Abstract
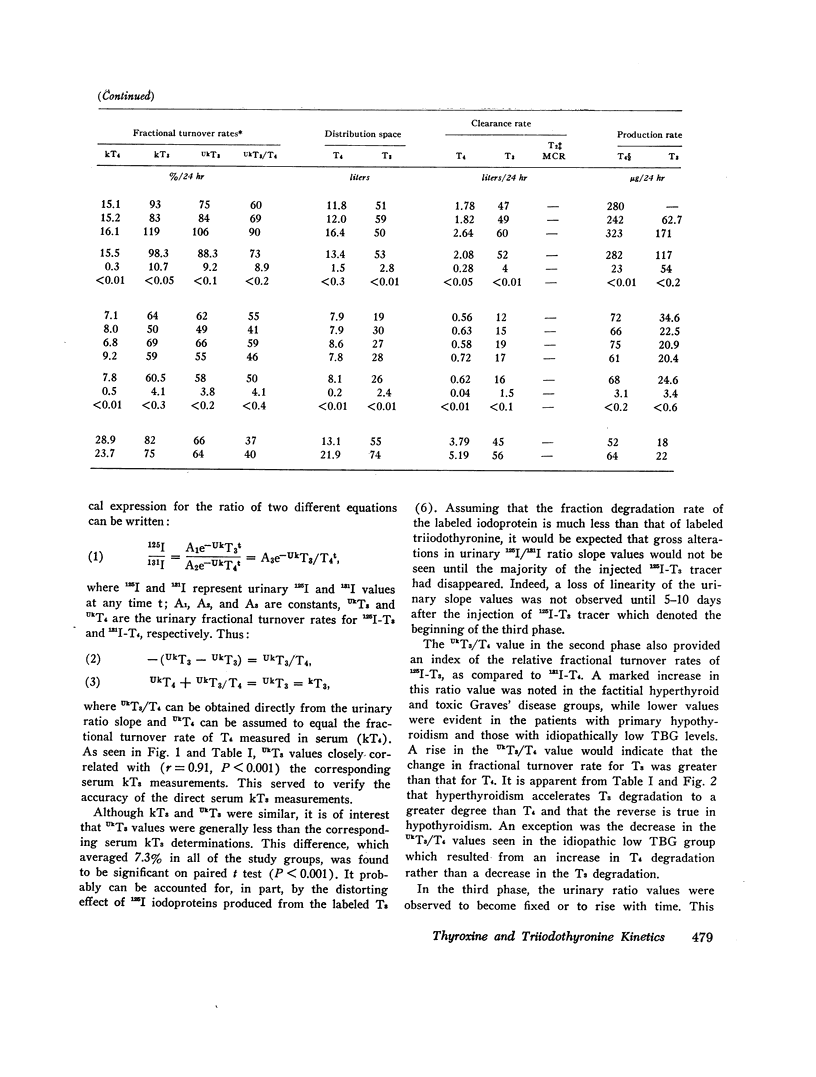
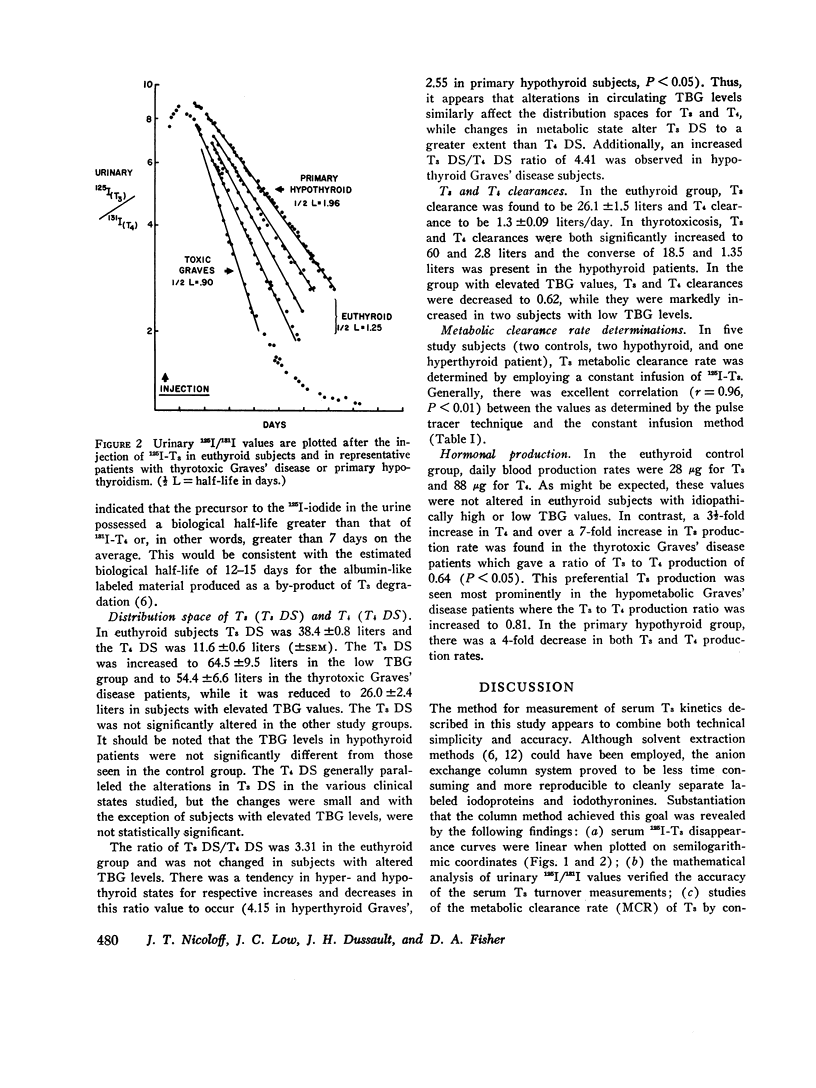
Serum triiodothyronine (T3) kinetics in man have been difficult to define presumably due to the interference of iodoproteins generated during the peripheral metabolism of T3. The use, in the present study, of an anion-column chromatographic method for separation of serum T3 as well as thyroxine (T4) from these iodoproteins has overcome this technical handicap. Simultaneous measurement of serum 125I-T3 and 131I-T4 kinetics were performed in 31 subjects from the clinical categories of euthyroid, primary hypothyroid, thyrotoxic and posttreatment hypothyroid Graves' disease, factitial thyrotoxic, and idiopathically high and low thyroxinebinding globulin states. The normal mean T3 fractional turnover rate (kT3) was 0.68 (half-life = 1.0 days), increased in toxic Graves' disease patients to 1.10 (half-life = 0.63 days), and decreased in primary hypothyroid patients to 0.50 (half-life = 1.38 days). The mean T3 equilibration time averaged 22 hr except in hypothyroid and high thyroxine-binding globulin (TBG) patients where the equilibration period was delayed by 10 hr. The mean T3 distribution space in normal subjects was 38.4 liters. This was reduced in subjects with high TBG levels (26 liters) and increased in patients with low TBG and in all hyperthyroid states (53-55 liters). The normal serum T3 concentration was estimated by radioimmunoassay to be 0.106 μg/100 ml. Combined with the mean T3 clearance value of 26.1 liters/day, the calculated T3 production rate was 27.6 μg/day. The mean T3 production rate increased to 201 μg/day in thyrotoxic Graves' disease patients and was reduced to 7.6 μg/day in primary hypothyroid subjects. T3 production rate was normal in subjects with altered TBG states. The ratio of T3 to T4 production rate in normal subjects was 0.31 and was unchanged in patients with altered TBG values. This ratio was increased in all Graves' disease patients with the highest value being 0.81 in the posttreatment hypothyroid Graves' disease group. This apparent preferential production of T3 may have been responsible for the retention of rapid turnover kinetics for T3 and T4 observed in treated Graves' disease patients. The finding that factitial thyrotoxic patients also displayed similar rapid T3 and T4 turnover kinetics indicates that these alterations are not a unique feature of Graves' disease per se. When comparing the peripheral turnover values for T3 and T4 in man, it is apparent that alterations in metabolic status and serum TBG concentration influence both hormones in a parallel manner; however, changes in metabolic status seem to have a greater influence on T3 kinetics while alterations in TBG concentrations have a greater effect on T4. These observations probably relate to the differences in TBG binding affinity and peripheral tissue distribution of these two hormones.
