regions.
Figure 1.
Data of Subject 1 after right upper quadrant BW pattern reversal. Magnetic field pattern and the corresponding MCE results, viewed from the back of the head, are integrated from 50 to 70 ms. The red contours in the magnetic field display indicate the magnetic flux coming out from the head and the blue contours the magnetic flux entering the head. The blue dots and lines on the individual MRIs represent the center and orientation of the estimated current. The red and black curves show the strengths of the anteromedial cuneus and left occipital (Occ.) sources as a function of time.
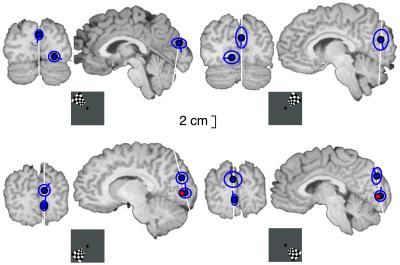
Fig. 2 presents the mean locations across subjects for the two sources activated by BW pattern reversals. The source at or close the anteromedial cuneus was found in 9 and 5 individuals after the left and right upper quadrant stimuli, respectively, but only in 5 and 3 subjects after the left and right lower quadrant stimuli. The inferior occipital source was found in 8 and 7 subjects after the left and right upper quadrant stimuli and in 8 and 10 subjects after the left and right lower quadrant stimuli, respectively. At best (after left upper quadrant stimuli), the simultaneous two sources were found in 7 subjects. The absence of the cuneus source in 5–7 subjects after lower visual field stimuli could reflect the shorter intersource distance after lower than upper field stimuli. In subjects who showed two sources, the intersource distances were on average only 25–26 mm for lower field stimuli but 32–40 mm for upper field stimuli. If the distance had been smaller in other source pairs, the estimation method might have faced difficulties in differentiating the two sources.
Figure 2.
The mean location, its SD, and current orientation (blue dot, circle, and line, respectively) of the cuneus and inferior occipital sources are displayed in a standard brain (18). The red dot displays the mean location of left and right V1 from Hasnain et al. (13), who reported, however, the average location of the upper and lower visual field representations.
The location of the inferior occipital source was more stable across individuals after lower than upper visual field stimuli (Fig. 2). After lower visual field stimuli, the mean location of the inferior source corresponded well to the human V1 in the medial occipital lobe (13), with the current directed upward. Assuming that this activation occurred in the upper lip of the calcarine fissure, the current direction would correspond to cortical current flow from the surface to the depth of the cortex. After upper visual field stimuli, the inferior source was lateral to the midline. Although the inferior occipital response could receive additional contribution from other visual areas, similar latencies for the upper and lower visual field responses and the group mean location close to calcarine fissure suggest considerable V1 contribution to the signal.
Location of the anteromedial cuneal source in Talairach space (ref. 20; x, y, and z coordinates 2, −79, and 23 mm after the left and −2, −77, and 26 mm after the right visual field stimulation; average for the upper and lower quadrant epochs) falls outside many positron emission tomography (13) and functional MRI (12, 15, 16) studies mapping the human occipital areas. These maps do not report areas in the anteromedial cuneus. However, Corbetta et al. (21) reported deactivation in functional MRI in similar regions (their figure 2 and table 1; 21 and 8 mm mean distance from our left and right cuneus sources) when covert shifts of attention and saccadic eye movements were compared. Thus, although our subjects had no task during stimulation, our activation in the medial cuneus could reflect automatic activation of a part of the attention- or visuomotor-related networks.
Although our cuneus source showed both lower and upper visual field representations similarly as the human area V3a does (15), the source location was 1–2 cm too medial for V3a, regardless of the quadrant stimulated (refs. 13 and 15; Fig. 2). Neither can the cuneus source be at V3, because the human V3 does not have upper visual field representation above the calcarine sulcus (15), and because our source was 2–3 cm too medial to the known location of the left and right human V3 (13).
In the monkey V6 (22), the representations of the upper and lower visual fields are clearly distinct, and in part even in different walls of the parieto-occipital sulcus, with a trend of more anterior locations for upper than lower visual field representations. Interestingly, the mean current directions at the cuneus source were opposite during the upper and lower visual field responses (Fig. 2), suggesting both that retinotopy occurs at the source area and that the responses could arise from opposite walls of a sulcus or a gyrus.
