Abstract
Background
Peri-implantitis has gained significant clinical attention in recent years. This disease is an inflammatory reaction to microorganisms around dental implants. Due to the limited accessibility, non-invasive antimicrobial strategies are of high interest. An unexpected approach to implant disinfection may evolve from electrolysis. Given the electrical conductivity of titanium implants, alkalinity or active oxidants can be generated in body fluids. We investigated the use of dental titanium implants as electrodes for the local generation of disinfectants. Our hypothesis was that electrolysis can reduce viable counts of adhering bacteria, and that this reduction should be greater if active oxidative species are generated.
Methodology/Principal Findings
As model systems, dental implants, covered with a mono-species biofilm of Escherichia coli C43, were placed in photographic gelatin prepared with physiological saline. Implants were treated by a continuous current of 0 - 10 mA for 15 minutes. The reduction of viable counts was investigated on cathodes and anodes. In separate experiments, the local change in pH was visualized using color indicators embedded in the gelatin. Oxidative species were qualitatively detected by potassium iodide-starch paper. The in situ generated alkaline environment around cathodic implants caused a reduction of up to 2 orders of magnitude in viable E. coli counts. On anodic implants, in contrast to cathodic counterparts, oxidative species were detected. Here, a current of merely 7.5 mA caused complete kill of the bacteria.
Conclusions/Significance
This laboratory study shows that electrochemical treatment may provide access to a new way to decontaminate dental implants in situ.
Introduction
Peri-implantitis is an inflammatory process of the tissues around an osseointegrated oral implant in function and results in loss of the supporting bone [1]. Bacterial colonization of dental implants and the infection of peri-implant tissues can lead to chronic bone destruction and may consequently lead to implant failure [2]. Current methods to treat peri-implantitis include mechanical decontamination and local antiseptic or antibiotic treatment (for reviews, see [3] and [4]). Suggested implant surface treatments are scaling, CO2-lasers, air abrasive powder, chlorhexidine or hydrogen peroxide irrigation, or local antibiotics. At present, most of the information on the effectiveness of such interventions derives from case reports, so that no evidence-based consensus has been reached as to which option is clinically most advantageous.
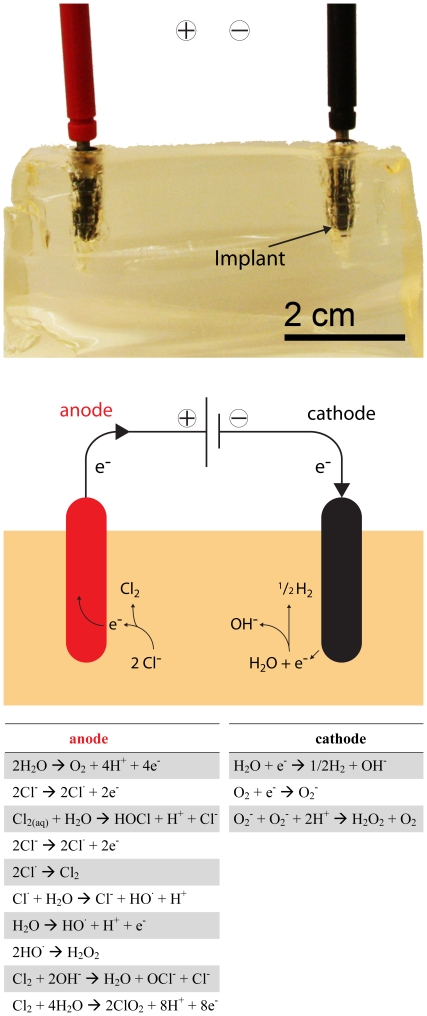
In spite of the finding that the pattern of spread of inflammation is different in periodontal and in peri-implant tissues [5], most of the proposed debridement protocols for dental implants have been derived from periodontology. There is, however, a pronounced difference between dental implants and teeth, namely the fact that the former are made of titanium, an electrically conducting metal. Accordingly, a conceivable alternative minimally invasive approach to reduce the number of viable microorganisms on dental implants could be electrochemistry. This purification process is well-known for water disinfection [6]–[9] and uses the possibility to generate active substances on-site on the electrode. Neat water can be decomposed into oxygen and hydrogen. At the cathode, water is decomposed into hydrogen and hydroxide ions, which creates an alkaline environment (high pH). At the anode, oxygen and protons are generated (low pH). In the presence of chloride ions (i.e. in a physiological environment) additional highly active oxidizing agents such as chlorine (Cl2), HOCl, OCl− or ClO2 are generated (Fig. 1). These are the key species involved in electrochemical disinfection [8], [10]. The formation of oxidative substances further depends on the quality and material of the electrode [11] and current/voltage. It has been reported for catheter disinfection that low amperage electric current can effectively inhibit bacterial growth [12]. The so-called iontophoresis makes use of microampere currents and has been applied successfully in an urinary catheter system [13], [14]. In both applications, the electrodes are in an undivided electrochemical cell, whereas in this work, two different and spatially separated environments are generated for the reduction of adhered microorganisms. In order to model a situation in a gingiva/implant site (patient), we used a physiological gelatin block with physiological saline and titanium implants produced for oral application.
