Summary
We have previously shown that Wnt5A-mediated signaling can promote melanoma metastasis. It has been shown that Wnt signaling is antagonized by the protein Klotho, which has been implicated in aging. We show here that in melanoma cells, expressions of Wnt5A and Klotho are inversely correlated. In the presence of recombinant Klotho (rKlotho) we show that Wnt5A internalization and signaling is decreased in high Wnt5A expressing cells. Moreover, in the presence of rKlotho, we observe an increase in Wnt5A remaining in the medium, coincident with an increase in sialidase activity and decrease in syndecan expression. These effects can be inhibited using a sialidase inhibitor. In addition to its effects on Wnt5A internalization, we also demonstrate that Klotho decreases melanoma cell invasive potential by a second mechanism, that involves the inhibition of calpain and a resultant decrease in filamin cleavage, which we demonstrate is critical for melanoma cell motility.
Significance
We report here a mechanism by which Klotho, the loss of which results in an accelerated aging phenotype, decreases the invasion of melanoma cells. It does this in a two-pronged manner, by inhibiting the internalization of Wnt5A, and also by inhibiting the cleavage of Filamin. Our data support those in which the aging microenvironment of a tumor has been shown to influence the metastatic process. Our results suggest that while Klotho can act to suppress Wnt signaling, Filamin cleavage and tumor progression in a “younger” microenvironment, loss of Klotho during aging could contribute to a microenvironment that promotes tumor invasion.
Introduction
The incidence of the vast majority of cancers, including melanoma, increases with advancing age. However, the mechanism by which aging influences the risk of developing cancer is not fully understood. Whether this is attributable to declines in immunity or other changes, such as changes in the aging microenvironment, remains unclear. Amongst the many proteins implicated in aging is the protein Klotho. Loss of Klotho function results in the early appearance of several pathologies associated with human aging including artherosclerosis, osteoporosis, and skin atrophy (Kuro-o et al., 1997), and mice lacking Klotho die prematurely around the age of 8–9 weeks (Nabeshima, 2006). Overexpression of Klotho, however, has been shown to increase lifespan by 20–30% on average compared to wild-type mice (Kurosu et al., 2005).
The Klotho gene codes for a single pass transmembrane protein and two forms of the protein have been identified: a transmembrane form present primarily in the kidneys, as well as a secreted form found in the blood circulation (Matsumura, 1998, Kurosu et al., 2005 and Imura et al., 2004). The existence of a third form, secreted and generated by alternative splicing was also reported, although its presence was never detected in the blood (Matsumura et al., 1998). The transmembrane form of Klotho acts as a co-factor for FGF23, an endocrine factor that lowers blood phosphate and vitamin D levels (Kurosu et al., 2006). The secreted form of Klotho was shown to have a putative sialidase activity and be involved in the regulation of glycoprotein function at the cell surface (Cha et al., 2008). Interestingly, secreted Klotho was reported to inhibit insulin/IGF-1 signaling (Kuro-o et al., 1997 and Kurosu et al., 2005), a pathway previously reported to be linked to aging. This inhibitory effect of Klotho on IGF-1 signaling was recently proposed as a mechanism for the reported tumor suppressor role of Klotho in breast cancer (Wolf et al., 2008).
A putative role for Klotho in cancer was also suggested in a recent study showing that downregulation of Klotho induced premature cellular senescence in a p53/p21-dependent mechanism (de Oliveira, 2006). Very recently, a study by Lee et al showed that Klotho is epigenetically silenced during cervical cancer progression (Lee et al, 2010). In addition, Klotho was shown to antagonize the activity of several members of the Wnt family (Liu et al., 2007), which have been shown to be involved in cancer progression. Our laboratory has had a long-standing interest in Wnt5A, a non-canonical Wnt protein, which we have shown to be involved in melanoma progression (Weeraratna et al., 2002), and Wnt5A overexpression is associated with a more aggressive form of the disease (Da Forno et al., 2008). We have previously shown that Wnt5A increased the metastatic ability of melanoma cells both in vitro (Weeraratna et al., 2002, Dissanayake et al., 2007) and in vivo (Dissanayake et al., 2008). In particular, we recently demonstrated that Wnt5A induced a migratory phenotype and increased cell motility via calpain-mediated cleavage of the cytoskeletal protein Filamin A (O'Connell et al., 2009a). We have also shown that the effects of Wnt5A on motility are augmented by its ability to interact with heparan sulfate proteoglycans, specifically syndecans (O'Connell et al., 2009b). Both of these observations hinted at a possible link between Klotho and Wnt5A, since Klotho has been shown to inhibit calpain activity, and also to have putative sialidase activity. Thus, we investigated the expression of Klotho in melanoma cells and its regulation in cells of differing metastatic potential expressing different levels of Wnt5A.
Results
Klotho expression is inversely correlated to Wnt5A expression in melanoma cells
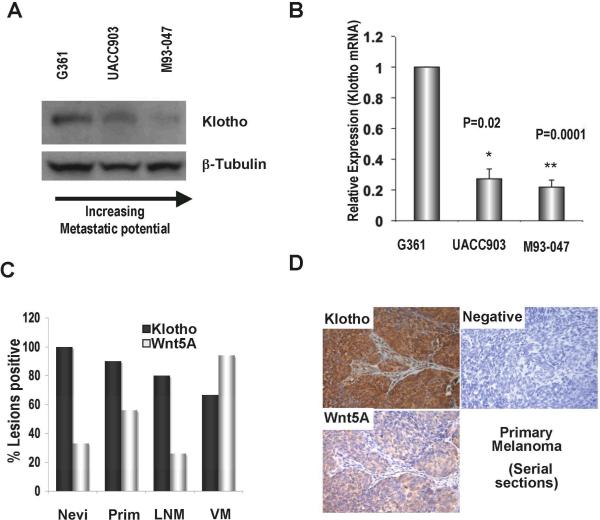
In previous studies (Dissanayake et al., 2007, Dissanayake et al, 2008), we have characterized a panel of melanoma cell lines with respect to their levels of Wnt5A, PKC, MART1 and invasive capacity, allowing us to establish a positive correlation between high levels of Wnt5A and PKC activation, low levels of MART1 expression, and an increase in metastatic potential. We therefore investigated whether Klotho was expressed in these melanoma cell lines, and if its expression was affected by that of Wnt5A. Western blot analysis of Klotho protein expression in melanoma cell lines showed that whereas Wnt5A-low G361 cells, with low metastatic ability, expressed high levels of Klotho, its expression was reduced in Wnt5A-medium UACC903 cells and considerably diminished in Wnt5A-high M93-047 cells (Figure 1A). To determine if changes in the levels of protein expression were the result of transcriptional regulation in these cells, we used real time PCR analysis to quantitate levels of Klotho gene expression. Levels of Klotho mRNA were reduced in both UACC903 and M93-047 cells as compared to Wnt5A-low G361 cells (Figure 1B). These results show that Klotho is expressed in melanoma cells, and that its expression is inversely correlated with levels of Wnt5A expression. Moreover, this inverse correlation in the expression of Wnt5A and Klotho is observed both at the protein and mRNA level. Because high expression of Wnt5A is correlated with increased metastatic potential in these melanoma cell lines, these results also suggest that Klotho expression might be reduced in more highly metastatic melanoma cells.
Figure 1. Melanoma cells express the protein Klotho and its expression decreases with increased metastatic potential.