In the stereotaxic isocontours displayed on the Visible Man atlas (8), the coordinates of the anteromedial cuneus source are located at the posterior bank of the parieto-occipital sulcus, which corresponds to the macaque area V6 (also known as PO) in the comparison map between monkey and man (23). In addition, based on histological evidence, this source area could be a homologue of monkey area V6.§ The backward shift of histologically defined area 19 during cortical phylogenesis (for reviews, see refs. 14 and 25) should have pushed the functional area V6 from the medial fundus of the parieto-occipital sulcus in monkeys (22) to the upper posterior wall of the parieto-occipital sulcus and thus to the anteromedial cuneus in humans. The neurons of V6 have large receptive fields (22), and lack enhanced foveal magnification both in monkeys (22) and humans (26), and the area has been suggested to participate in visuospatial analysis of environment for arm reaching and eye movements (27, 28). The human anteromedial cuneus could also contain an area or multiple areas with no equivalent in the macaque brain. The larger amount of neurons and longer distances between brain areas in humans than in monkeys implicate different transmission latencies between areas and thus different requirements for cortical organization in the two species when rapid processing is of essence.
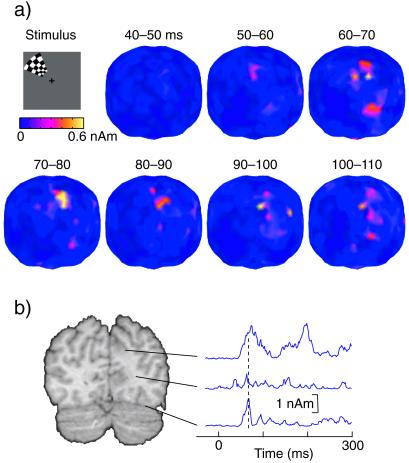
Fig. 3 presents the dynamics of processing in the two areas. The group average MCE to left upper quadrant stimuli showed the two maxima during the earliest response from 60 ms onward (Fig. 3a). The lower source fades out before 80 ms, whereas the upper source continues to be active up to about 100 ms. Attenuation of the MCE strength at sites located between the two maxima (middle trace in Fig. 3b) further supports discrete clusters during the initial activation; these clusters also display clearly different temporal behaviors. After upper quadrant BW stimuli, the occipital source became active on average at 56 (mean half-amplitude latency from individual data), and the cuneus source at 58 ms; the latency difference between the two sites was not statistically significant. This timing information leaves the pathway of retinal information to anteromedial cuneus unclear. Signals could either go through V1 and be rapidly fed forward (2, 29), or bypass V1 (30, 31).
Figure 3.
(a) Group average MCEs after left upper quadrant pattern reversals, integrated over successive 10-ms intervals after stimulus. (b) Spherical smooth-edged regions of interests with 8-mm radius are placed close the higher and lower current clusters and between. The ROIs are presented on the standard brain MRI (18). The right column shows the signal strength of these ROIs as a function of time.
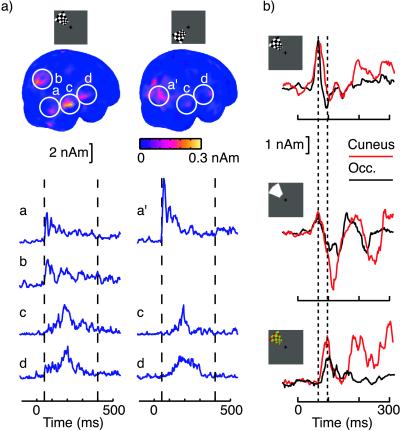
After 90 ms, the activation spread to other areas, predominantly in the contralateral hemisphere; in the time window of 100–200 ms the mean activation at the posterior brain was about four times stronger in the right than the left hemisphere (vol. 310 cm3). Whereas between 110 and 150 ms multiple structures contributed to the signal, later only few areas were prominently active at the right hemisphere. A movie of this activation is published on the PNAS web site as supplementary material (www.pnas.org). Fig. 4a depicts the strongest activations between 50 and 400 ms after left upper and lower quadrant BW stimuli. The posterior areas (a, a′, and b) were active clearly earlier than the temporo-occipital (c) and superior temporal (d) regions. The temporo-occipital source region was about 1 cm anterior and inferior to human MT/V5 (x, y, and z Talairach coordinates for the ROI c are 42, −67, and −4 mm, respectively; data have been compared with a summary of V5 coordinates in ref. 32). However, because of its size, the ROI c should include activation of the human V5/MT. This area has been shown to be active in monkeys almost immediately after V1 (2) and suggested to be active even before V1 in humans (33). However, the 170- to 220-ms latencies in our study are in line with earlier magnetoencephalographic data showing V5 activation at 160 ms for motion stimuli (32, 34). It is possible that our stimuli did not activate V5/MT, but the signals with longer latencies at the ROI c come from other functional areas. After left upper BW stimuli, the signals of the right superior temporal region increased slightly simultaneously with the signals of the posterior regions, but only in a few subjects. Of course, our data alone cannot exclude the possibility of other early responses, which might have been detected if more area-specific stimuli would have been applied or a much larger number of responses would have been averaged to improve the signal-to-noise ratio.
