Abstract
Our previous work indicated that pretreatment with the selective kappa opioid receptor (KOPr) agonist, U69593, attenuated the ability of priming injections of cocaine to reinstate extinguished cocaine-seeking behavior. The present study expanded these initial tests to include other traditional KOPr agonists, U50488H, spiradoline (SPR), and salvinorin A (Sal A), an active constituent of the plant Salvia divinorum. Following acquisition and stabilization of cocaine self-administration, cocaine-produced drug-seeking was measured. This test was conducted in a single day and comprised an initial phase of self-administration, followed by a phase of extinguished responding. The final phase examined reinstatement of extinguished cocaine self-administration followed by a priming injection of cocaine (20.0 mg/kg, intraperitoneal (I.P.)) in combination with the various KOPr agonists. Cocaine-induced drug-seeking was attenuated by pretreatment with U69593 (0.3 mg/kg, subcutaneous (S.C.)), U50488H (30.0 mg/kg, I.P.), SPR (1.0, 3.0 mg/kg, I.P.) and Sal A (0.3, 1.0 mg/kg, I.P.). Sal A (0.3, 1.0 mg/kg, I.P.) had no effect on operant responding to obtain sucrose reinforcement or on cocaine induced hyperactivity. These findings show that Sal A, like other traditional KOPr agonists attenuates cocaine-induced drug seeking behavior.
Keywords: cocaine self-administration, kappa opioid agonist, U69593, U50488H, spiradoline, salvinorin A, drug-seeking
1. Introduction
Prior studies have demonstrated that KOPr agonists attenuate some of the neuro-chemical and behavioral effects of drugs of abuse (Heidbreder et al., 1993,Heidbreder et al., 1995; Shippenberg et al., 1996; Thompson et al., 2000). These effects might be due to the interactions between KOPr and dopaminergic systems since pretreatment with kappa-opioid agonists decreased dopamine (DA) concentrations in terminal regions (Devine et al., 1993; Shippenberg et al., 1994; Maisonneuve et al., 1994; Heidbreder et al., 1994, 1996) and attenuated cocaine- (Shippenberg et al., 1994), amphetamine- (Gray et al., 1999) and heroin-(Xi et al., 1998) induced DA release in the ventral striatum. Also, many behavioral effects of psycho-stimulants like cocaine (Heidbreder et al., 1993,Heidbreder et al., 1995), amphetamine (Gray et al., 1999) and nicotine (Hahn et al., 2000) were decreased by prior administration of KOPr agonists. Additionally, pre-treatment with KOPr activating compounds attenuated cocaine (Glick et al., 1995; Mello and Negus, 1996; 1998; Negus et al., 1997; Schenk et al., 1999b; 2001), morphine (Glick et al., 1995) and heroin (Xi et al., 1998) self-administration in laboratory animals. These findings suggest the possible role of KOPr agonists as pharmacotherapeutics in the treatment of addiction (Mello and Negus, 1998; Prisinzano et al., 2005; Shippenberg et al., 2007; Willmore-Fordham et al., 2007; Prisinzano and Rothman, 2008; Tomasiewicz et al., 2008).
During a binge of cocaine self-administration in abusers there is tolerance to many of the subjective effects (Fischman et al., 1985). Self-administration and drug craving, however, continue relatively unabated. Since exposure to cocaine produces craving for more cocaine (Jaffe et al., 1989), it is possible that continued exposure to cocaine during a binge maintains a cycle of drug-seeking and drug-taking, even after tolerance develops. If so, identification of factors that reduce the ability of cocaine to produce drug-seeking would be an important step in the development of effective treatments for cocaine abuse.
Animal models of drug-seeking have contributed greatly to investigations of these factors. A number of laboratories have used procedures developed by de Wit and Stewart (1981) to demonstrate the ability of cocaine to reinstate extinguished cocaine self-administration behavior. This effect appears to be mediated, at least in part, by dopaminergic mechanisms since cocaine-produced drug-seeking was attenuated by pre-treatment with dopaminergic agonists and antagonists (Self et al., 1996; Khroyan et al., 2000; 2003; Alleweireldt et al., 2002).
Pretreatment with the kappa-opioid receptor agonist, U69593 attenuated cocaine-produced reinstatement of extinguished cocaine-taking behavior (Schenk et al., 1999b; 2000). This effect was somewhat specific since reinstatement produced by experimenter-administered injections of the dopamine uptake inhibitors, GBR 12909 or WIN 35428, was not attenuated by pretreatment with U69593 (Schenk et al., 2000). The attenuation of drug-seeking involved effects at central KOPr’s, as prior intracerebroventricular administration of nor-BNI, a kappa-opioid antagonist, reversed the effects of U69593 (Schenk et al., 1999b).
Sal A, a neoclerodane diterpene, is an active constituent of the hallucinogenic sage, Salvia divinorum, and has been used in traditional, spiritual and ethnopharmacological practices by the Mazatec Indians of Oaxaca, Mexico (Valdes et al., 1983, Valdes 1994; Siebert 1994). Sal A binds selectively to the KOPr (Roth et al., 2002; Yan and Roth, 2004; Prisinzano 2005) with greater efficacy than U69593 and U50488H (Chavkin et al., 2004). It has a rapid onset of action and a short elimination half life (56.6 ± 24.8 min) (Schmidt et al., 2005; Butelman et al., 2009). Accordingly, there has been recent interest in understanding the neuropharmacology of Sal A and comparing it with traditional KOPr agonists.
