Abstract
Air pollution is a serious environmental problem. Elderly subjects show increased cardiac morbidity and mortality associated with air pollution exposure. Mexico City (MC) residents are chronically exposed to high concentrations of fine particulate matter (PM2.5) and PM-associated lipopolysaccharides (PM-LPS). To test the hypothesis that chronic exposure to urban pollution produces myocardial inflammation, female Balb-c mice age 4 weeks were exposed for 16 months to two distinctly different polluted areas within MC: Southwest (SW) and Northwest (NW). SW mice were given either no treatment or chocolate 2g/9.5 mg polyphenols/3 times per week. Results were compared to mice kept in clean air. Key inflammatory mediator genes: cyclooxygenase-2 (COX-2), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), and the LPS receptor CD14 (cluster of differentiation antigen 14) were measured by real time polymerase chain reaction. Also explored were target NFκB (Nuclear Factor κ B), oxidative stress and antioxidant defense genes.
TNF-α, IL-6, and COX-2 were significantly increased in both NW and SWMC mice (p=0.0001). CD14 was up-regulated in SW mice in keeping with the high exposures to particulate matter associated endotoxin. Chocolate administration resulted in a significant down-regulation of TNF-α (p<0.0001), IL-6 (p=0.01), and IL-1β (p=0.02). The up-regulation of antioxidant enzymes and the down-regulation of potent oxidases, toll-like receptors, and pro-apoptotic signaling genes completed the protective profile.
Exposure to air pollution produces up-regulation of inflammatory myocardial genes and endotoxin plays a key role in the inflammatory response. Regular consumption of dark chocolate may reduce myocardial inflammation and have cardioprotective properties in the setting of air pollution exposures.
Keywords: air pollution, particulate matter, endotoxin, myocardial inflammation, IL-1β, TNF-α, IL-6, CD14, dark chocolate, cardioprotection
Introduction
Air pollution is a complex mixture of particulate matter (PM), gases, and organic compounds present in both outdoor and indoor air. In research concerning the health effects and pathology associated with air pollution, PM-associated effects are focused on PM2.5 (particles with a diameter less than 2.5μm) and ultrafine particles with a diameter less than 0.1μm (UFPM) (Brook, 2008; Grahame & Schlesinger, 2010; Hassing et al., 2009; Mills et al., 2009). The biological mechanisms linking air pollution to cardiovascular inflammation and pathology still remain largely unclear (Brook, 2008; Chahine et al., 2007; Grahame & Schlesinger, 2010; Hassing et al., 2009; Kodavanti et al., 2008; Mills et al., 2008; Ramana et al., 2007; Rich et al., 2006; Scapellato & Lotti, 2007; Simkhovich et al., 2007; Totlandsdal et al., 2008; Wellenius et al., 2007). Epidemiological and mechanistic animal studies from across the world have shown that both acute and chronic exposures to air pollution are associated with cardiovascular pathology. Accompanying air pollution exposure is an increased risk for cardiovascular (CV) events such as myocardial ischaemia and infarctions, heart failure, arrhythmias, and increases in cardiac mortality in the general population as well as in susceptible individuals, including the elderly (Brook, 2008; Chahine et al., 2007; Pope & Dockery, 2006; Rich et al., 2006; Simkhovich et al., 2007).
Three pathways are considered key in explaining the cardiovascular effects of PM: systemic inflammation, alterations in autonomic balance, and direct PM effects upon the pulmonary and systemic vasculature (Brook, 2008). Considerable uncertainty remains concerning the importance of inflammation as a key mechanism in acute cardiopulmonary PM toxicities in susceptible individuals (Scapellato & Lotti, 2007). In the inflammatory myocardial response, active participants include: circulating cells, plasma proteins, endothelial cells, myocardial cells, and extracellular matrices. Equally important in the context of air pollution and PM exposure is particle composition and size as well as the characterization of the soluble and insoluble fractions (Mills et al., 2008; Scapellato & Lotti, 2007). It is well known that PM components such as lipopolysaccharides (LPS) and metals are pivotal players in the production of cytokines and free radical formation, and that ultrafine particles have the ability to produce more severe inflammation than larger size PM (Brook, 2008; Kodavanti et al., 2008; Mills et al., 2008; Scapellato & Lotti, 2007; Totlandsdal et al., 2008). UFPM has a significant inflammatory effect in primary cardiac cells and interleukin-1β (IL-1β) appears to be a critical cytokine in triggering a particle-induced release of interleukin-6 (IL-6) (Totlandsdal et al., 2008).