Figure 4.
(a) Group average MCE after left upper and left lower quadrant pattern reversals, integrated from 50 to 400 ms after stimulus. Note that averaging the signal from 50 to 400 ms makes the earliest transient activation poorly visible. The four distinct ROIs are in the posterior inferior occipital cortex (a and a′), in the anteromedial cuneus (b), in the temporooccipital junction (c), and in the superior temporal sulcus (d). After left lower quadrant stimuli, the group average MCE separate poorly the cuneus and occipital sources because the two areas are close together and there is some interindividual variance in locations—the two distinct sources were better separable in individual data. (b) Group average amplitude as a function of time for BW pattern reversal, luminance onset, and equiluminant RG pattern reversal stimuli at the left upper quadrant. The dashed vertical lines indicate latencies at 67 and 97 ms. The amplitude curves reflect group average activity at the mean locations of the individual sources, determined from BW data.
The initial dual activation of the occipital cortex and of the anteromedial cuneus was best distinguished after reversals of BW checkerboard patterns, but it was also present after the luminance onsets and reversals of equiluminant RG patterns. Fig. 4b displays activation of the two sources for the three stimuli; the source locations and current orientations were determined from the individual BW pattern reversal data. These two areas were always simultaneously active, but responses of both areas were about 30 ms delayed for the equiluminant stimuli. This delay may correspond to slower conduction of signals through the wavelength-sensitive parvocellular than the broad-band magnocellular retinocortical pathway (35) or to a weaker retinocortical signal at equiluminance (36). At longer latencies, the cortical dynamics was different for each stimulus.
The novel finding of early activation in the human anteromedial cuneus is interesting in the light of earlier monkey data. Activation latencies in monkey V6 have not been reported. Whereas in monkeys the V5/MT and superior temporal sulcus respond to stationary stimuli shortly after V1 (2, 3), we found prominent activation considerably later in the human V5/MT and superior temporal sulcus areas, suggesting differences in the processing dynamics between humans and monkeys. In monkeys, the first responses at V1 occur around 31–34 ms (2, 29) to luminance stimuli and the first responses to pattern reversal stimuli peak in lamina 4C of V1 around 50 ms after the stimulus, corresponding most likely to the 70-ms evoked potential in human scalp recordings (37). However, the cortical activation latencies depend on stimulus characteristics, such as contrast and luminance, hindering direct comparison across studies. Neuromagnetic signals at the human parieto-occipital sulcus region have been detected earlier after luminance stimuli (26, 38), eyeblinks (39), and saccades (40). The responses in those studies reached their maxima between 130 and 285 ms after the stimuli, and thus showed considerably longer latencies than what we found at the anteromedial occipital lobe.
Apparently, our first 56-ms response can be considered early, and it could even correspond to disynaptic activation immediately after the first afferent signals coming through the magnocellular pathway (29). Furthermore, a relatively early activated higher-order area could interact with V1 and thereby modulate the processing of the later arriving parvocellular (main) volley of afferent information (41), thus affecting the quality or quantity of visual information reaching later processing stages.