Sal A dose dependently produced antinociception in tail flick, hot plate and acetic acid induced writhing in mice that was antagonized by nor-BNI (McCurdy et al., 2006; John et al., 2006). Sal A also produced discriminative stimulus effect in rats (Willmore-Fordham et al., 2007; Baker et al., 2009) and rhesus monkey’s (Butelman et al., 2004), decreased mobility in the forced swim test (Carlezon et al., 2006) and produced motor inco-ordination in mice (Fantegrossi et al., 2005; Zhang et al., 2005). The aim of our current study was to determine whether Sal A has similar effects to other KOPr agonists, U69593, U50488H and SPR on cocaine produced drug-seeking in rats.
2. METHODS
2.1. Subjects
Male Sprague-Dawley rats weighing 325–350 g were used. They were housed individually in hanging polycarbonate cages. The humidity (55%) and temperature (19–21°C) were controlled and food and water were freely available except during testing. Animals tested for sucrose self-administration had free access to water all the times within the home cage and were maintained at approximately 85% of their initial feeding weight during the experiments by restricting access to food. The animal colony was maintained in the animal facility at School of Psychology, Victoria University of Wellington. All the experiments were conducted in accordance with the guidelines of the Animal Ethics Committee of Victoria University of Wellington. Lights were maintained on a 12:12 hr cycle with lights on at 0700.
2.2. Surgery
Under deep anesthesia produced by ketamine/xylacine (90/9 mg/kg, I.P.), a silastic catheter was placed in the right jugular vein. The external jugular vein was isolated, the catheter was inserted and the distal end (22 gauge stainless steel tubing) was passed subcutaneously to an exposed portion of the skull where it was fixed to embedded jewele’s screws with dental acrylic.
Each day, the catheters were infused with 0.1 ml of a sterile saline solution containing heparin (30.0 U/ml), penicillin G Potassium (250,000 U/ml) and streptokinase (8000 U/ml) to prevent infection and the formation of clots and fibroids. The rats were allowed five days post surgery for recovery.
2.3. Apparatus
2.3.1. Cocaine Self administration
Self-administration testing was carried out in a humidity (55%) and temperature (19–21°C) controlled environment in standard operant chambers (Med Associates, ENV-001) equipped with 2 levers. Depression of one lever (the active lever) resulted in a 0.l ml intravenous infusion of cocaine HCl dissolved in sterile physiological saline containing heparin (3.0 U/ml). Infusions were of 12 s duration. Coincident with drug delivery was the illumination of a stimulus light located directly above the active lever. This stimulus light remained illuminated throughout each 12 s infusion. Depression of the other lever (the inactive lever) was without programmed consequence.
Rats were maintained in the animal colony except during testing. Immediately prior to each daily test, the catheter lines were infused with 0.1 ml of the heparin-penicillin-streptokinase solution. The stainless steel catheter was connected to a length of microbore tubing and connected to the syringe. At the end of each test, the lines were again infused with 0.1 ml of the heparin-penicillin-streptokinase solution, the stainless steel tubing was plugged and the animal was returned to its home cage. Drug delivery and data acquisition were controlled by Med Associates software. Cocaine deliveries were made via mechanical pumps (Razel, Model A with 1.0 rpm motor equipped with 20 ml syringe).
2.3.2. Sucrose self-administration
Training and testing procedures were conducted in eight standard operant chambers (Med Associates, ENV001) in an unlit, sound attenuating room. Operant chambers were quipped with two retractable levers and a sucrose bottle delivering 0.1 ml of 10% sucrose solution onto a tray on the chamber wall according to the imposed schedule of reinforcement. Sucrose delivery and data acquisition were controlled by Med Associates software. Experiments were conducted between 0900 and 1600 hours.
2.3.3. Locomotor activity tests
Eight Open field chambers (Med Associates) equipped with two banks of sixteen photocells on each wall were used to measure horizontal locomotion. The open field boxes were interfaced with a microcomputer located in an adjacent laboratory. Testing was conducted in the dark between 1000 and 1600 hours. White noise was continually present to mask extraneous disturbances.
2.4. Procedure
2.4.1 Cocaine Self administration training
Acquisition of cocaine self-administration was monitored during daily 2 hr sessions. Each session began with an experimenter-delivered infusion of cocaine (0.5 mg/kg/infusion). Thereafter, depression of the active lever produced automated cocaine infusions according to an FR-1 schedule of reinforcement. The criterion for acquisition of cocaine self-administration consisted of at least 20 reinforced responses (10 mg/kg) during the 2 hr session and a ratio of active: inactive lever responses of at least 2:1. Self-administration was considered acquired when these criteria were met for three consecutive days. Following acquisition, the response requirements were increased to FR-5. Daily two hr sessions were conducted until there was less than 20% variation in responding on three consecutive days. During training, the cocaine infusion was always paired with the illumination of a house light located directly above the active lever.