Figure 1. Simulated soft tissue (gelatin) with two dental implants.
The anode (top, left) and cathode (top, right) were connected as part of an electric circuit powered by an external controller (top). The most probable reactions occurring at the electrodes are displayed in a scheme of the electrolysis setup to further illustrate the process (middle) and an overview (below) of the most likely occurring reactions is shown, too (adapted from [8], [19]).
This study therefore describes the electrochemical reduction of adhered bacteria on dental implants. Also, the hypothesis that viable count reduction would be greater at the anode was tested. We expected oxidative substances generated at the anode to have a higher disinfecting capacity than the mere alkaline environment around cathodes.
Materials and Methods
Implant disinfection
To examine the possibility of using electric current to eliminate or at least significantly reduce bacteria on implant surfaces, standard dental titanium implants (Straumann SLA, Straumann AG, Basel, Switzerland) were coated with a mono-species biofilm of Escherichia coli (strain C43). Enteric bacteria such as E. coli are amongst the species most frequently isolated from peri-implantitis sites [15]. A mono-species biofilm was created according to a published protocol [16]. In brief, implants of 4.1 mm in diameter and 12 mm in length were autoclaved prior to application. A total of 9 implants were used for this study. Specimens were immersed in 1.7 ml horse serum (BioWhittaker, Walkersville MD, USA) diluted (1/10) in physiological saline (0.9% NaCl) for 2 hrs at 37°C in Eppendorf tubes to create a protein film on their surface. E. coli was grown in Difco LB Broth (Chemie Brunschwig, Basel, Switzerland), and diluted to 7.0–7.5 log10 colony-forming units (CFU)/ml prior to immersion in saline. Implants were immersed two times for 60 hrs in 1.7 ml of the E. coli suspension at 37°C. After 60 hrs each set of implants was dipped thrice in MilliQ water to remove the loosely adherent bacteria. To imitate inflammatory soft tissues around the implant, photographic gelatin (Ballistic grade A, Gelita, Eberbach, Germany) was prepared according to the manufacturer's guideline with saline (20 wt% gelatin and 80 wt% saline). Holes were cut and filled with 20–40 µl of saline to ensure good contact of the implant with gelatin. Implants were placed in gelatin blocks with a space of 4 cm in between, and the electric circuit was connected (Fig. 1). Implants without electric circuit served as positive controls (maximal recovery of bacteria). Electrochemical treatments were done in triplicates. On each experimental day, implants were assigned to new treatment groups. A continuous current of 2 mA, 5 mA, 7.5 mA or 10 mA was applied for 15 min. Subsequently, the implants were immersed in 1.7 ml of saline, vortexed for 30 sec and ultrasonicated for 5 sec (80 W, UP400S, Hielscher, Teltow, Germany) to remove adhering microorganisms. Dilution series were plated on tryptic soy agar plates (VWR, Sentmenat, Barcelona, Spain) and incubated for 12 hrs at 37°C, revealing the reduction of viable bacteria compared to positive control treatments (no current). Purity of growth was checked by assessing colony morphology and by Gram staining.