According to our findings, an area at the human anteromedial cuneus has the temporal position needed to interact with the lowest levels of cortical hierarchy before processing proceeds more widely to other visually responsive areas. In monkeys, the first responses in V1 specific to figure boundary arise with about 60–70 ms latency, about 30 ms after the first response (19). In our data, the initial dual activation lasted less than 30 ms. Thus these two areas would have time to interact before the edges of a figure are detected in V1. The methods and results of this study open one window for exploring the hypothesis of stimulus-driven early top-down priming of V1 processing (41, 24) in human subjects.
Supplementary Material
Acknowledgments
We thank Jean Bullier for valuable comments on the manuscript and Jaakko Järvinen for help in the data analysis. The MRIs were obtained at the Department of Radiology, Helsinki University Central Hospital. The source coordinate transformations are based on work conducted within European Computerized Brain Database project, EC 4th Framework program/Biotech 2, No EN D 100413. This study has been supported by Academy of Finland, Ministry of Education, and Sigrid Jusélius Foundation.
Abbreviations
- MT
middle temporal area
- MCE
minimum current estimate
- ROI
region of interest
- BW
black and white
- RG
red and green
- V1
primary visual cortex
- V6
visual cortical area V6
Footnotes
Roland, P. E., Fredriksson, J., Svensson, P., Amunts, K., Cavada, C., Hari, R., Cowey, A., Crivello, F., Geyer, S., Kostopoulos, G., et al., 5th International Conference on Functional Mapping of the Human Brain, June 22–26, 1999, Düsseldorf, Neuroimage 9, S128 (abstr.).
Zeki, S. Proceedings of The Physiological Society, July 25–26, 1986, Cambridge, J. Physiol. (London) 381, 62P (abstr.).
References
- 1.Felleman D J, Van Essen D C. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 2.Schmolesky M T, Wang Y, Hanes D, Thompson K G, Leutgeb S, Schall J D, Leventhal A G. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder C E, Mehta A D, Givre S J. Cereb Cortex. 1998;8:575–592. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- 4.Orban A G. In: Cerebral Cortex. Rockland K S, Kaas J H, Peters A, editors. Vol. 12. London: Plenum; 1997. pp. 359–434. [Google Scholar]
- 5.Schall J D. In: Cerebral Cortex. Rockland K S, Kaas J H, Peters A, editors. Vol. 12. London: Plenum; 1997. pp. 527–638. [Google Scholar]
- 6.Zeki S. Nature (London) 1980;284:412–419. doi: 10.1038/284412a0. [DOI] [PubMed] [Google Scholar]
- 7.Desimone R, Schein S J. J Neurophysiol. 1987;57:835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- 8.Van Essen D C, Drury H A. J Neurosci. 1997;17:7079–7102. doi: 10.1523/JNEUROSCI.17-18-07079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uutela K, Hämäläinen M, Somersalo E. NeuroImage. 1999;10:173–180. doi: 10.1006/nimg.1999.0454. [DOI] [PubMed] [Google Scholar]
- 10.Schormann T, Henn S, Zilles K. Lect Notes Comput Sci. 1996;1131:337–342. [Google Scholar]
- 11.Woods R P, Grafton S T, Watson J D, Sicotte N L, Mazziotta J C. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Sereno M I, Dale A M, Reppas J B, Kwong K K, Belliveau J W, Brady T J, Rosen B R, Tootell R B H. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 13.Hasnain M K, Fox P T, Woldorff M G. Hum Brain Mapp. 1998;6:301–315. doi: 10.1002/(SICI)1097-0193(1998)6:4<301::AID-HBM8>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. NeuroImage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- 15.Tootell R B, Mendola J D, Hadjikhani N K, Ledden P J, Liu A K, Reppas J B, Sereno M I, Dale A M. J Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeYoe E A, Carman G J, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Proc Natl Acad Sci USA. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuura K, Okabe U. IEEE Trans Biomed Eng. 1995;42:608–615. doi: 10.1109/10.387200. [DOI] [PubMed] [Google Scholar]