(A) Total protein was isolated from melanoma cell lines of different metastatic potential and Klotho protein expression was measured by western blot analysis. β-Tubulin was used as a loading control. (B) Klotho mRNA expression by real-time PCR in G361, UACC903, and M93-047 melanoma cell lines normalized to 18S levels (n=4, error bars are standard deviation, *= p< 0.05, ** = p<0.005). (C) Percentages of lesions expressing Klotho, n= no of tumors per group. Prim= Primary melanoma, VM= Visceral metastases, LNM= Lymph node metastases. (D) Serial sections of primary melanoma stained for both Klotho and Wnt5A expression.
To assess this prediction in human melanoma samples, we used a melanoma tissue microarray to stain for Klotho expression. We have previously demonstrated that the vast majority of visceral metastases (VM) in this array (94%; 34/36) stain strongly positive for Wnt5A while only 33% of nevi, and 56% of primary lesions (PRIM) stain even weakly positive for Wnt5A (Dissanayake et al., 2008). By contrast, 100% of nevi (n=23), and 90% of primary lesions (n=21), stain strongly positive for Klotho, and only 66% of visceral metastases (n=36) stain positive, with generally weaker intensity. Graphing these data show a trend of decreasing Klotho expression, which is concomitant with an increase in Wnt5A expression (Figure 1 C). As previously shown (Dissanayake et al., 2008), lymph node metastases (LNM) rarely express Wnt5A (only 26%, or 6/23), while over 80% of lymph node metastases express Klotho. Klotho staining is shown in a primary melanoma sample, and compared to Wnt5A staining in a corresponding serial section (Figure 1D). These data confirm an inverse relationship between Klotho, Wnt5A and the metastatic capacity of melanoma cells.
Wnt5A regulates Klotho expression in melanoma cells
Since we observed a decrease in Klotho expression in Wnt5A-high cell lines, we examined whether Wnt5A could regulate Klotho in melanoma cells. Using immunofluorescent confocal microscopy, we confirmed that Wnt5A-high M93-047 or UACC903 cells have very low levels of Klotho (Figure 2A). Conversely, Klotho was expressed in low-Wnt5A G361 cells and localized predominantly in the cytoplasm and around the nucleus, as previously reported (Liu et al., 2007) (Figure 2B, left panel). To determine if this was a cell-specific or Wnt5A-related effect, G361 cells were treated with recombinant Wnt5A (rWnt5A), which resulted in a decrease in Klotho expression (Figure 2B, right panel). This decrease in Klotho protein expression in the presence of Wnt5A was also shown by Western blot analysis (Figure 2C). We then asked whether Wnt5A could affect Klotho mRNA expression in Wnt5A-low/ Klotho-high cells. Indeed, treatment of G361 cells with recombinant Wnt5A resulted in a decrease in Klotho gene expression (Figure 2D). Wnt5A-high M93-047 cells were then transfected with siRNA that we have previously shown specifically downregulates Wnt5A expression (Dissanayake et al., 2007). Upon Wnt5A knockdown, Klotho gene expression was increased (Figure 2E), as was Klotho protein expression (figure 2F). Taken together, these results suggest that Wnt5A antagonizes the expression of Klotho.
Figure 2. Wnt5A decreases Klotho expression.
The expression and localization of Klotho (red) was examined in melanoma cell lines expressing different levels of Wnt5A using immunofluorescent confocal microscopy. (A) The expression of Klotho was low in M93-047 and UACC903 cell lines and (B) was abrogated in G361 cells following treatment with recombinant Wnt5A for 16 hours. (C) Total protein lysates from G361 cells were analyzed by Western blot for the expression of Klotho, which was decreased following treatment with recombinant Wnt5A for 16 hrs. β-Tubulin was used as a loading control. (D) Analysis of Klotho mRNA expression measured by real-time PCR analysis shows that treatment of G361 cells with recombinant Wnt5A decreased Klotho expression (n=3, error bars are standard error of the mean, * = p<0.05). Transfection of Wnt5A-expressing M93-047 with Wnt5A siRNA for 48 hrs resulted in an increase in (E) Klotho mRNA expression (Klotho mRNA expression was normalized to 18S levels (n=3, error bars are standard error of the mean, ** = p<0.005)), as well as (F) Klotho protein expression.
Klotho Prevents HSPG-mediated Wnt5A uptake and signaling
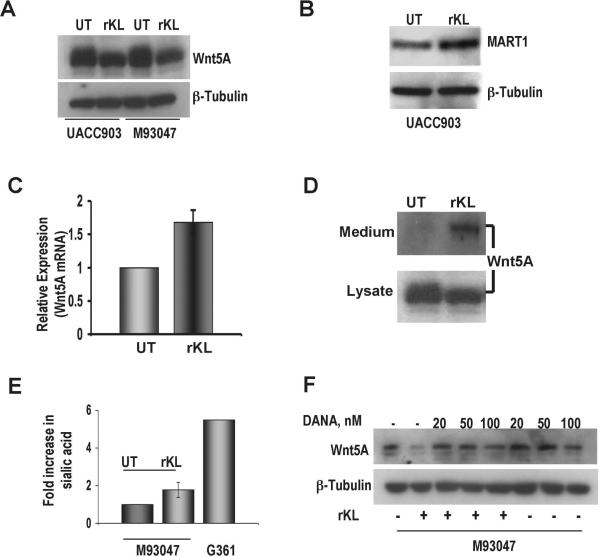
Since Klotho was previously reported to negatively regulate Wnt signaling in a mouse model (Liu et al., 2007), we also investigated the effect of Klotho on Wnt5A expression in our human melanoma cell lines. Wnt5A-high, Klotho-low M93-047 cells were treated with recombinant Klotho (rKlotho). Western analysis showed a reduction in Wnt5A expression in the presence of rKlotho in UACC903 and M93-047 cells (Figure 3A), and specifically of the upper, glycosylated band, often indicative of Wnt5A activity (Dissanayake et al., 2007). We have previously demonstrated that Wnt5A activation results in an increase in phospho-PKC, and more robustly, a down-regulation of MART1. Although M93-047 cells are completely negative for MART1, UACC903 cells have low levels of this protein. Treatment of UACC903 cells with rKlotho effectively increases the expression of MART1 (Figure 3B), indicating that Wnt5A signaling is inhibited. Interestingly, gene expression of Wnt5A was not decreased by rKlotho, suggesting that regulation of Wnt5A by Klotho may be post-translational (Figure 3C). We have previously demonstrated that heparan sulfate proteoglycans (HSPGs), specifically syndecan 1 and 4, are essential for Wnt5A internalization and signaling (O'Connell et al., 2009b). In addition to weak glucuronidase activity (Tohyama et al., 2004), Klotho was shown to affect glycosylation status of proteins by modification of N-linked glycans (Chang et al., 2005), which was further shown to implicate removal of sialic acid from glycan chains (Cha et al., 2008). This reported sialidase activity led us to hypothesize that Klotho may be either directly affecting the glycosylation of Wnt5A, required for its secretion, or affecting the HSPGs that play a role in Wnt5A internalization and signaling. We therefore looked at Wnt5A expression in the culture medium. In the presence of rKlotho we observed an increase in Wnt5A expression in the medium (Figure 3D). This suggested that glycosylation of Wnt5A itself was unaffected, since it could still be efficiently secreted into the medium, and pointed instead to the possibility that the internalization of Wnt5A, potentially due to sialidation of HSPGs, was being affected. We assayed sialidase activity in melanoma cells. G361 cells released much higher amounts of sialic acid than M93-047 cells. Treating M93-047 cells with rKlotho resulted in an increase in their sialidase activity (Figure 3E). Next, we used a sialidase inhibitor DANA (2-deoxy-2,3-dehydro-N-acetylneuraminic acid), that has been previously shown to inhibit Klotho activity (Cha et al., 2008). When M93-047 cells are treated with DANA alone there is no decrease in the level of Wnt5A, and when treated with rKlotho, Wnt5A decreases dramatically. When cells are treated with rKlotho in the presence of DANA, rKlotho is no longer able to inhibit the expression of Wnt5A (Figure 3F), suggesting that the effect of Klotho on Wnt5A may be attributable to its sialidase activity.