2.4.2. Cocaine reinstatement test
Once responding on the FR-5 schedule was stable, the effect of prior administration of the kappa-opioid agonists, U69593, U50488H, SPR and Sal A on drug-seeking produced by cocaine was measured. As with our previous studies (Schenk et al., 1999b; 2000), this test was conducted in a single day and consisted of three phases. The first phase was comprised of a one hr period of cocaine self-administration (0.5 mg/kg/infusion, FR-5 schedule of reinforcement) in which the light stimulus was paired with cocaine infusions. After the one hr self-administration period, the cocaine solution was replaced with heperanized saline and responding was reinforced with this vehicle infusion (FR-5 schedule of reinforcement) and illumination of the light stimulus. Responding during this phase was measured for 3 hrs. At the start of the third phase during which responding was again reinforced according to an FR-5 schedule of reinforcement with an infusion of vehicle solution and illumination of the light stimulus, separate groups of rats (n=5–7 per group) received an injection of U69593 (0.03, 0.1 or 0.3 mg/kg, S.C.), U50488H (3.0, 10.0 or 30.0 mg/kg, I.P.), SPR (0.3, 1.0 or 3.0 mg/kg, I.P.), Sal A (0.1, 0.3 and 1 mg/kg, I.P.) or vehicle. These injections were administered either 5 (Sal A), 15 (U69593) or 30 (U50488H, SPR) min prior to an injection of cocaine HCl (20 mg/kg, I.P.). Responding was measured for 60 min following the cocaine injection.
2.4.3. Sucrose reinforcement training and test
Animals were trained to self-administer sucrose using an auto-shaping procedure. Training sessions were 45 min duration on each of 10 training days. Once stable responding was produced, the animals were maintained on an FR1 schedule of reinforcement during which depression of the active lever (left lever) delivered 0.1 ml of a 10% sucrose solution. Following acquisition, the response requirements were increased to FR-5. Daily one hr sessions were conducted until there was less than 20% variation in responding for three consecutive days. Once responding on the FR-5 schedule was stable, the effect of Sal A on sucrose-reinforced responding was measured. Rats received an injection of either Sal A (0.3 or 1.0 mg/kg, I.P.) or vehicle 5 min prior to sucrose self-administration testing and the number of responses was measured for 60 min.
2.4.4. Procedure for cocaine-produced locomotor activity
On the test day, separate groups of rats (n=6 per group) received an injection of Sal A (0.0 or 0.3 mg/kg, I.P.) 5 minutes prior to an injection of cocaine (20 mg/kg, I.P.). Immediately following the second injection, the rats were placed in the activity chambers and total activity, a compilation of horizontal and vertical activity, was measured for a period of 60 min.
2.5. Data Analysis
The number of responses produced during the one-hour period following the cocaine injection (20 mg/kg, I.P.) at the start of phase 3 was measured. Sucrose-reinforced responding was measured for a one-hour period following Sal A or vehicle exposure. Statistical analysis for cocaine-induced reinstatement and sucrose reinforcement consisted of one-way ANOVAs followed by Tukey post-hoc comparisons where appropriate. Student t-test was applied for locomotion test.
2.6. Drugs
Cocaine HCl (Merck Pharmaceuticals, Palmerston North, New Zealand), SPR and U50488H (Sigma-Aldrich, St. Louis, MO) were dissolved in physiological saline. U69593 (National Institute of Drug Abuse) was dissolved in an aqueous solution of 25% propylene glycol. Sal A (isolated by Dr. Thomas E. Prisinzano, University of Kansas, Lawrence, KS) was suspended in 75% DMSO. Sucrose was dissolved in tap water. Intravenous infusions were in a volume of 100 μl and S.C. or I.P. injections were in a volume of 1 ml/kg. All drug weights refer to the salt.
3. Results
Figure 1 shows the responding during phases 1 and 2 for a representative group of rats from the present study. During the 1 hr period of cocaine self-administration, responding was high. When saline was substituted for cocaine during phase 2, responding decreased to less than 20 responses during hr 3.
Fig. 1.
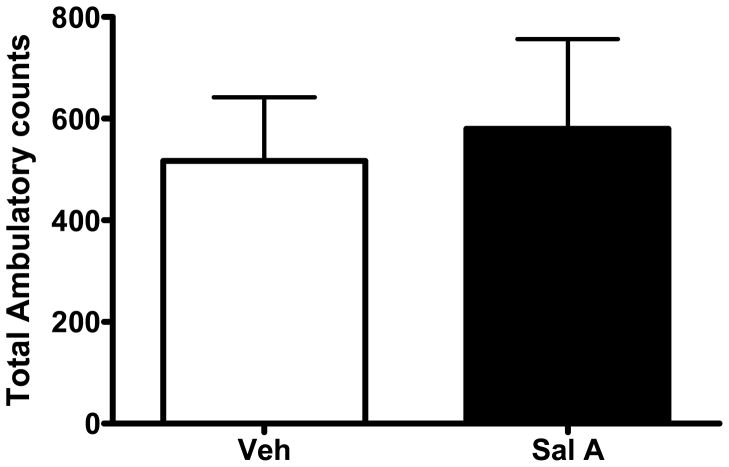
Effect of salvinorin A (Sal A) on spontaneous locomotion. Total activity (+SEM) recorded for 60 min following vehicle (Veh) and Sal A (0.3 mg/kg) pre-treatment. Student t-test. N= 7 for each group.