- 18.Roland P E, Graufelds C J, Wåhlin J, Ingelman L, Andersson M, Ledberg A, Pedersen J, Åkerman S, Dabringhaus A, Zilles K. Hum Brain Mapp. 1994;1:173–184. doi: 10.1002/hbm.460010303. [DOI] [PubMed] [Google Scholar]
- 19.Lamme V A, Rodriguez-Rodriguez V, Spekreijse H. Cereb Cortex. 1999;9:406–413. doi: 10.1093/cercor/9.4.406. [DOI] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 21.Corbetta M, Akbudak E, Conturo T E, Snyder A Z, Ollinger J M, Drury H A, Linenweber M R, Petersen S E, Raichle M E, VanEssen D C, Shulman G L. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 22.Galletti C, Fattori P, Gamberini M, Kutz D F. Eur J Neurosci. 1999;11:3922–3936. doi: 10.1046/j.1460-9568.1999.00817.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Essen D C, Drury H A, Joshi S, Miller M I. Proc Natl Acad Sci USA. 1998;95:788–795. doi: 10.1073/pnas.95.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullman S. Cereb Cortex. 1995;5:1–11. doi: 10.1093/cercor/5.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Mountcastle V B. Perceptual Neuroscience: The Cerebral Cortex. Cambridge, MA: Harvard Univ. Press; 1998. [Google Scholar]
- 26.Portin K, Hari R. Proc R Soc London B. 1999;266:981–985. doi: 10.1098/rspb.1999.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galletti C, Battaglini P P, Fattori P. Eur J Neurosci. 1995;7:2486–2501. doi: 10.1111/j.1460-9568.1995.tb01047.x. [DOI] [PubMed] [Google Scholar]
- 28.Shipp S, Blanton M, Zeki S. Eur J Neurosci. 1998;10:3171–3193. doi: 10.1046/j.1460-9568.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- 29.Nowak L G, Munk M H, Girard P, Bullier J. Vis Neurosci. 1995;12:371–384. doi: 10.1017/s095252380000804x. [DOI] [PubMed] [Google Scholar]
- 30.Bullier J, Girard P, Salin P-A. In: Cerebral Cortex. Peters A, Rockland KS, editors. Vol. 10. New York: Plenum; 1994. pp. 301–330. [Google Scholar]
- 31.Barbur J L, Watson J D G, Frackowiak R S J, Zeki S. Brain. 1993;116:1293–1302. doi: 10.1093/brain/116.6.1293. [DOI] [PubMed] [Google Scholar]
- 32.Vanni S, Uusitalo M A, Kiesilä P, Hari R. NeuroReport. 1997;8:1939–1942. doi: 10.1097/00001756-199705260-00029. [DOI] [PubMed] [Google Scholar]
- 33.Beckers G, Zeki S. Brain. 1995;118:49–60. doi: 10.1093/brain/118.1.49. [DOI] [PubMed] [Google Scholar]
- 34.Uusitalo M A, Jousmäki V, Hari R. Neurosci Lett. 1997;224:45–48. doi: 10.1016/s0304-3940(97)13445-0. [DOI] [PubMed] [Google Scholar]
- 35.Schiller P H, Malpeli J G. J Neurophysiol. 1978;41:788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- 36.Logothetis N K, Schiller P H, Charles E R, Hurlbert A C. Science. 1990;247:214–217. doi: 10.1126/science.2294602. [DOI] [PubMed] [Google Scholar]
- 37.Schroeder C E, Tenke C E, Givre S J, Arezzo J C, Vaughan H G., Jr Vision Res. 1991;31:1143–1157. doi: 10.1016/0042-6989(91)90040-c. [DOI] [PubMed] [Google Scholar]
- 38.Portin K, Salenius S, Salmelin R, Hari R. Cereb Cortex. 1998;8:253–260. doi: 10.1093/cercor/8.3.253. [DOI] [PubMed] [Google Scholar]
- 39.Hari R, Salmelin R, Tissari S O, Kajola M, Virsu V. Nature (London) 1994;367:121–122. doi: 10.1038/367121b0. [DOI] [PubMed] [Google Scholar]
- 40.Jousmäki V, Hämäläinen M, Hari R. NeuroReport. 1996;7:2961–2964. doi: 10.1097/00001756-199611250-00032. [DOI] [PubMed] [Google Scholar]
- 41.Nowak L G, Bullier J. In: Cerebral Cortex. Rockland K S, Kaas J H, Peters A, editors. Vol. 12. London: Plenum; 1997. pp. 205–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.