Data pertaining to viable E. coli counts are presented as log10 colony-forming units (CFU). Mean values between test and control treatments at each time point were compared using one-way analysis of variance (ANOVA) followed by Bonferroni's correction for multiple testing. The alpha-type error was set at 0.05.
Visualization of electrolysis
The gelatin around the implant was analyzed using pH indicators and a calibrated pH electrode (Seven Easy, Mettler-Toledo, Greifensee, Switzerland). Gelatin was produced as described above, using two indicators (thymol blue and bromocresol green) to visualize the pH change in the environment around the implant. Implants were treated with 10 mA for 15 minutes. Then, bigger holes (9 mm) were cut in the ballistic gelatin blocks (no indicator) to use larger amounts of saline in order to be able to check the pH precisely using a calibrated electrode. To this end, a treatment with 10 mA and 15 min was carried out while the pH was measured every minute. During measurement of the pH the current was turned off and the implants were removed so as to not influence the pH electrode.
As suggested in the literature, oxidative species are generated at the anode. Potassium iodide-starch paper, that shows the color dark blue if exposed to oxidants like chlorine, was placed above both electrodes to check the formation of oxidative species. Eppendorf tubes were used to cover the paper and the implant and to capture evolved oxidants.
Results
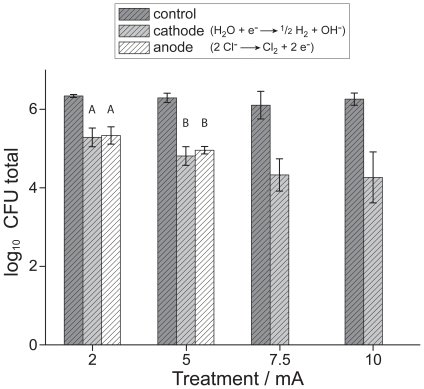
Standard dental implants served as electrodes for the electrolysis of physiological saline around the dental implants and in the simulated soft tissue (gelatin). In contrast to the positive control treatment, i.e. when no current applied, both anode and cathode electrodes induced a reduction of bacterial counts for different currents during the 15 minutes treatment (Fig. 2). At the cathode, viable bacteria decreased with increasing current. At the maximum current of 10 mA, viable bacteria were reduced by 2 orders of magnitude (99% in total counts) compared to the positive control. The difference in log10 CFU was significant (p<0.05) compared to the control treatment already with a continuous current of 2 mA. The electrochemical disinfection at the anode caused a statistically similar reduction of E. coli counts at 2 and 5 mA. A treatment with 7.5 mA or 10 mA resulted in a complete kill of all viable bacteria and complete disinfection (Fig. 2, p<0.05 compared to log10 CFU values at the cathode and implants with no current applied).
Figure 2. Reduction of E. Coli adhered on dental implants.
Viable E. coli counts were reduced on implants after current treatment for 15 minutes (mean log10 CFU (colony-forming units) values (N = 3) and standard deviation). Pronounced differences arised for anodic (oxidative environment) and cathodic (alkaline environment) implants. A full disinfection could be obtained if an implant was used as anode and at a current of at least 7.5 mA. Same capital letters above data sets indicate no statistically significance (p>0.05).
