Abstract
The purpose of this study was to evaluate the role of gastrin in the genesis of lower esophageal sphincter (LES) pressure by the use of a high titer gastrin antiserum. Intravenous infusions of increasing amounts of rabbit gastrin antiserum, but not control antiserum, produced graded reductions in the resting LES pressure in anesthetized opossums. A maximal inhibition in LES pressure of 80.0±3.1% (mean ±SE) was achieved when gastrin antiserum was administered in an amount estimated to bind almost all endogenous circulating gastrin in the opossum. Gastrin antiserum also inhibited the LES response to endogenous gastrin release (gastric deacidification) and to exogenous intravenous administration of gastrin I. The inhibition of the LES response to exogenous gastrin I by gastrin antiserum could be eliminated by giving excess gastrin I. Studies performed in vitro showed that gastrin antiserum inhibited the contractile response of LES circular muscle to gastrin I, but not to acetylcholine. These studies indicate that gastrin antiserum: (a) specifically antagonized the response of LES circular muscle to gastrin, in vitro; (b) diminished the LES response to the endogenous release and to the exogenous administration of gastrin; and (c) markedly reduced the resting level of LES pressure. We conclude that endogenous gastrin is the major determinant of resting LES pressure.
Full text
PDF

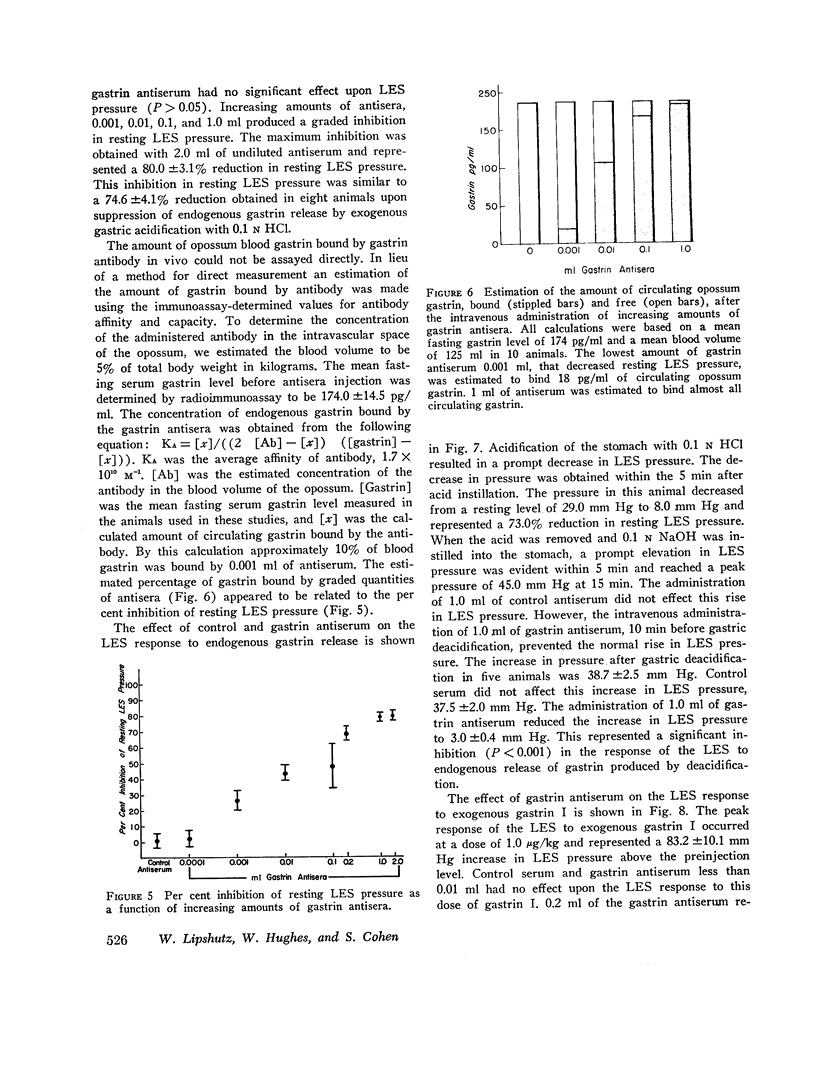
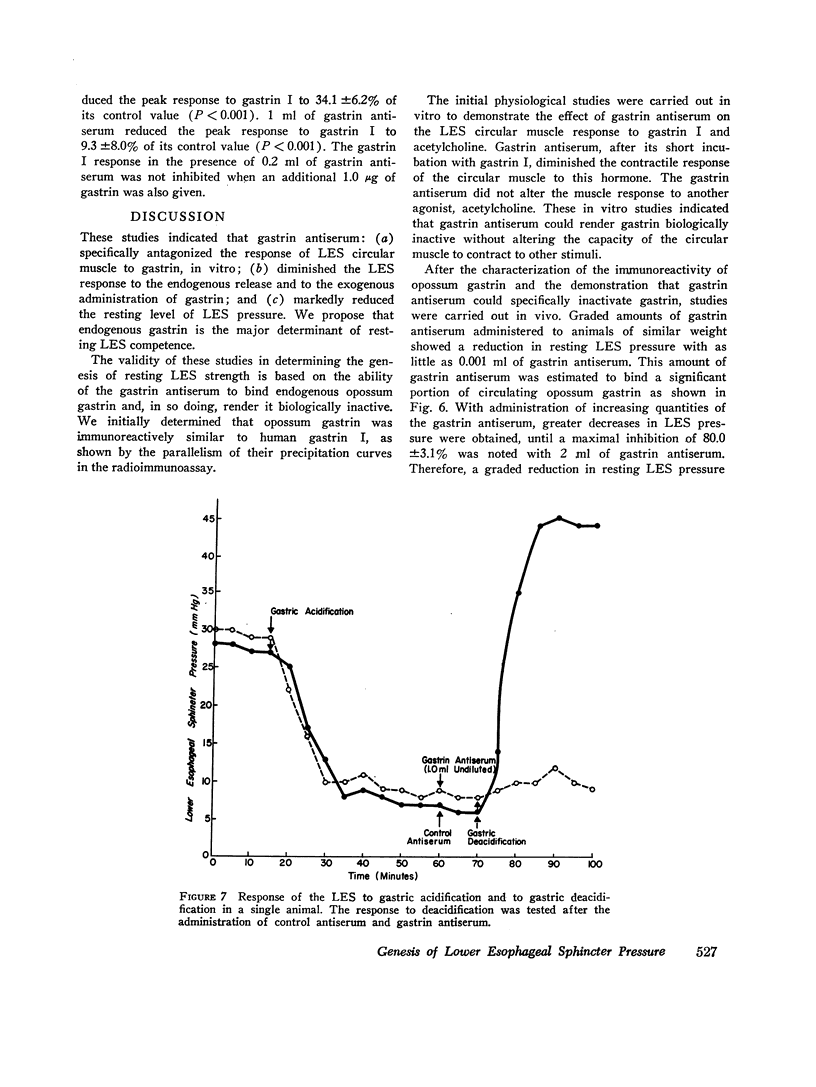
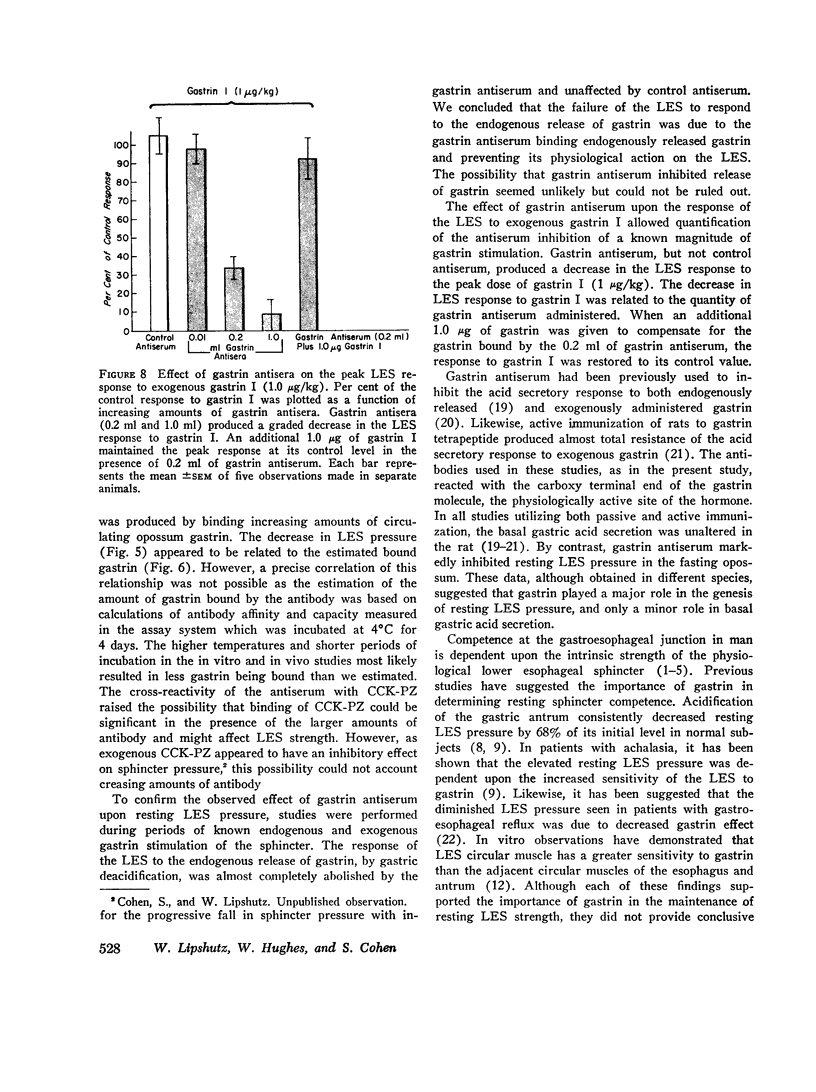

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castell D. O., Harris L. D. Hormonal control of gastroesophageal-sphincter strength. N Engl J Med. 1970 Apr 16;282(16):886–889. doi: 10.1056/NEJM197004162821602. [DOI] [PubMed] [Google Scholar]