Figure 3. Klotho decreases Wnt5A expression.
Total protein lysates from UACC903 and/or M93-047 cell lines were used to analyze Wnt5A and MART-1 protein expressions following treatment with recombinant Klotho for 16 hrs. (A) Wnt5A expression was decreased in both cell lines following rKlotho treatment. (B) MART-1 protein expression was increased in UACC903 following rKlotho treatment. β-Tubulin was used as a loading control. (C) Real-time PCR analysis of Wnt5A mRNA in M93-047 cells. Wnt5A expression was not significantly decreased in the presence of rKlotho. mRNA expression for Klotho was normalized to 18S levels (n=3, error bars are standard error of the mean). (D) Cells were treated with rKlotho for 16h, and the medium of the cells was examined for Wnt5A release. In the presence of Klotho, Wnt5A accumulates in the medium (upper panel), which correlates with a decrease in Wnt5A expression in total protein lysate (bottom panel). (E) Following appropriate treatment of cells, culture medium was collected and analyzed for sialidase activity as measured by sialic acid release. (Each sample was assayed in triplicate, n=3, error bars are standard error of the mean, * = p<0.05). (F) M93047 cells were treated for 16 hrs with recombinant Klotho in the presence or absence of DANA and total protein lysates were used to analyze Wnt5A protein expression. In the presence of DANA, the Klotho-induced decrease in Wnt5A expression is no longer observed.
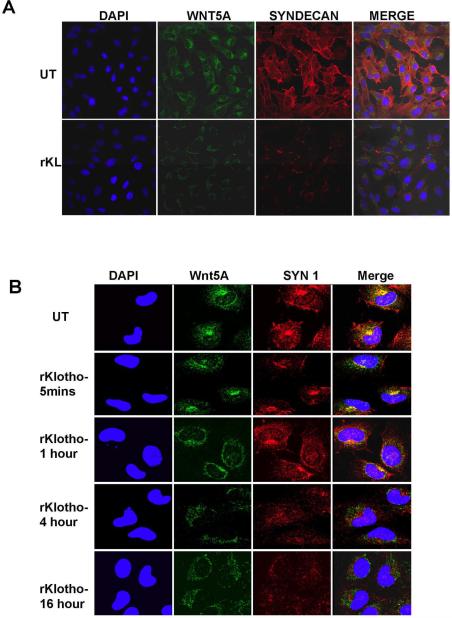
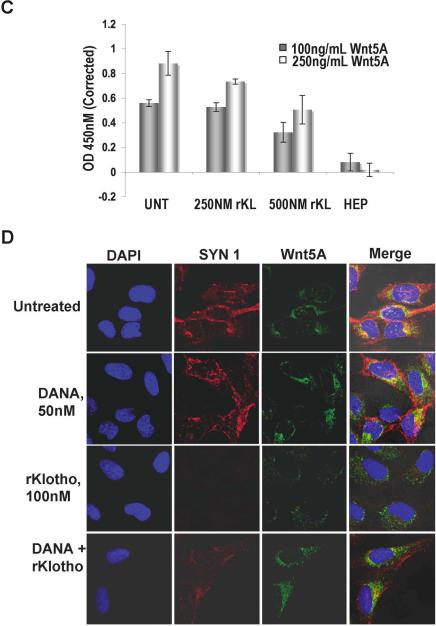
To assess if this increased sialidase activity and the increased expression of Wnt5A in the medium upon Klotho treatment could be due to the reduced ability of Wnt5A to bind to syndecans, and thus internalized, we used immunofluorescent confocal microscopy to look at syndecan −1 and −4 expression and localization in Wnt5A-high M93-047 cells. As previously reported (O'Connell et al., 2009b), the expression of both syndecans was high in untreated M93-047 cells (in the interest of space, syndecan 1 is shown in Figure 4A, and syndecan 4 in Supplementary Figure 1). In the presence of rKlotho however, in addition to a decrease in Wnt5A expression and internalization, we also observed a decrease in the expression of both syndecans (Figure 4A, and Supplementary Figure 1). However, mRNA levels of syndecans remain unaffected by Klotho, suggesting that this decrease is post-translational. We have previously shown that although it is difficult to show binding of Wnt5A to syndecans, given the nature of the interaction (protein binding to sugar chains rather than protein to protein binding), when Wnt5A and syndecans bind, they are internalized together and co-localize within the cell. We treated the cells with Klotho, and examined the co-localization profiles of Wnt5A and syndecan over a time course. Untreated cells show tight co-localization of red and green signal (forming yellow), representing Wnt5A (green) and syndecan (red, Figure 4B, panel 1). As early as 5 minutes after treatment with Klotho, Wnt5A and syndecan start to lose co-localization (Figure 4B, panel 2). By 1 hour, there is a significant decrease in co-localization, and Wnt5A can be seen mainly in the cytoplasm rather than in its typical perinuclear location (Figure 4B, panel 3), By 4 hours, while some syndecan expression is sustained, all perinuclear Wnt5A and co-localization of syndecan and Wnt5A is abolished, and by 16 hours as also shown in Figure 4A, both Wnt5A and syndecan expression is significantly reduced. To confirm that Klotho was affecting the binding of Wnt5A to syndecan, a solid-phase binding assay was performed as described in detail in Materials and Methods. Briefly, recombinant syndecan 1 was bound to a plate, and then rWnt5A was bound to the immobilized syndecan, in the presence or absence of rKlotho. The data demonstrate that Klotho can inhibit the binding of Wnt5A to syndecan in a dose-dependent manner (Figure 4C).
Figure 4. Klotho prevents HSPG-mediated Wnt5A uptake and signaling.
The expression and localization of Syndecan-1 (red) and Wnt5A (green) were examined in M93-047 cells using immunofluorescent confocal microscopy. (A) Treatment of M93-047 cells with recombinant Klotho for 16hrs shows that the decrease in Wnt5A expression in the presence of Klotho correlates with a decrease in the expression of Syndecan-1. (B) Co-localization of Wnt5A and syndecan-1 is affected as early as 5 mins after adding recombinant Klotho. (C) A solid phase binding assay demonstrates that in the presence of rKlotho, the binding of rWnt5A to rSyndecan is inhibited in a dose-dependent manner. (D) In the presence of DANA (50nM), treatment with recombinant klotho for 16 hrs no longer affects Wnt5A and syndecan expression levels as well as their co-localization.
Finally, to ascertain whether it is the sialidase activity of Klotho that has this effect, cells were treated with the sialidase inhibitor DANA, at 50nM. Untreated cells, and those treated with DANA alone (Figure 4C, panels 1 and 2 respectively) demonstrate clear co-localization and expression of Wnt5A and syndecan (yellow). Klotho treatment for 16 hours ablates this (Figure 4C, panel 3). However, if the cells are treated with Klotho in the presence of the sialidase inhibitor DANA, then co-localization of Wnt5A and syndecan is restored, albeit not to the levels of untreated cells (Figure 4C, panel 4, yellow). Taken together, these data strongly suggest that Klotho regulates the internalization of Wnt5A by the sialidation of syndecans.