Full text
PDF

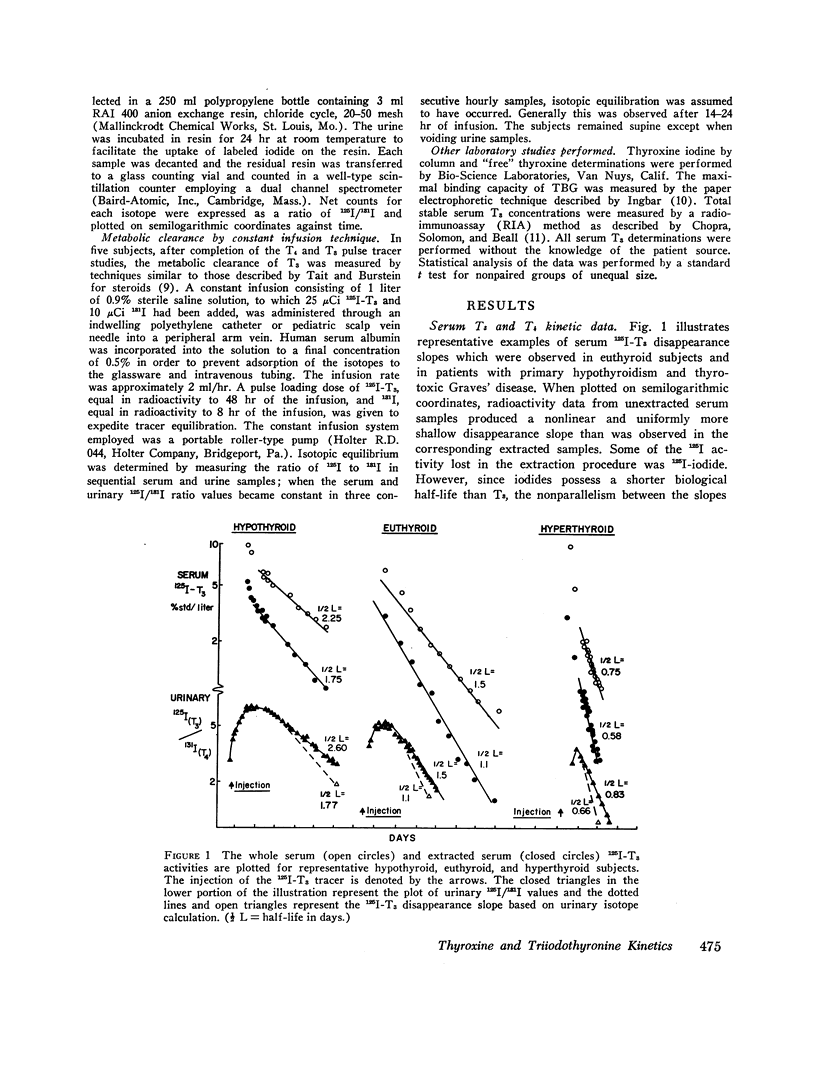
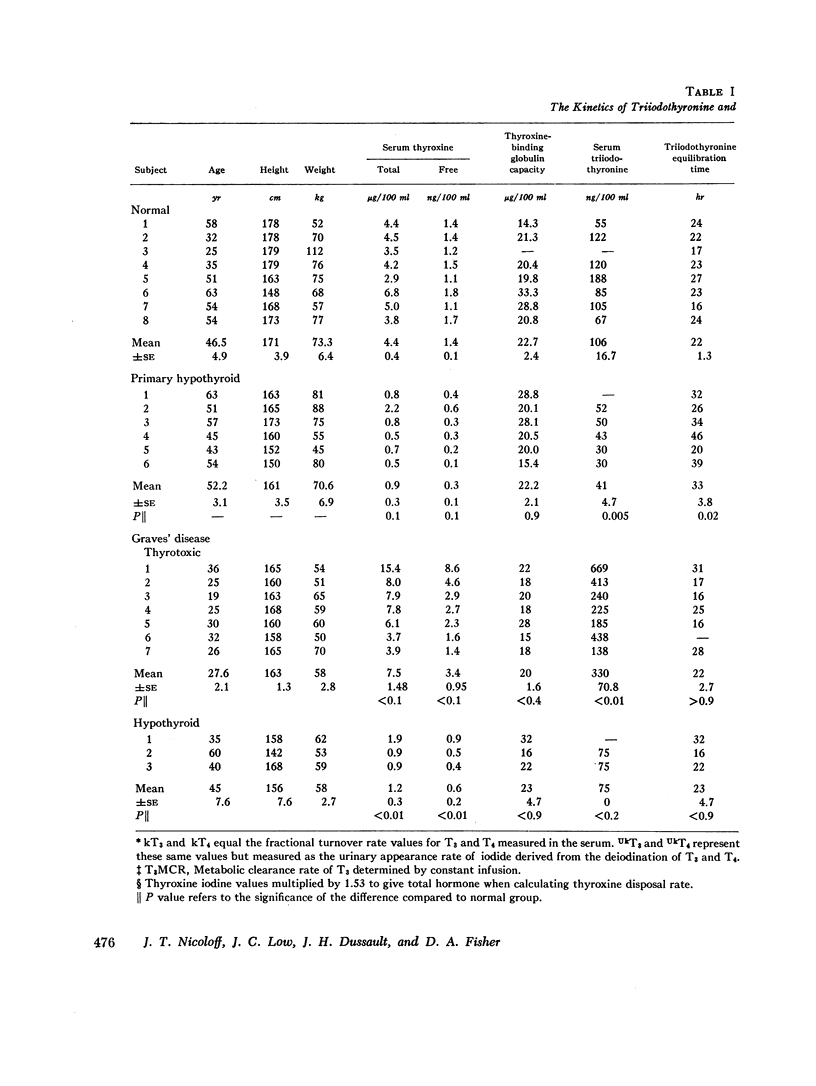
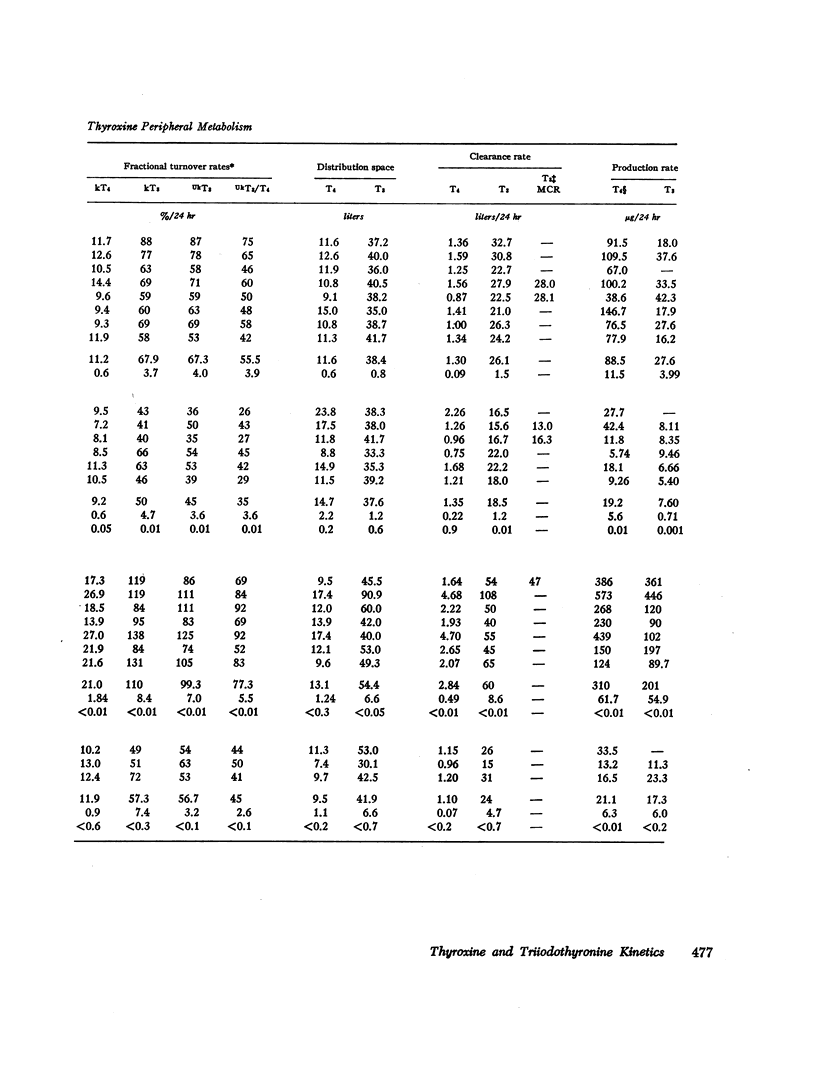
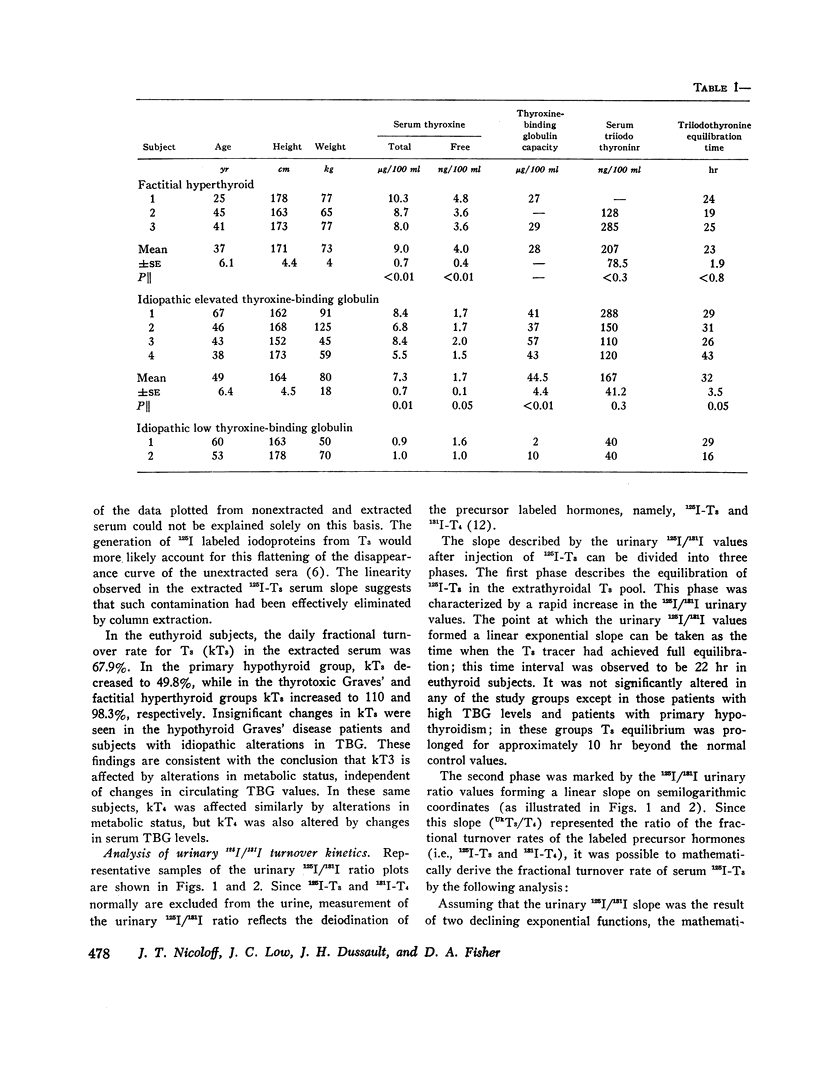



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braverman L. E., Foster A. E., Ingbar S. H. Thyroid hormone transport in the serum of patients with thyrotoxic Graves' disease before and after treatment. J Clin Invest. 1968 Jun;47(6):1349–1357. doi: 10.1172/JCI105827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. R., Steinberg M., Searle G. L. The distribution kinetics of triiodothyronine: studies of euthyroid subjects with decreased plasma thyroxine-binding globulin and patients with Graves' disease. J Clin Invest. 1970 Jun;49(6):1041–1050. doi: 10.1172/JCI106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J., Solomon D. H., Beall G. N. Radioimmunoassay for measurement of triiodothyronine in human serum. J Clin Invest. 1971 Oct;50(10):2033–2041. doi: 10.1172/JCI106696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER D. A., ODDIE T. H. WHOLE-BODY COUNTING OF 131-I-LABELED TRIIODOTHYRONINE. J Clin Endocrinol Metab. 1964 Aug;24:733–739. doi: 10.1210/jcem-24-8-733. [DOI] [PubMed] [Google Scholar]