- Christensen J., Lund G. F. Esophageal responses to distension and electrical stimulation. J Clin Invest. 1969 Feb;48(2):408–419. doi: 10.1172/JCI105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Harris L. D. Does hiatus hernia affect competence of the gastroesophageal sphincter? N Engl J Med. 1971 May 13;284(19):1053–1056. doi: 10.1056/NEJM197105132841902. [DOI] [PubMed] [Google Scholar]
- Cohen S., Harris L. D. Lower esophageal sphincter pressure as an index of lower esophageal sphincter strength. Gastroenterology. 1970 Feb;58(2):157–162. [PubMed] [Google Scholar]
- Cohen S., Lipshutz W. Hormonal regulation of human lower esophageal sphincter competence: interaction of gastrin and secretin. J Clin Invest. 1971 Feb;50(2):449–454. doi: 10.1172/JCI106512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Lipshutz W., Hughes W. Role of gastrin supersensitivity in the pathogenesis of lower esophageal sphincter hypertension in achalasia. J Clin Invest. 1971 Jun;50(6):1241–1247. doi: 10.1172/JCI106601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles G. R., Mason M. C., Humphries C., Clark C. G. Action of gastrin on the lower oesophageal sphincter in man. Gut. 1969 Sep;10(9):730–734. doi: 10.1136/gut.10.9.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F., Horton P. F., Pope C. E., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970 Feb;58(2):163–174. [PubMed] [Google Scholar]
- Jaffe B. M., McGuigan J. E., Newton W. T. Gastrin resistance following immunization to the C-terminal tetrapeptide amide of gastrin. Surgery. 1971 Feb;69(2):232–237. [PubMed] [Google Scholar]