Klotho negatively regulates the Wnt5A-mediated cleavage of Filamin A
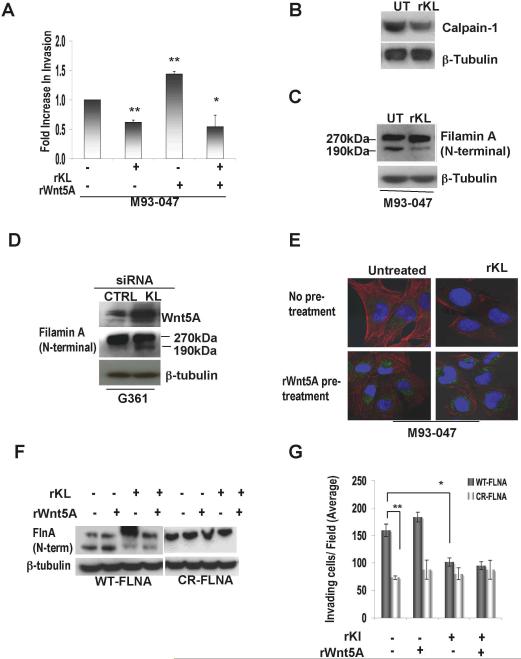
Having established in previous studies a major role for Wnt5A in cell motility and melanoma metastasis, we investigated whether Klotho reduction of Wnt5A could decrease the invasive potential of highly invasive M93-047 cells. Highly metastatic melanoma cells that were treated with rKlotho showed a decrease in the number of cells that migrated through the transwell (Figure 5A). These results suggested that Klotho could affect Wnt5A-mediated melanoma cell invasiveness. In previous experiments we have shown that adding excess rWnt5A can overcome decreases in syndecans, because there is so much Wnt5A, that at least a portion can bind to the receptor and be internalized, reconstituting signaling (O'Connell et al 2009b). However, in the presence of Klotho, treating cells with excess Wnt5A cannot restore motility, strongly suggesting that Klotho may be acting by a second mechanism independent of its effects on the internalization of Wnt5A via syndecans.
Figure 5. Klotho negatively regulates the Wnt5A-mediated Filamin A cleavage and invasive potential.
(A) M93-047 cells were subjected to a matrigel invasion assay. Invasion was measured by counting the cells in the bottom well 48 hrs after seeding on the matrigel-coated transwell filter. For each well, cells were counted in four different fields and averaged. The results are the average of three separate assays in which each condition was performed in triplicate. Error bars are standard error of the mean, * = p<0.05, **=p<0.005. (B) Calpain-1 protein expression was measured by Western blot analysis and was decreased in the presence of Klotho. (C) Total protein lysates from M93-047 cells were analyzed by Western blot for the presence of Filamin A cleavage. The N-terminal Filamin A cleavage fragment was identified as a 190 kDa band, and cleavage was decreased in the presence of Klotho. β-Tubulin was used as a loading control. (D) Expression of the N-terminal Filamin A cleavage fragment was increased in G361 cells transfected with Klotho SiRNA (E) The effect of excess rWnt5A on the Klotho-mediated expression and localization of filamin was examined in M93-047 cells. (F) M2 cells were transfected with either wildtype (WT) or Calpain resistant (CR) Filamin A (FLNA) treated with recombinant Klotho and /or recombinant Wnt5A for 16 hrs. (G) Total protein lysates were analyzed for the expression of N-terminal Filamin A cleavage and, (H) the invasive potential of the cells was measured using a matrigel invasion assay as described in (A). Error bars are standard error of the mean, * = p<0.05, **=p<0.005.
We have recently shown that one of the ways in which Wnt5A mediates melanoma cell motility is via the μ-calpain-mediated cleavage of Filamin A, resulting in remodeling of the cytoskeleton (O'Connell et al., 2009a). Interestingly, Klotho deficiency, which we show in this study to be associated with high levels of Wnt5A, was reported by others to result in the activation of μ-calpain (Manya et al., 2002). Treatment of Wnt5A high melanoma cells with recombinant Klotho results in a decrease in calpain 1 protein expression (Figure 5B). We therefore investigated the effect of Klotho on Filamin cleavage, a downstream result of calpain activity. We analyzed the expression of cleaved Filamin by Western blot analysis. Following treatment of M93-047 or UACC903 cells with recombinant Klotho we observed a decrease in the expression of the 190 kDa band corresponding to the cleaved N-terminal product of Filamin A cleavage (Figure 5C). Immunofluorescent analysis was also used to visualize whether Klotho decreases filamin cleavage. Filamin expression in untreated Wnt5A-high M93-047 cells was very diffuse throughout the cytoplasm (Supplementary Figure 2A, upper panel, green staining). This diffuse expression of Filamin is associated with high Wnt5A (Supplementary Figure 2A, upper panel, red staining), and corresponds to increased Filamin cleavage as described previously (O'Connell et al., 2009a). Upon treatment of these cells with rKlotho, the expression of Filamin A was relocalized to the edges of the cell (Supplementary Figure 2A, lower panel, green staining), which we have previously reported to correlate with a decrease in Filamin A cleavage (O'Connell et al., 2009a). Wnt5A protein levels were also decreased by rKlotho (Supplementary Figure 2A, lower panel, red staining).
In order to confirm the effect of Klotho on Filamin cleavage, small interfering RNAs targeted against Klotho, were used on G361 cells, which normally express low levels of Filamin A cleavage, low levels of Wnt5A and high levels of Klotho. Transfection of G361 cells with Klotho siRNA caused ~60% knockdown of Klotho (Supplementary Figure 2B), which led to an increase in Wnt5A expression as well as an increase in Filamin A cleavage, as measured by Western blot analysis (Figure 5D). These data could also be further confirmed by immunofluorescent analysis (Supplementary Figure 2C), where siRNA against Klotho caused an increase in Filamin cleavage as shown by the increased diffusion of Filamin staining upon Klotho knockdown. Finally, we sought to determine if Klotho was modulating these effects via the reduction of Wnt5A internalization as demonstrated in Figure 3, or if these effects on Filamin were partially independent, due to Klotho effects on calpain. We treated M93-047 cells with rKlotho in the presence of excess rWnt5A. We show here that despite some internalization of excess Wnt5A (Figure 5E, green staining), Filamin cleavage is still inhibited by rKlotho (Figure 5E, red staining), implying that Klotho effects on Filamin cannot be fully overcome by Wnt5A.
In order to confirm that these effects of Klotho involved specifically the calpain-mediated cleavage of filamin, we obtained M2 melanoma cells, that, while devoid of filamin, have active calpain signaling (Mammoto et al, 2007). Transfecting M2 cells with a wild type, full length clone of Filamin (Woo et al, 2004) resulted in the expression of large amounts of cleaved filamin in M2 cells (Figure 5F). Treatment of the cells with rWnt5A did not increase the already substantial cleavage much further, but treatment of Klotho, as seen before, significantly inhibited the cleavage of filamin, an effect that could not be restored in the presence of Wnt5A. We then transfected the cells with a calpain-resistant Filamin clone (Mammoto et al, 2007). Filamin remains uncleaved, due to a deletion in the calpain cleavage site, and neither treatment with Klotho nor Wnt5A affects this (Figure 5G). To assess the effects of Klotho on motility of cells containing cleavage-sensitive (WT) filamin as compared to calpain-resistant (CR) filamin, we repeated the same experiment, and subjected the cells to a transwell assay. Where Filamin is cleaved cells are highly motile, however, transfection of these cells with a calpain-resistant, uncleavable Filamin construct significantly reduces the ability of the cells to invade. Furthermore, rKlotho treatment also inhibits motility in cells transfected with wild type filamin, presumably due to decreases in Filamin cleavage (Figure 5H). It should be noted that while untransfected M2 cells are slightly less motile than WT-Filamin expressing cells (Supplementary Figure 2D), and more motile than CR-Filamin expressing cells, these data fall outside of statistical significance (p>0.09 and p>0.07 respectively) allowing us to more accurately assess the direct effect of Klotho on cleaved Filamin-mediated motility.