In the clinical setting of sepsis, LPS is critical for the induction of inflammation and cardiomyopathy (Ramana et al., 2008). Vascular endothelial and smooth muscle cells respond to LPS by interacting with soluble CD14 and a subsequent transcription of inflammatory and immune-response genes via the NFκB pathway. These responsive genes include IL-1β and tumor necrosis factor-α (TNF-α) (Ramana et al., 2008). As was noted in Rozenberg et al. in 2006, severe myocardial dysfunction was exhibited by isolated perfused hearts of senescent rats exposed to relatively low doses of intravenous LPS 12 hours after the LPS treatment. The myocardial impact of chronic low doses of LPS, however—specifically in the context of environmental exposures—is unknown.
Air quality in Mexico City (MC) has been recognized among the worst in the world (Bravo & Torres-Jardón, 2002; Molina et al., 2007). MC residents experience year round exposures to air pollutant concentrations above the Environmental Protection Agency’s (EPA) current National Air Ambient Quality Standards (NAAQS). High concentrations of PM2.5, as well as significant levels of PM associated with LPS (PM-LPS) are present in MC air, and marked differences in the profile of air pollutants have been reported within metropolitan Mexico City (Bravo & Torres-Jardón, 2002; Bonner et al., 1998; Osornio-Vargas et al., 2003; Querol et al. 2008; Rauch et al., 2006; Rosas-Perez et al., 2007).
The search for potentially beneficial drugs useful to ameliorate the CV effects of air pollution represents an enormous clinical challenge. Dark chocolate is rich in polyphenols (McShea et al., 2008) and its administration consistently results in changes in biomarkers related to oxidative stress, lipids, and/or vascular function (Akita et al., 2008; Allen et al., 2008; Bahadorani & Hilliker 2008; Bisson et al., 2008; Flammer et al., 2007; Hermann et al., 2006; Jalil & Ismail 2008; Mursu et al., 2004; Rozan et al., 2007). Chocolate thus may offer some CV protection to susceptible individuals in the setting of severe air pollution.
Given the importance of LPS as a key risk factor for myocardial dysfunction (Marshall et al., 2009; Ramana et al., 2008; Rozenberg et al., 2006) and the significant historical differences in PM-LPS between southwest (SW) and northwest (NW) regions in Mexico City (Bonner et al., 1998; Osornio-Vargas et al., 2003), the goal for this work was to define the myocardial effects of mice lifetime exposures to the polluted atmosphere of two different regions within Mexico City in comparison to mice exposed to clean air. Balb-c female mice were continuously exposed for 16 months starting at 1 month of age to the different environments. In addition, mice exposed to the SW region of MC were given either no treatment or administrated dark chocolate orally. Myocardial mRNA was measured for four key inflammatory genes: cyclooxygenase-2 (COX-2), IL-1β, IL-6, and TNF-α as well as the LPS receptor CD14. Target NFκB, oxidative stress and antioxidant defense genes were also explored.
It was hypothesized that myocardial inflammation in older mice would be significantly higher in those mice exposed to the SWMC atmosphere with high concentrations of endotoxin in comparison to the NWMC mice and clean air controls. In keeping with this hypothesis, it was also expected to see a significant myocardial up-regulation of CD14 in the endotoxin highly exposed cohort, given the role of CD14 as an endotoxin receptor.
The goals of the present study are threefold. First, to determine by real time polymerase chain reaction (RT-PCR) the amount of gene expression of key inflammatory mediators: COX-2, IL-1β, IL-6, TNF-α, and the LPS receptor CD14, in two cohorts of Balb-c mice exposed to the SW and NWMC atmosphere versus mice kept in clean air. Second, to explore key pathways likely involved in the inflammatory myocardial responses associated with urban pollution exposures. In addressing this goal NFκB, oxidative, and antioxidant defense gene PCR arrays were selected. Third, to define if the chronic administration of dark chocolate rich in polyphenols will decrease the myocardial inflammation associated with urban exposures.