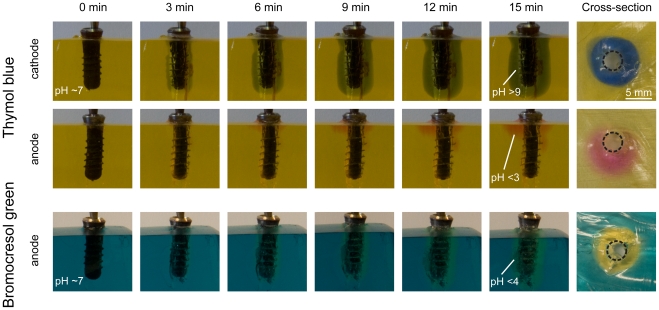
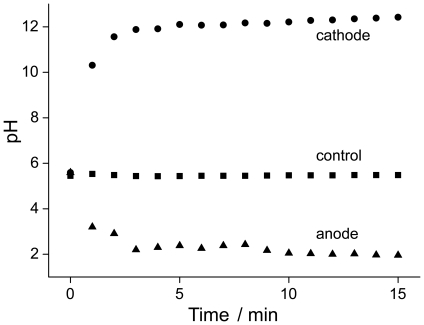
To illustrate what happens during the electrochemical disinfection in the vicinity of the implant, a number of well-established physico-chemical methods was used. Doping the gelatin with pH-sensitive dyes allowed mapping local changes in acidity (low pH) or alkalinity (high pH), that can both contribute to local implant disinfection. After electrochemical treatment, both pH sensitive dyes changed their color in the appropriate pH region. Thymol blue has two color transitions, from pink over yellow to blue (from low to high pH). Bromocresol green changes from blue to yellow at pH: 4–5. At the cathode, thymol blue indicated an alkaline environment above pH: 9 (Fig. 3). With increasing treatment time, the zone of changed pH increased and formed a circular high-pH area around the implant after 15 minutes (see Fig. 3, top right). On the counter electrode (anode) a low pH (<3) was confirmed locally by the pH-sensitive dye's change to pink. The pronounced local changes in pH were confirmed by using a second pH-sensitive dye. Bromocresol green also indicated an acidic environment at the anode (Fig. 3). A constant circle of yellow-tinted gelatin was observed after 15 minutes of treatment. Quantitative pH measurement with a pH-sensitive electrode revealed a rapid and pronounced drop (pH: ∼2) at the anode as opposed to an increase at the cathode (pH: ∼12) after starting the electrochemical treatment (Fig. 4).
Figure 3. Proof of pH changes during electrochemical implant treatment.
Photographic images of dental implants in simulated soft tissue using pH-sensitive dyes to visualize local pH changes. The dark blue color for thymol blue indicated a pH above 9 (alkaline) while the pink color confirmed a pH below 3 (strongly acidic). Confirmation with a second pH-sensitive dye, bromocresol green allowed mapping a similar acidic pH at the anode. For both dyes, a homogenous, circular simulated soft tissue section of high/low pH evolved around the implant insertion hole.
Figure 4. Quantitative pH evolution in the saline around the implant.
The evolution of pH in a larger saline environment around the implant measured each minute by a pH microelectrode confirmed the opposing pH drifts at the two implants serving as electrodes.
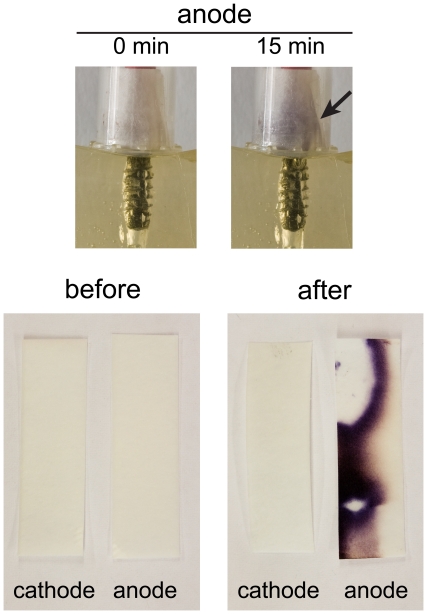
The presence of oxidizing species was locally monitored by the use of potassium iodide-starch paper [17]. This well established and sensitive method relies on the oxidation of iodide to molecular iodine (oxidizer +2I− → I2+ red. oxidizer) and subsequent formation of I3 − ions (I−+I2 → I3 −), that form a highly-colored complex with starch (dark blue areas in Fig. 5). If KI-starch paper was placed above both electrodes, the dark blue color appeared only at the anode (i.e. the site of electrochemical oxidation). The staining was only visible on the inner side of the paper, which was exposed to the oxidative species deriving from the electrolysis (Fig. 5).
Figure 5. Production of oxidizing species in the vicinity of the implants.
Potassium iodide-starch paper before and after the treatment of implants in simulated soft tissue showed a dark blue coloration for the anode after electrolysis. This is in agreement with electrochemical oxidation taking place exclusively at the anode.