Taken together, these results show that Klotho expression leads to a decrease in Filamin A cleavage, via the inhibition of calpain, as well as decreases in Wnt5A internalization, further contributing to Klotho-mediated suppression of motility.
Discussion
Several studies have focused on Klotho and its role in the aging phenotype. In particular, its expression has been shown to decrease with advancing age, and Klotho knockout mice exhibit age-related degeneration, including muscle degeneration, increased artherosclerosis, and loss of skin integrity (Kuro-o et al., 1997). In contrast, Wnt5A, the increased expression of which can promote melanoma metastasis, is increased in the skin of aged mice (data not shown). An interaction between the Wnt pathway and Klotho was described in a study by Liu et al., in which the authors report the suppression of Wnt biological activity in the presence of Klotho, when both of these molecules are artificially expressed (Liu et al., 2007). In this study, we show that Klotho and Wnt5A, a member of the non-canonical Wnt pathway, are endogenously expressed in melanoma cells of different invasive capacity, and can regulate each other. Interestingly, Wnt5A regulates Klotho at the transcriptional level. Over expression of Wnt5A has been previously shown to be associated with the downregulation of a large number of genes (Dissanayake et al., 2007), probably through epigenetic silencing, although the mechanism in melanoma cells has not yet been described. A very recent paper indicates that Klotho is epigenetically silenced in late-stage cervical cancer largely via histone deacetylation activity (Lee et al, 2010). Whether or not this is a mechanism via which Wnt5A can transcriptionally regulate Klotho is under investigation in our laboratory.
Our results clearly indicate that expression of Klotho is lost as cells become more metastatic, and describe a mechanism whereby this loss may contribute to Wnt5A-mediated metastasis. Coincident with this, others have suggested a tumor suppressor function for Klotho, in particular based on its decreased expression in more advanced lung cancer (Chen et al, 2010), cervical cancer (Lee et al, 2010), and breast cancer (Wolf et al., 2008). The loss of Klotho expression in more metastatic and higher Wnt5A expressing melanoma cells suggests one of two things: 1) in less metastatic cells, high expression of Klotho inhibits Wnt5A expression, but as the cells become more metastatic, loss of Klotho results in increased levels of Wnt5A, or 2) low levels of Wnt5A in less metastatic cells permit Klotho expression, but as the cells become more metastatic, higher levels of Wnt5A inhibit Klotho expression. These two possibilities are not mutually exclusive, and indeed, these two molecules may exist in a regulatory feedback loop.
The inhibition of Wnt signaling by Klotho was previously reported (Liu et al., 2007) and hypothesized to be the result of reduced ability of Wnt to bind to its receptor. In a recent study, we showed a role for heparan sulfate proteoglycans (HSPGs) in Wnt5A signaling in melanoma metastasis (O'Connell et al., 2009b). In particular, we showed that the expression of syndecans 1 and 4 was increased in more metastatic melanoma cell lines and promoted the binding and internalization of Wnt5A, and thus, an increase in Wnt5A signaling (O'Connell et al., 2009b). Knockout of syndecans 1 and 4 decreased Wnt5A internalization, signaling, and effects on motility. This could be overcome by adding back excess recombinant Wnt5A. Klotho has been shown to affect the glycosylation status of glycoproteins by modification of N-linked glycans (Chang et al., 2005). It has also been shown to have both glucuronidase (Tohyama et al., 2004), (Chang et al., 2005) and sialidase activity (Cha et al., 2008), which in turn have been shown to affect the glycosylation and expression of heparan sulfate proteoglycans. We hypothesized that Klotho may affect the glycosylation and sialylation of syndecans and limit their ability to promote Wnt5A internalization, resulting in reduced Wnt5A signaling. We show here that the presence of Klotho increases the release of Wnt5A in the medium, suggesting a decrease in receptor-mediated internalization, and this effect correlates with a decrease in syndecan-1 and syndecan-4 protein expression. We also show that this decrease in syndecan and Wnt5A internalization in the presence of Klotho correlates with an increase in sialidase activity that can be specifically attributed to Klotho, and the use of a sialidase inhibitor can promote the co-localization and internalization of syndecan and Wnt5A. Hence, we clearly implicate Klotho sialidase activity in reducing syndecan 1 and 4-mediated Wnt5A signaling. These results are particularly interesting when considering the correlation between excessive sialic acid expression and increased metastatic ability (reviewed in Miyagi et al., 2004, Miyagi et al., 2008). The sialidase NEU1 for example, can act as a metastasis suppressor in colon cancer. Increases in sialic acid residues have been shown to augment melanoma metastasis (Uemura et al., 2009). It is possible that, in melanoma, the sialidase activity of Klotho plays a similar role to NEU1, one of the effects of which is a decrease in the internalization and subsequent signaling of Wnt5A.
In addition to inhibiting the internalization of Wnt5A, we show here that Klotho has a second effect on melanoma metastasis, related to, but not dependent upon Wnt5A signaling. We have previously shown that Wnt5A signaling induced Filamin A cleavage, which resulted in increased melanoma cell motility (O'Connell et al., 2009a) and that this Wnt5A-induced cleavage of Filamin A was dependent on the activation of calpain-1. Several studies have shown increased calpain activity during aging (Baudry et al., 1986, Lynch et al., 1986), consistent with an increased intracellular or calcium influx in aging tissues (Besse et al., 1994, Blalock et al., 1999, Ouanounou et al., 1999 and Romero et al., 2002). In turn, deficiency in Klotho protein was reported by others to lead to the overactivation of calpain-1 (Manya et al., 2002). We show here that Klotho expression results in a decrease in calpain-1 expression in melanoma cells. Although this decrease in expression is not a direct measure of activity, in the presence of Klotho we also observe a decrease in the cleavage of Filamin A, an effect that we have previously attributed to calpain activity (O'Connell et al., 2009a). Treatment with excess rWnt5A, which we have shown can overcome decreases in syndecan-mediated internalization (O'Connell et al., 2009b), simply by the increase in ligand availability, cannot rescue the effect of Klotho pre-treatment on Filamin A in this case, and more importantly cannot restore motility to the melanoma cells (Figure 5). These data suggest that the effect of Klotho on Filamin A has less to do with its effects on Wnt5A than its direct effects on calpain, providing a second impediment to melanoma metastasis. This has huge implications for the potential use of Klotho as an anti-metastatic agent.