- Farmer T. A., Jr, Smitherman T. C., Beschi R. J., Pittman J. A., Jr Effect of triiodothyronine administration on serum PBI in hypothyroid patients maintained on constant doses of thyroxine. J Clin Endocrinol Metab. 1969 Jun;29(6):781–785. doi: 10.1210/jcem-29-6-781. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Dussault J. H. Contribution of methodological artifacts to the measurement of T3 concentration in serum. J Clin Endocrinol Metab. 1971 May;32(5):675–679. doi: 10.1210/jcem-32-5-675. [DOI] [PubMed] [Google Scholar]
- Gharib H., Mayberry W. E., Ryan R. J. Radioimmunoassay for triiodothyronine: A preliminary report. J Clin Endocrinol Metab. 1970 Dec;31(6):709–712. doi: 10.1210/jcem-31-6-709. [DOI] [PubMed] [Google Scholar]
- Gregerman R. I., Solomon N. Acceleration of thyroxine and triiodothyronine turnover during bacterial pulmonary infections and fever: implications for the functional state of the thyroid during stress and in senescence. J Clin Endocrinol Metab. 1967 Jan;27(1):93–105. doi: 10.1210/jcem-27-1-93. [DOI] [PubMed] [Google Scholar]
- Hollander C. S. On the nature of the circulating thyroid hormone: clinical studies of triiodothyronine and thyroxine in serum using gas chromatographic methods. Trans Assoc Am Physicians. 1968;81:76–91. [PubMed] [Google Scholar]