Discussion
Similar to teeth, oral implants are at risk of becoming colonized by biofilms that cause inflammation of their supporting tissues. Failing implants are associated with microbial colonization [18]. In the current study, a common approach for water purification was adapted to kill bacteria adhering to dental implants. In agreement with the hypothesis of this investigation, in situ generated active oxidants at the anode (Fig. 1) indeed caused higher bacterial reduction than the mere alkaline environment emerging at the cathode.
Implants that served as cathode showed a maximum reduction of 2 orders of magnitude for the electrochemical treatment (Fig. 2). This reduction can be attributed to the alkaline environment generated at this electrode (Fig. 1), which is only partially tolerated by microorganisms. In addition to mere alkalinity, the production of reactive oxygen species could have equally contributed to bacterial reduction [19]. At the anode, E. coli counts were statistically similar to the cathode for 2 mA and 5 mA, but treatment with 7.5 mA and 10 mA resulted in complete implant disinfection (Fig. 2). Similar results have been reported for a process called electro-sterilization that was used for root canal disinfection in the early 20th century [20]. For this treatment the anode was placed in the root canal because the antibacterial effect at the positive electrode (anode) was always distinctly greater than that at the cathode. With reasonable confidence, the here-observed disinfection at the anode can be attributed to the evolution of oxidative species (Fig. 5), such as aquatic chlorine formed by the electrolysis of saline. In addition to the chlorine species, hydroxide radicals or reactive oxygen species could have influenced the inactivation of E. coli at the implants serving as anode (Fig. 1). It has been shown that electrical current has a lethal activity on E. coli in medium. Besides the electrical current, chlorine can also be essential for the development of a killing effect in a treated medium [21]. Furthermore, not only inactivation of bacteria but also their detachment from the implant during the treatment could contribute to an antimicrobial effect at both electrodes [22]. The amount of electrolysis products is proportional to the amount of electrical charge, which could lead to an enhanced killing efficiency for increasing current, as was observed for the cathode and anode.
Compared to a clinical situation, where a mixture of microbial species is present at the implant site, only a single strain of E. coli was used in this study. Facultative enteric bacteria such as enterococci and escherichiae are commonly less susceptible to disinfectants than anaerobic taxa [16], [23], which are seen as the main causative agents of peri-implant disease [15]. However, single-species biofilms of facultative enteric bacteria can be as resistant to disinfectants as mixed-species biofilms [16], [23], and are thus ideal for this type of laboratory investigation. Nevertheless, future studies should also test the electrochemical disinfection of titanium implants contaminated by mixed biofilms, which represent the clinical situation more closely.
Water as well as urinary catheters have been treated with electrical current to reduce bacterial colonization [9], [14]. Both treatments used a continuous current set-up where either higher (water) or lower (catheter) currents were applied. However, for both methods undivided electrodes have been used. In this study, the electrolytic solution around the implants was separated from each other so that two different environments could evolve (Fig. 3). Davis et al. [13] reported that a saline solution became more basic during a similar treatment – an observation which is only comparable to one electrode in this study. The possible reason for the two different environments might be the spatial separation in the current investigation. This set-up mimicks the clinical situation, in which the implant (electrode) would also be spatially separated from the counterelectrode. The pH-sensitive dye in the gelatin clearly showed that an alkaline and acidic environment could evolve under these conditions (Fig. 3). At both electrodes the front of changed pH constantly grew with time.