Several lines of evidence indicate a link between advanced age and tumor onset and progression, and melanoma is no exception (Lachiewicz et al, 2008). In particular, a number of factors secreted by senescent cells in the direct microenvironment of melanoma cells can contribute to the acquisition of a more invasive phenotype (reviewed in (Campisi and d'Adda di Fagagna, 2007)). Loss of Klotho is associated with an aging phenotype, and older animals have less secreted Klotho in their serum (Xiao et al., 2004). It could be that in a “younger” microenvironment, Klotho is present and acts as a check on Wnt5A signaling, Filamin cleavage and metastatic progression. In a scenario where the microenvironment of the tumor has significant effects on its outcome, the progressive loss of Klotho in an aging microenvironment could potentially allow for an increase in Wnt5A expression, and calpain activity and subsequent Filamin cleavage, thus increasing melanoma progression. These data suggest that reconstitution of Klotho may have beneficial effects, not only for reversal of aging-related phenotypes, but also for the successful management of metastatic melanoma.
Materials and Methods
Cell Culture
UACC903 and M93-047 cell lines were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA), while G361 cells were maintained in McCoy's 5A medium (Invitrogen). M2 cells were maintained in MEM. All media were supplemented with 10% fetal Bovine serum (Invitrogen), 100 U ml-1 penicillin and streptomycin, and 4mM L-glutamine. All cell lines were cultured at 37°C in 5% CO2, and the medium was replaced every 2 days.
Wnt5A, Klotho, and DANA treatment
Cells were treated with 100ng/ml recombinant mouse Wnt5A (R&D systems, Minneapolis, MN) for 16 hours, or 250ng/ml recombinant mouse Klotho (R&D systems) for 16 hours, unless otherwise stated. Cells were treated with DANA (2-deoxy-2,3-dehydro-N-acetylneuraminic acid) (Sigma, St. Louis, MO) for 16 hours as co-treatment with recombinant Klotho.
siRNA and Filamin construct Transfection
Cells were transfected with high performance (HP)-validated negative control (CTRL), and Wnt5A siRNA (Qiagen, Valencia CA) and Klotho siRNA (Applied Biosystems/Ambion, Austin, TX) using Lipofectamine (Invitrogen) for 48–72 hours as described previously (Dissanayake et al., 2007). Wnt5A sequences have been previously described, and tested by microarray analysis for off-target effects (Dissanayake et al., 2007, Dissanayake et al, 2008) and are as follows: Sense: r(GGAUAACCUUgUAACAUAU)dTdT Antisense: r(AUAUgUUACAAggUUAUCC)dgdg. Two Klotho sequences (Ambion) were used to confirm specificity of observed effects and were as follows: SiRNA 1: sense:5'gUAUCAAUCUUUCgCgAUAtt -3' and antisense 5'- UAUCCgCAAAgAUUgAUACca -3' and SiRNA 2: Sense: 5'- gCAgAUCAgUUUgAgCCCAtt -3' Antisense: 5'UgggCUCAAACUgAUCUgCag -3'
Plasmids
The pRep4-FilaminA vector encoding the full-length, wildtype Filamin (Woo et al, 2004) was obtained from Dr. Michel Bernier (National Institute on Aging, Baltimore, MD). The pEGFP-Filamin-del2 vector, encoding calpain-resistant Filamin (Mammoto et al, 2007) was obtained from Dr. Akiko Mammoto (Childrens Hospital, Boston, MA). Full-length Filamin was excised by first linearizing the pRep4 vector with EcoRV, then excising full-length Filamin with NruI. XbaI and HindIII were used to sticky end digest the purified filamin fragment. The pEGFP-C1 vector was transformed into JM110 dam−/dcm− cells to demethylate the XbaI restriction site. Finally, XbaI/HindIII were used to digest the EGFP-C1 empty vector (Invitrogen) and the full-length filamin fragment was subcloned into the vector and transformed into DB10B electrocompetent cells (Invitrogen). Plasmids were transfected into Filamin-deficient M2 cells using Lipofectamine Plus for 24 hours as described previously (Dissanayake et al., 2007), then cells were treated as described in the text.
Immunofluorescence
Cells were seeded at a density of 3 × 105 cells per well (G361) and 2 × 105 cells per well (M93-047 and UACC903) into one-well chambers treated for the appropriate time. At the end of the treatment, cells were washed in phosphate buffered saline (PBS), fixed in ice - cold 95% methanol for 20 minutes at room temperature, washed in PBS, and blocked using sterile-filtered blocking buffer (0.2% triton X-100, 0.2% BSA, 0.2% casein, 0.2% gelatin, and 0.02% sodium azide) for 1 hour at room temperature. Biotinylated Wnt5A (5 μg/ml, R&D), Klotho (5 μg/ml, Abcam, Cambridge, MA), Filamin A (1:100, Millipore, Billerica, MA), syndecan-1 (1:100, R&D), and syndecan-4 (1:100, R&D) primary antibodies were diluted in blocking buffer, and slides were incubated overnight at 4°C. Cells were washed in PBS and incubated with Alexa Fluor-568 (1:2,000) or Alexa-488 (1:2,000) conjugated secondary antibodies (Invitrogen) in blocking buffer for 1 hour at room temperature. The cells were then washed in PBS and mounted in Prolong Gold antifade reagent containing 4,6-diamidino-2-phenyindole (Invitrogen) and cured for at least 24 hours at room temperature in the dark. Images were taken using a Zeiss Meta 510 confocal microscope.
RNA extraction, cDNA synthesis, and real-time PCR
RNA was extracted using Trizol (Invitrogen) and the RNeasy Mini kit (Qiagen). As described previously (O'Connell et al., 2009a), cDNA was synthesized from 1 μg of RNA treated with DNase (Invitrogen) for 10 minutes. Gene expression was quantified using the SYBR green method of real-time PCR, and specific mRNA levels were normalized to 18S mRNA following the absolute quantitation method. Reactions were performed in triplicate using 100 nM gene-specific primers or universal 18S primers (Ambion). Real time PCR reactions were performed using an ABI prism 7300 sequence detection system under the following cycling conditions: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, and 60°C for 1 minute. Sequences of the primers used were as follows: Klotho, Sense: 5'TCTCAGTTTACCGACC TGAATGT-3'; antisense: 5'GCAAAGTCAACACAGTAGGA-3': Wnt5A: Sense: 5'-AGGGCTCCTACGAGA GTGCT-3'; antisense: 5'- GACACC CCATGGCACTTG-3'.
Western Blotting
Cells were lysed or culture medium was collected and concentrated in microcon YM10 columns, as described previously (O'Connell et al., 2009b). 50–75 μg total protein was run on a 4–20% Tris-glycine gel (Invitrogen). Proteins were then transferred, and blocked as described previously (O'Connell et al., 2009a). Primary antibodies were used at the following concentrations: Biotinylated Wnt5A (500 ng/ml, R&D systems), Klotho (1 μg/ml, Abcam), N-terminal Filamin A (1:2000, Millipore), calpain-1 (1:1000, Abcam) and MART-1 (1:500, Abcam). All primary antibodies were incubated overnight at 4°C, after which the membranes were washed in TBS-T and probed with the appropriate secondary antibody.
Invasion Assays
Invasion assays were performed using transwell migration chambers (Corning Life Sciences, Lowell, MA, USA). The filters were coated with 150 μl of 80 μg/ml reconstituted basement membrane (Matrigel) (Becton Dickinson, Franklin Lakes, NJ, USA). Prior to performing the assay, cells were serum starved for 16 hours and treated with recombinant Klotho protein (250 ng/mL) or recombinant Wnt5A (100 ng/ml) for 6 hours prior to seeding 100,000 cells in serum- free medium on the transwell membrane. Recombinant Klotho or recombinant Wnt5A was also added to the cells on the transwell membrane. The same medium, but containing 10% fetal bovine serum was added to the well beneath the filter to act as chemoattractant. The cells were then placed in a 37°C humidified incubator for 48 hours, following which the cells at the bottom of the well were washed and fixed and stained with crystal violet (0.5% in 40% Methanol) for 20 minutes. The number of cells was counted in four different fields and averaged for each of three experiments.