- INGBAR S. H., FREINKEL N. Studies of thyroid function and the peripheral metabolism of I 131-labeled thyroxine in patients with treated Graves disease. J Clin Invest. 1958 Nov;37(11):1603–1614. doi: 10.1172/JCI103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingbar S. H. CLINICAL AND PHYSIOLOGICAL OBSERVATIONS IN A PATIENT WITH AN IDIOPATHIC DECREASE IN THE THYROXINE-BINDING GLOBULIN OF PLASMA. J Clin Invest. 1961 Nov;40(11):2053–2063. doi: 10.1172/JCI104431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutras D. A., Berman M., Sfontouris J., Rigopoulos G. A., Koukoulommati A. S., Malamos B. Endemic goiter in Greece: thyroid hormone kinetics. J Clin Endocrinol Metab. 1970 Apr;30(4):479–487. doi: 10.1210/jcem-30-4-479. [DOI] [PubMed] [Google Scholar]
- Nauman J. A., Nauman A., Werner S. C. Total and free triiodothyronine in human serum. J Clin Invest. 1967 Aug;46(8):1346–1355. doi: 10.1172/JCI105627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoloff J. T., Dowling J. T. Estimation of thyroxine distribution in man. J Clin Invest. 1968 Jan;47(1):26–37. doi: 10.1172/JCI105712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoloff J. T., Dowling J. T. Studies of peripheral thyroxine distribution in thyrotoxicosis and hypothyroidism. J Clin Invest. 1968 Sep;47(9):2000–2015. doi: 10.1172/JCI105887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERLING K., CHODOS R. B. Radiothyroxine turnover studies in myxedema, thyrotoxicosis, and hypermetabolism without endocrine disease. J Clin Invest. 