In water treatment, hypochlorite or chlorine are routinely measured with the so-called DPD method [24]. That test, however, is very sensitive to the acidic environment that evolved around the anode implant and hence could not be applied. Normal hypochlorite or aquatic chlorine is stable at basic conditions [25], which were not present in the environment around the anode under the current conditions (Fig. 3 and 4). It has been reported by Czarnetzki et al. [26], who analyzed the evolution of hypochlorite, chlorate and oxygen from the electrolysis of a NaCl solution, that it was necessary to compensate the pH of the anolyte with an alkaline solution. This, however, was obviously not indicated in this study because we ultimately intend to directly use electrochemical implant disinfection in a living tissue environment. The in situ generated chlorine species have most probably reacted with constituents of the gelatin, or other species [13]. Furthermore, gaseous chlorine could have escaped from the electrolyte. Potassium iodide-starch paper was used to assess oxidant evolution (Fig. 5). The dark blue coloration proves that iodide had been oxidized, which unambiguously confirms oxidant formation. It must be noted that this method can not distinguish between hypochlorite or chlorine evolution. However, since only chlorine is gaseous and the oxidant must have left the liquid to reach the KI-starch paper, it is likely to assume the presence of chlorine (Cl2) in this system.
The here applied current was between 2 mA and 10 mA, a range which is tolerable for human beings assuming a covered distance of the electric current in the human body (from the hand to the feet), according to the standard [27]. There is no physiological impact expected for this range of current. In fact, similar currents were routinely applied during electromedication in root canal treatments, using a hand-held cathode and a root canal instrument as the anode [28]. However, aiming for a new possibility to treat peri-implantitis, further experiments have to be carried out due to the difference in distance and tissue between the above mentioned standard and the conditions in this study. The current was set and the resistance was given by the implant, gelatin and saline. The voltage tuned itself in between 4 and 20 V with increasing treatment time, so that the resistance varied between 2 and 6 kΩ. This electrical resistance is similar to the one reported between a root canal terminus and a connected lip clip [29]. In contrast to the reported iontophoresis for urinay catheters with a much lower current (microampere range) [14], the here presented disinfection treatment is much quicker and thus potentially feasible during a dental visit.
Future studies should aim to assess the current approach in an animal model for peri-implantitis.
Acknowledgments
We are grateful to Dr. Alexander Schaetz and Andreas Dutly for valuable discussions. The generous provision of expired implants by Straumann AG, Basel is kindly acknowledged.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was financially supported by the Department of Preventive Dentistry and Oral Epidemiology, University of Zurich, Center for Dental Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lang NP, Karring T, editors. Albrektsson T, Isidor F. Consensus report of session IV. Proceedings of the 1st European workshop on periodontology; 1993 Feb 01-04; Thurgau, Switzerland. Quintessence Publ Co Inc. pp. 365–369.
- 2.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63–76. doi: 10.1111/j.1600-0757.1998.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 3.Mombelli A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 2002;28:177–189. doi: 10.1034/j.1600-0757.2002.280107.x. [DOI] [PubMed] [Google Scholar]
- 4.Esposito M, Grusovin MG, Kakisis I, Coulthard P, Worthington HV. Interventions for replacing missing teeth: treatment of perimplantitis. Cochrane Database Syst Rev. 2008;2:CD004970. doi: 10.1002/14651858.CD004970.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implant Res. 1992;3:9–16. doi: 10.1034/j.1600-0501.1992.030102.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajeshwar K, Ibanez JG, Swain GM. Electrochemistry and the environment. J Appl Electrochem. 1994;24:1077–1091. [Google Scholar]
- 7.Drees KP, Abbaszadegan M, Maier RM. Comparative electrochemical inactivation of bacteria and bacteriophage. Water Res. 2003;37:2291–2300. doi: 10.1016/S0043-1354(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Huitle CA, Brillas E. Electrochemical alternatives for drinking water disinfection. Angew Chem-Int Edit. 2008;47:1998–2005. doi: 10.1002/anie.200703621. [DOI] [PubMed] [Google Scholar]