Figure 2 shows the number of saline-reinforced responses produced during the 1 hr period following the injection of cocaine at the start of Phase 3 for rats that received systemic administration of the KOPr agonists, U69593, U50488H, SPR and SalA. Each of the KOPr agonists produced a dose-dependent reduction of cocaine-produced reinstatement (U50488H: F(3,17)=4.277, p<0.05; U69593: F(3,20)=3.103, p<0.05); SPR: (F(3,21)=7.899, p<0.01; Sal A: (F(3,23)= 79.33, p<0.0001). Doses of 30.0 mg/kg U50488H, 0.3 mg/kg U69593, 1.0 or 3.0 mg/kg SPR and 0.3 or 1.0 mg/kg Sal A significantly decreased drug-seeking (p<0.05).
Fig. 2.
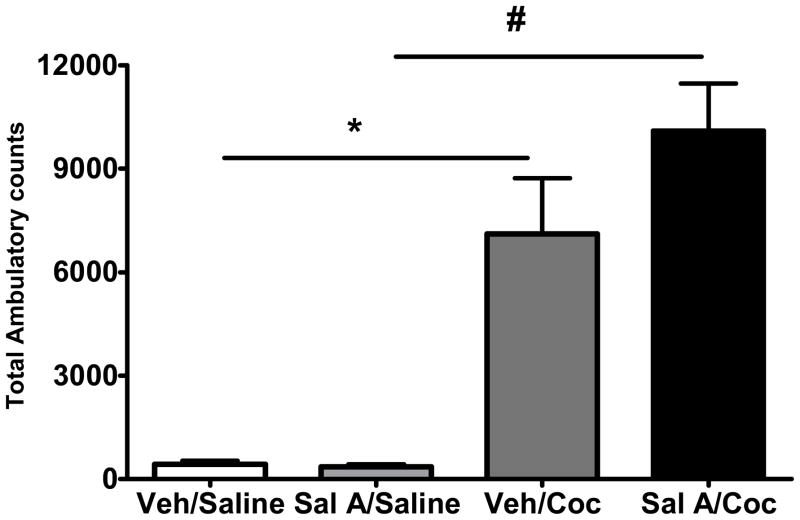
Effect of salvinorin A (Sal A) on cocaine induced hyperactivity. Drug naive rats were initially injected with vehicle (Veh, 75% DMSO) or Sal A (0.3 mg/kg) followed by saline (1 ml/kg) or cocaine (Coc, 20 mg/kg) and total activity was monitored for 60 min. Data expressed as mean total activity (+SEM). *p<0.05, data compared with Veh/Saline treated group, #p<0.05, data compared with Sal A/Saline treated group. One way ANOVA followed by Tukey test. n= 6–7 for each group.
Figure 3 shows the number of sucrose-reinforced responses produced during the 1 hr period following the injection of Sal A (0.3 and 1.0 mg/kg, I.P.). Sal A did not significantly decrease sucrose self-administration (F(2,27)=0.06, p=0.942).
Fig. 3.
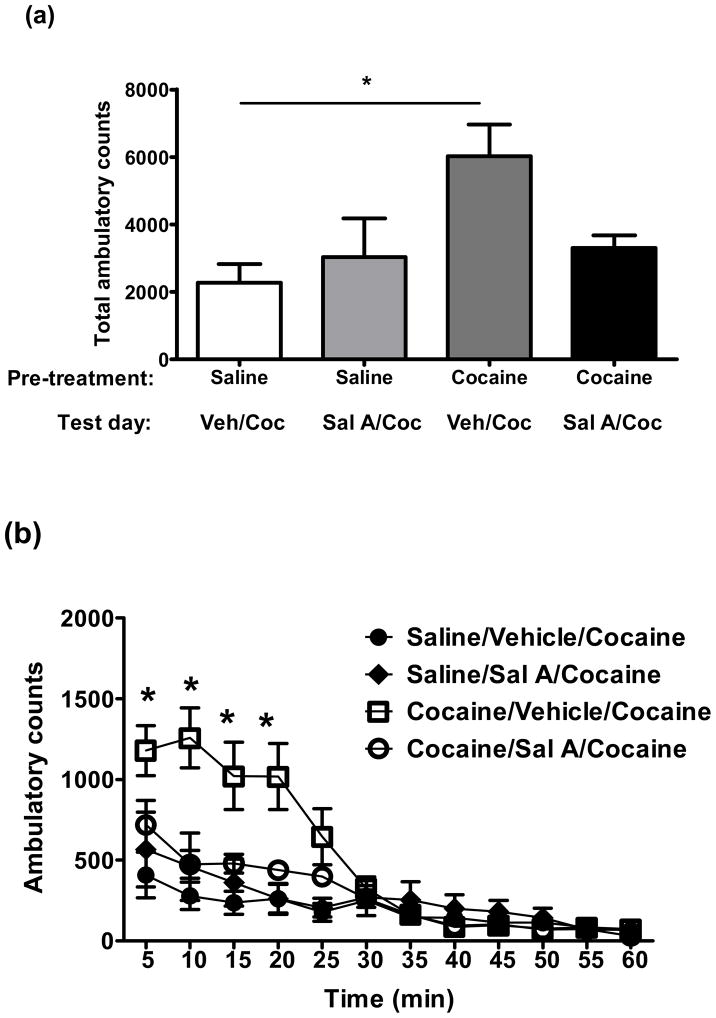
Effect of salvinorin A (Sal A) on expression of cocaine sensitization. Rats were injected with saline (1 ml/kg) or cocaine (20 mg/kg) for 5 consecutive days. Animals remained drug free from day 6–9. On day 10, rats were injected with either vehicle (Veh, 75% DMSO) or Sal A (0.3 mg/kg) and 5 min later were injected with cocaine (Coc, 20 mg/kg) and activity was measured for 60 min. (a) Data expressed as mean total activity (+SEM). *p<0.05, data compared with Saline/Vehicle/Cocaine treated group. One way ANOVA followed by Tukey test. (b) Time course measurement of mean (±SEM) of locomotion activity over a period of 5 min interval. *p<0.05, vs. Cocaine/Sal A/Cocaine treated group. Two way ANOVA followed by Bonferroni post hoc test. n= 6-8 for each group.