- Jaffe B. M., Newton W. T., McGuigan J. E. Inhibition of endogenous gastrin activity by antibodies to the carboxyl-terminal tetrapeptide amide of gastrin. Gastroenterology. 1970 Feb;58(2):151–156. [PubMed] [Google Scholar]
- Jaffe B. M., Newton W. T., McGuigan J. E. Inhibition of gastrin activity by incubation with antibodies to the C-terminal tetrapeptide of gastrin. Surgery. 1969 Apr;65(4):633–639. [PubMed] [Google Scholar]
- Lind J. F., Cotton D. J., Blanchard R., Crispin J. S., Dimopolos G. E. Effect of thoracic displacement and vagotomy on the canine gastresophageal junctional zone. Gastroenterology. 1969 Jun;56(6):1078–1085. [PubMed] [Google Scholar]
- Lipshutz W., Cohen S. Physiological determinants of lower esophageal sphincter function. Gastroenterology. 1971 Jul;61(1):16–24. [PubMed] [Google Scholar]
- McGuigan J. E. Immunochemical studies with synthetic human gastrin. Gastroenterology. 1968 Jun;54(6):1005–1011. [PubMed] [Google Scholar]
- McGuigan J. E., Trudeau W. L. Studies with antibodies to gastrin. Radioimmunoassay in human serum and physiological studies. Gastroenterology. 1970 Feb;58(2):139–150. [PubMed] [Google Scholar]
- Pope C. E., 2nd A dynamic test of sphincter strength: its application to the lower esophageal sphincter. Gastroenterology. 1967 May;52(5):779–786. [PubMed] [Google Scholar]
- Rodbard D., Bridson W., Rayford P. L. Rapid calculation of radioimmunoassay results. J Lab Clin Med. 1969 Nov;74(5):770–781. [PubMed] [Google Scholar]
- Winans C. S., Harris L. D. Quantitation of lower esophageal sphincter competence. Gastroenterology. 1967 May;52(5):773–778. [PubMed] [Google Scholar]