1956 Jul;35(7):806–813. doi: 10.1172/JCI103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERLING K., LASHOF J. C., MAN E. B. Disappearance from serum of I131-labeled l-thyroxine and l-triiodothyronine in euthyroid subjects. J Clin Invest. 1954 Jul;33(7):1031–1035. doi: 10.1172/JCI102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussler G. C., Vance V. K. Effect of thyroid-suppressive doses of triiodothyronine on thyroxine turnover and on the free thyroxine fraction. J Clin Invest. 1968 Apr;47(4):720–728. doi: 10.1172/JCI105767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling K., Bellabarba D., Newman E. S., Brenner M. A. Determination of triiodothyronine concentration in human serum. J Clin Invest. 1969 Jun;48(6):1150–1158. doi: 10.1172/JCI106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Formation of iodoprotein during the peripheral metabolism of 3,5,3'-triiodo-L-thyronine-125I in the euthyroid man and rat. J Clin Invest. 1969 Apr;48(4):685–695. doi: 10.1172/JCI106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISWELL J. G., CORONHO V. Disappearance of I-131-triiodo-thyronine from the plasma in the presence of fever. J Clin Endocrinol Metab. 1962 Jun;22:657–659. doi: 10.1210/jcem-22-6-657. [DOI] [PubMed] [Google Scholar]