Figure 4 shows the effect of Sal A (0.0 or 0.3 mg/kg, I.P.) on cocaine-produced locomotor activity. Sal A failed to decrease cocaine-produced hyperactivity. (p=0.85).
Fig. 4.
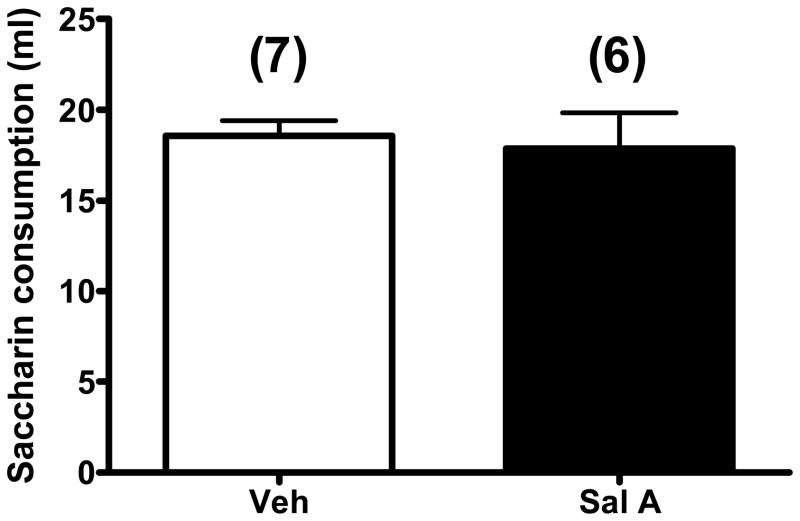
Effect of salvinorin A (Sal A) on conditioned taste aversion. Saccharin consumption in ml (+SEM) by rats treated with either vehicle (Veh) or Sal A (0.3 mg/kg) on test day. Student t-test. Numbers in parenthesis indicate sample size.
4. Discussion
As has previously been shown, cocaine reinstated extinguished cocaine-taking behavior (Worley et al., 1994; Schenk and Partridge 1999a; Schenk et al., 1999b). This effect was attenuated by pretreatment with KOPr agonists. High doses of SPR might have produced a non-selective effect on motor behavior as suggested by the decreased responding observed in a drug discrimination study (Holtzman et al., 1991). The attenuation of drug-seeking was also produced by a dose of SPR (1.0 mg/kg) that did not decrease responding in a drug discrimination task (Holtzman 2000). It is therefore unlikely that the decrease in cocaine-seeking represents a generalized inability to perform the lever press operant at the dose used in this study. Similarly, decreased drug-seeking was produced by doses of U69593 and U50488H that failed to produce a generalized decrease in motor activity (Schenk et al., 1999b; unpublished findings).
In a previous study, U50488H administered to mice 60 min prior to cocaine potentiated cocaine-induced conditioned place preference (CPP) but when administered 15 min prior to cocaine it suppressed the cocaine-CPP (McLaughlin et al. 2006). In this study we measured cocaine-induced drug-seeking during a 60 min period following U50488H administration and also observed a small but non-significant increase in responding.
In drug discrimination tests, Sal A and its synthetic derivatives substituted completely for U69593 (Baker et al., 2009), suggesting an effect mediated by the KOPr. Because of the interaction between KOPr and dopaminergic mechanisms, a focus on the effects of Sal A on dopamine-mediated behaviors has been of interest. Cocaine-seeking has been attributed to dopaminergic mechanisms and there are data to support the idea that Sal A modulates the effects of cocaine through interactions with D1 receptor mediated signaling in the dorsal striatum (Chartoff et al., 2008; Gehrke et al., 2008). Low doses of Sal A (40 μg/kg) increased DA levels in nucleus accumbens (NAc) (Braida et al., 2008) whereas higher doses decreased NAc DA levels (Zhang et. al., 2005). Thus, the ability of moderate to high doses of Sal A to decrease cocaine-produced drug-seeking might be due to its effects on the dopaminergic system.