Solid-phase binding assay
Solid-phase binding assys were performed by adapting the protocol of Hindson et al (Hindson et al, 2005). Ninety-six-well high-binding ELISA plates (Costar, Cambridge, MA) were coated with 10 μg/mL (25 μL/well) recombinant human Syndecan 1 (R&D Systems) overnight at 4°C. Plates were then rinsed three times with TBST (50 mM Tris-HCl, pH 7.4; 0.15 M NaCl; 0.05% Tween 20) and blocked with 5% BSA in TBST for 1 hour. The plates were washed briefly once, and then incubated for 2 hours at 37°C in 50 nM Tris-acetate buffer (pH 8) with or without Heparinase (5 ng/mL), or with or without recombinant human Klotho (R&D Systems) at either 250ng/mL, or 500ng/mL. After washing three times with TBST, the wells were incubated with either 100ng/ mL or 500ng/mL recombinant Wnt5A (R&D Systems) for 90 minutes. After washing three more times, the plates were incubated with biotinylated Wnt5A antibody (R&D Systems, 1:500) overnight at 4oC. The next day plates were washed, and incubated with HRP-anti-goat, diluted 1:1000 in TBST for 40 minutes before washing and visualizing with TMB microwell 2-component peroxidase substrate system (KPL, Gaithersburg, MD) according to the manufacturer's instructions. The reaction was stopped using an equal volume (100 μL) of sulfuric acid. Absorbance was read at 450 nm, and is presented as the OD450, corrected for blank (BSA only) wells. Other negative controls included 1) Syndecan with no Wnt5A, but all other treatments as well as 2) Syndecan, Wnt5A, all other treatments but no primary antibody. Both of these conditions ranked at or below BSA only background values. All data points were collected in triplicate.
Sialidase Assay
One mL of culture medium was collected for the conditions described, and concentrated to 30 μL in centricon YM10 columns (Millipore). Measurement of free sialic acid in culture medium was performed using the Sialic Acid (NANA) Assay Kit from BIOVISION (Mountain View, CA), which uses a colorimetric measurement of the sialic acid-dependent oxidation of a specific probe. The absolute amount of sialic acid in samples was determined by running a standard curve with known concentrations of sialic acid standards in parallel with the unknown samples. Each sample was assayed in triplicate and the data shown is the average of three separate experiments.
Supplementary Material
The expression and localization of Syndecan-4 (red) and Wnt5A (green) were examined in M93-047 cells using immunofluorescent confocal microscopy. Treatment of M93-047 cells with recombinant Klotho for 16hrs shows that the decrease in Wnt5A expression in the presence of Klotho correlates with a decrease in the expression of Syndecan-4.
(A) The effect of Klotho on the localization and expression of Filamin A (green) was examined in M93-047 cells. Filamin A shows an irregular, diffuse pattern in the untreated cells, but becomes more regular and relocates to the periphery of the cells as Wnt5A expression (red) is reduced in the presence of Klotho.
(B) G361 cells were treated with 200 nM control (CTRL) or Klotho (KL) siRNA for 48 or 72h. Total RNA was isolated and real-time PCR analysis of Klotho expression was performed. Levels were normalized to 18S expression. (C) Knockdown of Klotho in G361 cells results in Filamin becoming expressed in a more diffuse pattern throughout the cells. (D) M2 cells were either left untransfected (UT) or transfected with either wildtype (WT) or Calpain resistant (CR) Filamin A (FLNA), and then subjected to a transwell invasion assay.
Acknowledgements
We thank Dr. Dan Longo (National Institute on Aging, Baltimore, MD) for helpful discussions on this manuscript. We also thank Dr. Michel Bernier (National Institute on Aging, Baltimore, MD) for the M2 cells, and full length, wild type pRep4-Filamin A construct, and Dr. Akiko Mammoto (Childrens Hospital, Boston, MA) for the pEGFPFilamin-del2 calpain-resistant Filamin A construct. This work was funded by the Intramural Research Program of the National Institute on Aging, Baltimore, MD.
References
- BAUDRY M, DUBRIN R, BEASLEY L, LEON M, LYNCH G. Low levels of calpain activity in Chiroptera brain: implications for mechanisms of aging. Neurobiol Aging. 1986;7:255–8. doi: 10.1016/0197-4580(86)90004-7. [DOI] [PubMed] [Google Scholar]
- BESSE S, DELCAYRE C, CHEVALIER B, HARDOUIN S, HEYMES C, BOURGEOIS F, MOALIC JM, SWYNGHEDAUW B. Is the senescent heart overloaded and already failing? Cardiovasc Drugs Ther. 1994;8:581–7. doi: 10.1007/BF00877412. [DOI] [PubMed] [Google Scholar]
- BLALOCK EM, PORTER NM, LANDFIELD PW. Decreased G-protein-mediated regulation and shift in calcium channel types with age in hippocampal cultures. J Neurosci. 1999;19:8674–84. doi: 10.1523/JNEUROSCI.19-19-08674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPISI J, D'ADDA DI FAGAGNA F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- CHA SK, ORTEGA B, KUROSU H, ROSENBLATT KP, KURO OM, HUANG CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105:9805–10. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG Q, HOEFS S, VAN DER KEMP AW, TOPALA CN, BINDELS RJ, HOENDEROP JG. The beta-glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–3. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- CHEN B, WANG X, ZHAO W, WU J. Klotho inhibits growth and promotes apoptosis in human lung cancer cell line A549. J Exp Clin Cancer Res. 2010;29:99. doi: 10.1186/1756-9966-29-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DA FORNO PD, PRINGLE JH, HUTCHINSON P, OSBORN J, HUANG Q, POTTER L, HANCOX RA, FLETCHER A, SALDANHA GS. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14:5825–32. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- DE OLIVEIRA RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580:5753–8. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- DISSANAYAKE SK, OLKHANUD PB, O'CONNELL MP, CARTER A, FRENCH AD, CAMILLI TC, EMECHE CD, HEWITT KJ, ROSENTHAL DT, LEOTLELA PD, WADE MS, YANG SW, BRANT L, NICKOLOFF BJ, MESSINA JL, BIRAGYN A, HOEK KS, TAUB DD, LONGO DL, SONDAK VK, HEWITT SM, WEERARATNA AT. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68:10205–14. doi: 10.1158/0008-5472.CAN-08-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISSANAYAKE SK, WADE M, JOHNSON CE, O'CONNELL MP, LEOTLELA PD, FRENCH AD, SHAH KV, HEWITT KJ, ROSENTHAL DT, INDIG FE, JIANG Y, NICKOLOFF BJ, TAUB DD, TRENT JM, MOON RT, BITTNER M, WEERARATNA AT. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259–71. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINDSON VJ, GALLAGHER JT, HALFTER W, BISHOP PN. Opticin Binds to Heparan and Chondroitin Sulfate Proteoglycans. Investigative Ophthalmology and Visual Science. 2005;46:4417–4423. doi: 10.1167/iovs.05-0883. [DOI] [PubMed] [Google Scholar]