- Wahner H. W., Gorman C. A. Interpretation of serum tri-iodothyronine levels measured by the sterling technic. N Engl J Med. 1971 Feb 4;284(5):225–230. doi: 10.1056/NEJM197102042840501. [DOI] [PubMed] [Google Scholar]
- Weil J. V., Byrne-Quinn E., Sodal I. E., Friesen W. O., Underhill B., Filley G. F., Grover R. F. Hypoxic ventilatory drive in normal man. J Clin Invest. 1970 Jun;49(6):1061–1072. doi: 10.1172/JCI106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. D., Chavré V. J., Wolfe M. A simple method for estimating serum thyroxine concentration in thyroid disease and iodine-treated patients. J Clin Endocrinol Metab. 1966 Sep;26(9):986–993. doi: 10.1210/jcem-26-9-986. [DOI] [PubMed] [Google Scholar]
- Woeber K. A., Sobel R. J., Ingbar S. H., Sterling K. The peripheral metabolism of triiodothyronine in normal subjects and in patients with hyperthyroidism. J Clin Invest. 1970 Apr;49(4):643–649. doi: 10.1172/JCI106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninovich A. A., Volpe R., Ezrin C. Effects of variations of thyroxine-binding globulin capacity on the disappearance of triiodothyronine from the plasma. J Clin Endocrinol Metab. 1969 Dec;29(12):1601–1607. doi: 10.1210/jcem-29-12-1601. [DOI] [PubMed] [Google Scholar]