Alternatively, the decrease in drug-seeking might reflect a non specific effect. This possibility was tested by examining the effects of Sal A on responding maintained by a sucrose reinforcer. Conditions for this test were comparable to conditions of the reinstatement tests. In this experiment, responding maintained by sucrose was high and comparable to responding maintained by cocaine. Doses of Sal A (0.3, 1.0 mg/kg I.P.) that decreased cocaine-seeking failed to alter sucrose reinforced responding.
Additionally, KOPr activation has shown to produce motor inco-ordination (Fantegrossi et al., 2005). A more recent report, however, failed to find any effect of Sal A on locomotor activity (Baker et al., 2009). The effect of Sal A on cocaine-produced hyperactivity was measured in the present study under conditions that were comparable to the conditions of the reinstatement tests. Sal A did not attenuate cocaine-produced hyperactivity. Therefore, the decrease in drug-seeking produced by Sal A cannot be attributed to either a generalized inability to perform the lever press operant or a disruption of motor activity.
Reinstatement of conditioned place preference (CPP) following extinction has also been used as a model of drug-seeking (Kreibich and Blendy, 2004) and a modulation of drug-seeking behaviors by traditional KOPr agonists has been demonstrated using this model. The effects appear, however, to be opposite to those produced when drug-seeking in a self-administration paradigm are measured. For example, foot-shock or forced swim stress were effective in reinstating both extinguished place preference (McLaughlin et al., 2003; 2006; Redila and Chavkin, 2008) and self-administration (Beardsley et al., 2005). The effect in the CPP paradigm was, however, blocked by pretreatment with the KOPr antagonists, nor-BNI (Redila and Chavkin, 2008; McLaughlin et al., 2003). The effect of KOPr antagonists might depend on the drug-seeking stimulus since the KOPr antagonist, JDTic inhibited stress induced drug seeking but had no effect on cocaine-produced reinstatement (Beardsley et al., 2005).
Further, KOPr knock out mice were insensitive to stress-produced reinstatement, as measured in the CPP paradigm, supporting the idea that reinstatement of CPP required KOPr activation. A further difference in the role of KOPr in reinstatement as measured in the CPP and self-administration paradigms is that U50488H potentiated the cocaine-produced place preference whereas it decreased drug-seeking following extinction of self-administration (present study). Additional studies will have to be conducted in order to tease apart the different aspects of the two paradigms.
In conclusion, Sal A, like traditional KOPr agonists attenuated cocaine-induced cocaine seeking. Further studies on Sal A and its structural derivatives will help us understand the mechanism by which KOPr agonists attenuate drug-seeking behaviors and to determine weather Sal A produces the same magnitude of adverse effects that have limited the development of other KOPr agonists as anti-addiction pharmacotherapies.
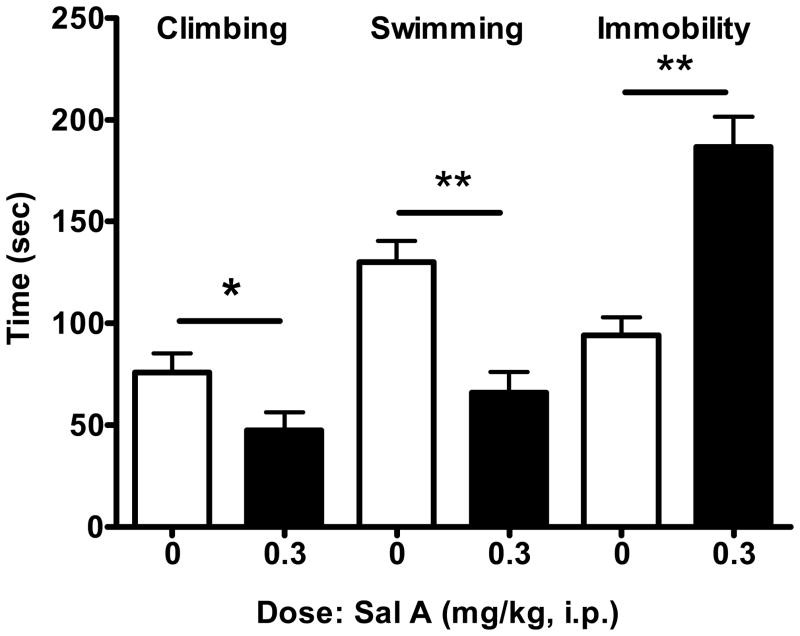
Fig. 5.
Effect of single injection of salvinorin A (Sal A) on forced swim test (FST) in drug naive rats (n=6). On test day, animals were injected with Sal A and 5 min later were subjected to FST. Data expressed as mean time (sec) (+SEM) for climbing, swimming and immobility behaviours during 5 min of FST. *p<0.05, **p<0.01. Data for 0.3 mg/kg compared with 0 mg/kg for climbing, swimming and immobility. Mann Whitney test. n= 6 for each group.