- IMURA A, IWANO A, TOHYAMA O, TSUJI Y, NOZAKI K, HASHIMOTO N, FUJIMORI T, NABESHIMA Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–7. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- KURO-O M, MATSUMURA Y, AIZAWA H, KAWAGUCHI H, SUGA T, UTSUGI T, OHYAMA Y, KURABAYASHI M, KANAME T, KUME E, IWASAKI H, IIDA A, SHIRAKI-IIDA T, NISHIKAWA S, NAGAI R, NABESHIMA YI. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- KUROSU H, OGAWA Y, MIYOSHI M, YAMAMOTO M, NANDI A, ROSENBLATT KP, BAUM MG, SCHIAVI S, HU MC, MOE OW, KURO-O M. Regulation of fibroblast growth factor-23 signaling by Klotho. J Biol Chem. 2006;281:6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUROSU H, YAMAMOTO M, CLARK JD, PASTOR JV, NANDI A, GURNANI P, MCGUINNESS OP, CHIKUDA H, YAMAGUCHI M, KAWAGUCHI H, SHIMOMURA I, TAKAYAMA Y, HERZ J, KAHN CR, ROSENBLATT KP, KURO-O M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACHIEWICZ AM, BERWICK M, WIGGINS CL, THOMAS NE. Epidemiologic Support for Melanoma Heterogeneity Using the Surveillance, Epidemiology, and End Results Program. Journal of Investigative Dermatology. 2008;128:1340–1342. doi: 10.1038/jid.2008.18. [DOI] [PubMed] [Google Scholar]
- LEE J, JEONG DJ, KIM J, LEE S, PARK JH, CHANG B, JUNG SI, YI L, HAN Y, YANG Y, KIM KI, LIM JS, YANG I, JEON S, BAE DH, KIM CJ, LEE MS. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer. 2010;9:109. doi: 10.1186/1476-4598-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU H, FERGUSSON MM, CASTILHO RM, LIU J, CAO L, CHEN J, MALIDE D, ROVIRA II, SCHIMEL D, KUO CJ, GUTKIND JS, HWANG PM, FINKEL T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- LYNCH CJ, SOBO GE, EXTON JH. An endogenous Ca2+-sensitive proteinase converts the hepatic alpha 1-adrenergic receptor to guanine nucleotide-insensitive forms. Biochim Biophys Acta. 1986;885:110–20. doi: 10.1016/0167-4889(86)90045-5. [DOI] [PubMed] [Google Scholar]
- MAMMOTO A, HUANG S, INGBER DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. Journal of Cell Science. 2007;120:456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- MANYA H, INOMATA M, FUJIMORI T, DOHMAE N, SATO Y, TAKIO K, NABESHIMA Y, ENDO T. Klotho protein deficiency leads to overactivation of mu-calpain. J Biol Chem. 2002;277:35503–8. doi: 10.1074/jbc.M206033200. [DOI] [PubMed] [Google Scholar]
- MATSUMURA Y, AIZAWA H, SHIRAKI-IIDA T, NAGAI R, KURO-O M, NABESHIMA Y. Identification of the human Klotho gene and its two transcripts encoding membrane and secreted Klotho protein. Biochem Biophys Res Commun. 1998;242:626–30. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- MIYAGI T, WADA T, YAMAGUCHI K, HATA K. Sialidase and malignancy: a minireview. Glycoconj J. 2004;20:189–98. doi: 10.1023/B:GLYC.0000024250.48506.bf. [DOI] [PubMed] [Google Scholar]
- MIYAGI T, WADA T, YAMAGUCHI K, SHIOZAKI K, SATO I, KAKUGAWA Y, YAMANAMI H, FUJIYA T. Human sialidase as a cancer marker. Proteomics. 2008;8:3303–11. doi: 10.1002/pmic.200800248. [DOI] [PubMed] [Google Scholar]
- NABESHIMA Y. Toward a better understanding of Klotho. Sci Aging Knowledge Environ. 2006;2006:pe11. doi: 10.1126/sageke.2006.8.pe11. [DOI] [PubMed] [Google Scholar]
- O'CONNELL MP, FIORI JL, BAUGHER KM, INDIG FE, FRENCH AD, CAMILLI TC, FRANK BP, EARLEY R, HOEK KS, HASSKAMP JH, ELIAS EG, TAUB DD, BERNIER M, WEERARATNA AT. Wnt5A activates the calpain-mediated cleavage of filamin A. J Invest Dermatol. 2009a;129:1782–9. doi: 10.1038/jid.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONNELL MP, FIORI JL, KERSHNER EK, FRANK BP, INDIG FE, TAUB DD, HOEK KS, WEERARATNA AT. Heparan sulfate proteoglycan modulation of Wnt5A signal transduction in metastatic melanoma cells. J Biol Chem. 2009b;284:28704–12. doi: 10.1074/jbc.M109.028498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUANOUNOU A, ZHANG L, CHARLTON MP, CARLEN PL. Differential modulation of synaptic transmission by calcium chelators in young and aged hippocampal CA1 neurons: evidence for altered calcium homeostasis in aging. J Neurosci. 1999;19:906–15. doi: 10.1523/JNEUROSCI.19-03-00906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMERO PJ, SALAS V, HERNANDEZ C. Calcium pump phosphoenzyme from young and old human red cells. Cell Biol Int. 2002;26:945–9. doi: 10.1006/cbir.2002.0932. [DOI] [PubMed] [Google Scholar]
- TOHYAMA O, IMURA A, IWANO A, FREUND JN, HENRISSAT B, FUJIMORI T, NABESHIMA Y. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem. 2004;279:9777–84. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- UEMURA T, SHIOZAKI K, YAMAGUCHI K, MIYAZAKI S, SATOMI S, KATO K, SAKURABA H, MIYAGI T. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28:1218–29. doi: 10.1038/onc.2008.471. [DOI] [PubMed] [Google Scholar]
- WEERARATNA AT, JIANG Y, HOSTETTER G, ROSENBLATT K, DURAY P, BITTNER M, TRENT JM. Wnt5A signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- WOLF I, LEVANON-COHEN S, BOSE S, LIGUMSKY H, SREDNI B, KANETY H, KURO-O M, KARLAN B, KAUFMAN B, KOEFFLER HP, RUBINEK T. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- WOO MS, OHTA Y, RABINOVITZ I, STOSSEL TP, BLENIS J. Ribosomal S6 Kinase (RSK) Regulates Phosphorylation of Filamin A on an Important Regulatory Site. Molecular And Cellular Biology. 2004;24(7):3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIAO NM, ZHANG YM, ZHENG Q, GU J. Klotho is a serum factor related to human aging. Chin Med J (Engl) 2004;117:742–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression and localization of Syndecan-4 (red) and Wnt5A (green) were examined in M93-047 cells using immunofluorescent confocal microscopy. Treatment of M93-047 cells with recombinant Klotho for 16hrs shows that the decrease in Wnt5A expression in the presence of Klotho correlates with a decrease in the expression of Syndecan-4.
(A) The effect of Klotho on the localization and expression of Filamin A (green) was examined in M93-047 cells. Filamin A shows an irregular, diffuse pattern in the untreated cells, but becomes more regular and relocates to the periphery of the cells as Wnt5A expression (red) is reduced in the presence of Klotho.
(B) G361 cells were treated with 200 nM control (CTRL) or Klotho (KL) siRNA for 48 or 72h. Total RNA was isolated and real-time PCR analysis of Klotho expression was performed. Levels were normalized to 18S expression. (C) Knockdown of Klotho in G361 cells results in Filamin becoming expressed in a more diffuse pattern throughout the cells. (D) M2 cells were either left untransfected (UT) or transfected with either wildtype (WT) or Calpain resistant (CR) Filamin A (FLNA), and then subjected to a transwell invasion assay.