- 9.Patermarakis G, Fountoukidis E. Disinfection of water by electrochemical treatment. Water Res. 1990;24:1491–1496. [Google Scholar]
- 10.Bergmann H, Koparal S. The formation of chlorine dioxide in the electrochemical treatment of drinking water for disinfection. Electrochim Acta. 2005;50:5218–5228. [Google Scholar]
- 11.Jeong J, Kim C, Yoon J. The effect of electrode material on the generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Res. 2009;43:895–901. doi: 10.1016/j.watres.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Liu WK, Brown MRW, Elliott TSJ. Mechanisms of the bactericidal activity of low amperage electric current (DC). J Antimicrob Chemother. 1997;39:687–695. doi: 10.1093/jac/39.6.687. [DOI] [PubMed] [Google Scholar]
- 13.Davis CP, Shirtliff ME, Trieff NM, Hoskins SL, Warren MM. Quantification, qualification, and microbial killing efficiencies of antimicrobial chlorine-based substances produced by iontophoresis. Antimicrob Agents Chemother. 1994;38:2768–2774. doi: 10.1128/aac.38.12.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis CP, Arnett D, Warren MM. Iontophoretic killing of Escherichia coli in static fluid and in a model catheter system. J Clin Microbiol. 1982;15:891–894. doi: 10.1128/jcm.15.5.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonhardt A, Renvert S, Dahlen G. Microbial findings at failing implants. Clin Oral Implant Res. 1999;10:339–345. doi: 10.1034/j.1600-0501.1999.100501.x. [DOI] [PubMed] [Google Scholar]
- 16.Brandle N, Zehnder M, Weiger R, Waltimo T. Impact of growth conditions on susceptibility of five microbial species to alkaline stress. J Endod. 2008;34:579–582. doi: 10.1016/j.joen.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Schöne E. Experimentaluntersuchungen über das Wasserstoffhyperoxyd. Liebigs Ann. 1879;195:228–252. [Google Scholar]
- 18.Mombelli A, Lang NP. Microbial aspects of implant dentistry. Periodontol 2000. 1994;4:74–80. doi: 10.1111/j.1600-0757.1994.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 19.Urano H, Ishikawa H, Fukuzaki S. Involvement of radical species in inactivation of Vibrio parahaemolyticus in saline solutions by direct-current electric treatment. J Biosci Bioeng. 2006;102:457–463. doi: 10.1263/jbb.102.457. [DOI] [PubMed] [Google Scholar]
- 20.Grossman LI, Appleton JLT. Experimental and applied studies in electro-sterilization (Part I). Dent Cosmos. 1931;73:147–160. [Google Scholar]
- 21.Pareilleux A, Sicard N. Lethal effects of electric current on Escherichia coli. Appl Microbiol. 1970;19:421–424. doi: 10.1128/am.19.3.421-424.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong SH, Jeong J, Shim S, Kang H, Kwon S, et al. Effect of electric currents on bacterial detachment and inactivation. Biotechnol Bioeng. 2008;100:379–386. doi: 10.1002/bit.21760. [DOI] [PubMed] [Google Scholar]
- 23.Siren EK, Haapasalo MPP, Ranta K, Salmi P, Kerosuo ENJ. Microbiological findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. Int Endod J. 1997;30:91–95. [PubMed] [Google Scholar]
- 24.Public Health Association, American Water Works Association, Water Environment Federation. DPD colorimetric method. In: Clescerl L, Greenberg A, Eaton A, editors. Standard methods for the examination of water and wastewater. 20th ed. Washington D.C.: American Public Health Association; 1999. pp. chapter part 4–63. [Google Scholar]
- 25.Dychdala G. Chlorine and chlorine compounds. In: Block S, editor. Disinfection, sterilization and preservation. Philadelphia: Lea & Febiger; 1991. pp. 131–51. [Google Scholar]
- 26.Czarnetzki LR, Janssen LJJ. Formation of hypochlorite, chlorate and oxygen during NaCl electrolysis from alkaline solutions at an RuO2/TiO2 anode. J Appl Electrochem. 1992;22:315–324. [Google Scholar]
- 27.German Institute for Standardization. Frankfurt: DIN German Institute for Standardization; 2005. Effects of current on human beings and livestock - Part 1: General aspects.59 [Google Scholar]
- 28.Sommer RF, Ostrander FD, Crowley MC. Electromedication. In: Sommer RF, Ostrander FD, Crowley MC, editors. Clinical endodontics: a manual of scientific endodontics. 2nd ed. Philadelphia and London: Saunders Company; 1961. pp. 199–203. [Google Scholar]
- 29.Sunada I. New method for measuring the length of the root canal. J Dent Res. 1962;41:375–387. [Google Scholar]