Table 1.
Experimental groups for the effects of Sal A on cocaine induced hyperactivity.
| Acute pre-treatment (ip) | Challenge injection (ip) |
|---|---|
| Veh | Saline (1 ml/kg) |
| Sal A | Saline (1 ml/kg) |
| Veh | Cocaine(20 mg/kg) |
| Sal A | Cocaine (20 mg/kg) |
Veh, vehicle (75% DMSO); Sal A, Salvinorin A (0.3 mg/kg)
Table 2.
Experimental groups for the effects of Sal A on expression of cocaine sensitization.
| Pretreatment (day 1–5) (ip) | Acute treatment (day 10) (ip) | Challenge injection (day 10)(ip) |
|---|---|---|
| Saline (1 ml/kg) | Veh | Cocaine (20 mg/kg) |
| Saline (1 ml/kg) | Sal A | Cocaine (20 mg/kg) |
| Cocaine (20 mg/kg) | Veh | Cocaine (20 mg/kg) |
| Cocaine (20 mg/kg) | Sal A | Cocaine (20 mg/kg) |
Veh, vehicle (75% DMSO); Sal A, Salvinorin A (0.3 mg/kg)
Acknowledgments
This work was funded by Wellington Medical Research Foundation, The Neurological Foundation of New Zealand and grant DA018151 (T.E.P.) from the National Institute on Drug Abuse (NIDA). The authors are thankful for the technical assistance of Mr. Alex Howard, Mr. Lincoln Hely and Mr. Richard Moore.
Contributor Information
Aashish S. Morani, Email: aashish.morani@vuw.ac.nz.
Bronwyn Kivell, Email: Bronwyn.kivell@vuw.ac.nz.
Thomas E. Prisinzano, Email: prisinza@ku.edu.
Susan Schenk, Email: Susan.schenk@vuw.ac.nz.
References
- Alleweireldt A, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2002;159(3):284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Baker L, Panos J, Killinger B, Peet M, Bell L, Haliw L, Walker S. Comparison of the discriminative stimulus effects of salvinorin A and its derivatives to U69593 and U50488 in rats. Psychopharmacology (Berl) 2009;203(3):203–211. doi: 10.1007/s00213-008-1458-3. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183(1):118–26. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Capurro V, Fadda P, Rubino T, Mascia P, Zani A, Gori E, Fratta W, Parolaro D, Sala M. Involvement of kappa-Opioid and Endocannabinoid System on Salvinorin A-Induced Reward. Biol Psychiatry. 2008;63(3):286–292. doi: 10.1016/j.biopsych.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris T, Kreek MJ. The plant-derived hallucinogen, salvinorin A, produces kappa-opioid agonist-like discriminative effects in rhesus monkeys. Psychopharmacology (Berl) 2004;172(2):220–224. doi: 10.1007/s00213-003-1638-0. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Prisinzano TE, Deng H, Rus S, Kreek MJ. Unconditioned behavioral effects of the powerful kappa-opioid hallucinogen salvinorin A in non-human primates: Fast onset and entry into cerebrospinal fluid. J Pharmacol Exp Ther. 2009;328(2):588–597. doi: 10.1124/jpet.108.145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WJ, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee YWD, Cohen BM. Depressive-like effects of the Kappa-opioid receptor agonist Salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316(1):440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33(11):2676–87. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. Salvinorin A, an active component of the hallucinogenic sage Salvia divinorum is a highly efficacious kappa-opioid receptor agonist: Structural and functional considerations. J Pharmacol Exp Ther. 2004;308(3):1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266(3):1236–1246. [PubMed] [Google Scholar]
- Fantegrossi W, Kugle KM, Valdes LJ, III, Koreeda M, Woods JH. Kappa-opioid receptor-mediated effects of the plant-derived hallucinogen, salvinorin A, on inverted screen performance in the mouse. Behav Pharmacol. 2005;16(8):627–633. doi: 10.1097/00008877-200512000-00005. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Javaid J, Hatano Y, Davis J. Acute tolerance development to the cardiovascular and subjective effects of cocaine. J Pharmacol Exp Ther. 1985;235(3):677–82. [PubMed] [Google Scholar]
- Gehrke BJ, Chefer VI, Shippenberg TS. Effects of acute and repeated administration of salvinorin A on dopamine function in the rat dorsal striatum. Psychopharmacology (Berl) 2008;197(3):509–517. doi: 10.1007/s00213-007-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa-opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681(1–2):147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The kappa-opioid agonist, U69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem. 1999;73(3):1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- Hahn B, Stolerman IP, Shoaib M. Kappa-opioid receptor modulation of nicotine-induced behaviour. Neuropharmacology. 2000;39(13):2848–2855. doi: 10.1016/s0028-3908(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616(1–2):335–338. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Shippenberg TS. U69593 prevents cocaine sensitization by normalizing basal accumbens dopamine. Neuroreport. 1994;5(14):1797–800. doi: 10.1097/00001756-199409080-00028. [DOI] [PubMed] [Google Scholar]
- Hiedbreder CA, Babovic-Vuksanovic D, Shoaib M, Shippenberg TS. Development of behavioral sensitization to cocaine: influence of Kappa-opioid receptor agonists. J Pharmacol Exp Ther. 1995;275(1):150–163. [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278(2):490–502. [PubMed] [Google Scholar]
- Holtzman SG, Cook L, Steinfels GF. Discriminative stimulus effects of spiradoline, a kappa-opioid agonist. Psychopharmacology (Berl) 1991;105(4):447–52. doi: 10.1007/BF02244362. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Further characterization of the discriminative stimulus effects of spiradoline. Pharmacol Biochem Behav. 2000;66(3):517–22. doi: 10.1016/s0091-3057(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97(1):59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- John TF, French LG, Erlichman JS. The antinociceptive effect of Salvinorin A in mice. Eur J Pharmacol. 2006;545(2–3):129–133. doi: 10.1016/j.ejphar.2006.06.077. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: Effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294(2):680–687. [PubMed] [Google Scholar]
- Khroyan TV, Platt DM, Rowlett JK, Spealman RD. Attenuation of relapse to cocaine seeking by dopamine D1 receptor agonists and antagonists in non-human primates. Psychopharmacology (Berl) 2003;168(1–2):124–131. doi: 10.1007/s00213-002-1365-y. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci. 2004;24(30):6686–92. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50488, a kappa-opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181(1–2):57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol Biochem Behav. 2006;83(1):109–113. doi: 10.1016/j.pbb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23(13):5674–83. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31(6):1241–8. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14(6):375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of kappa-opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther. 1998;286(2):812–824. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa-opioids on cocaine self- administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282(1):44–55. [PubMed] [Google Scholar]
- Prisinzano TE, Tidgewell K, Harding WW. Kappa-opioids as potential treatments for stimulant dependance. AAPS J. 2005;7(3):E592–599. doi: 10.1208/aapsj070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisinzano TE. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005;78(5):527–531. doi: 10.1016/j.lfs.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Prisinzano TE, Rothman RB. Salvinorin A analogues as probes in opioid pharmacology. Chem Rev. 2008;108(5):1732–1743. doi: 10.1021/cr0782269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200(1):59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: A potent naturally occurring nonnitrogenous Kappa-opioid selective agonist. Proc Natl Acad Sci USA. 2002;99(18):11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) 1999;147(3):285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144(4):339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. Reinstatement of extinguished drug-taking behaviour in rats: effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology (Berl) 2000;151(1):85–90. doi: 10.1007/s002130000476. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Effect of the kappa-opioid receptor agonist, U69593, on reinstatement of extinguished amphetamine self-administration behavior. Pharmacol Biochem Behav. 2001;68(4):629–634. doi: 10.1016/s0091-3057(00)00478-0. [DOI] [PubMed] [Google Scholar]
- Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, Prisinzano TE. Pharmacokinetics of the plant-derived kappa-opioid hallucinogen Salvinorin A in non-human primates. Synapse. 2005;58(3):208–210. doi: 10.1002/syn.20191. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271(5255):1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Spanagel R, Heidbreder CA. Modulation of mesolimbic dopamine release by endogeneous opioids; role in drug-induced sensitization and dependance. In: Louilot A, Durkin T, Spampinato U, Cardor M, editors. In monitoring molecules in neuroscience; Proc. 6th International Conference on In Vivo methods; 1994. pp. 123–125. [Google Scholar]
- Shippenberg TS, LeFevour A, Heidbreder CA. Kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276(2):545–554. [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116(2):306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43(1):53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20(24):9333–40. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The Kappa-Opioid Agonist U69,593 Blocks Cocaine-Induced Enhancement of Brain Stimulation Reward. Biol Psychiatry. 2008;64(11):982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés LJ, III, Diaz J, Paul AG. Ethnopharmacology of ska Maria Pastora (Salvia divinorum, Epling AND Jativa-M. ) J Ethnopharmacol. 1983;7(3):287–312. doi: 10.1016/0378-8741(83)90004-1. [DOI] [PubMed] [Google Scholar]
- Valdes LJ., III Salvia divinorum and the unique diterpene hallucinogen, Salvinorin (divinorin) A. J Psychoactive Drugs. 1994;26(3):277–283. doi: 10.1080/02791072.1994.10472441. [DOI] [PubMed] [Google Scholar]
- Willmore-Fordham CB, Krall DM, McCurdy CR, Kinder DH. The hallucinogen derived from Salvia divinorum, salvinorin A, has kappa-opioid agonist discriminative stimulus effects in rats. Neuropharmacology. 2007;53(4):481–486. doi: 10.1016/j.neuropharm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Worley CM, Valadez A, Schenk S. Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav. 1994;48(1):217–221. doi: 10.1016/0091-3057(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Fuller SA, Stein EA. Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: An in vivo fast-cyclic voltammetry study. J Pharmacol Exp Ther. 1998;284(1):151–161. [PubMed] [Google Scholar]
- Yan F, Roth BL. Salvinorin A: A novel and highly selective kappa-opioid receptor agonist. Life Sci. 2004;75(22):2615–2619. doi: 10.1016/j.lfs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa-opioid receptors. Psychopharmacology (Berl) 2005;179(3):551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]