The ultimate concern is the human long-term consequences of myocardial inflammation found in association with air pollutant exposures and how to protect susceptible populations particularly the elderly. This information is relevant to the millions of people exposed to polluted urban environments, as well as to household, environmental, and occupational settings with high concentrations of endotoxin.
Materials and Methods
Mice
Four to five week-old female Balb-c mice were purchased from Taconic Laboratories (Germantown, NY). Animals were maintained under specific pathogen-free conditions. The protocol was approved by the Institutional Animal Care and Use Committee from both the University of Montana and the National Institute of Pediatrics (INP) in Mexico City. Animals were housed under Institutional Animal Care and Use Committee-approved conditions in a secured animal facility and were maintained at 20–22 °C on a 12-h light–dark cycle with food and water available ad libitum. Clean air mice were exposed to 100% fresh air distributed through a Heating, Ventilating, and Air Conditioning system (HVAC) and into housing rooms. The non-recirculating air is passed through a 35% coarse mechanical filter (macro-environment of mice). Individual mouse cages (microenvironment) receive room air that diffuses through a HEPA filter in the mouse box lid. Mice housed in the animal facility in Mexico City (SW and NW) were exposed 24/7 to the polluted air in an outdoor/indoor housing room. Mexico City mice were maintained at 20–22 °C on a 12-h light–dark cycle with food and water available ad libitum. Fifty-six mice were divided into 4 groups: i) animals exposed to clean air and receiving no treatment, ii) mice exposed 24/7 to the NWMC atmosphere (Legaria monitoring station), iii) mice exposed 24/7 to the SWMC atmosphere (INP) receiving no treatment, and iv) mice exposed 24/7 to the SWMC atmosphere treated orally with dark chocolate—60% cocoa solids, 2g/9.5mg polyphenols—three times per week. The chocolate used in this study was purchased from D’Vicar, Coyoacán, Mexico City. Animals were maintained under veterinarian care and weights were recorded each month. An average of 10–11 animals were sacrificed per group at 17 months. The SWMC animal facility is located approximately 8.5km downwind from the NWMC facility.
Study city and air quality data
Mexico City (MC) represents an extreme of urban growth and environmental pollution (Bravo-Alvarez & Torres-Jardón, 2002; Molina et al., 2007). Lying in an elevated basin at an altitude of around 2240m above sea level, the city covers an area of approximately 2000 km2 and is surrounded by a series of volcanic and discontinuous mountain ranges that limit the natural ventilation of the basin. The population grew from fewer than 3 million in 1950 to over 18 million in 2000. As a consequence of the increase in population and the associated industrialization, the basin has more than 30,000 industrial facilities and 4 million vehicles with an estimated annual emission of 2.6 million tons of particulate and gaseous air pollutants (Bravo-Alvarez & Torres-Jardón, 2002).
Residents in MC have been chronically exposed to significant concentrations of particulate matter and LPS for the last two decades. PM2.5 concentrations in MC are above the current US NAAQS. Endotoxin (LPS) is a toxic, pro-inflammatory compound that has been detected in outdoor and indoor air and dust in homes and occupational settings (Mueller-Anneling L, et al., 2004). Threshold ambient endotoxin concentrations of 17 EU/m3 are associated with acute airway obstruction in potato-processing workers (Mueller-Anneling L, et al., 2004; Zock JP et al., 1998). Lipopolysaccharides detected in PM10 samples show an average of 17.95ng/mg of PM10 and south Mexico City PM samples show the highest endotoxin concentrations at 59 EU/mg PM10 (Bonner et al., 1998; Osornio-Vargas et al., 2003; Rosas-Pérez et al., 2007). Mexico City has significant sources of environmental endotoxin including daily outdoor deposits of 500 metric tons of animal and human fecal material (Estrada-Garcia et al., 2002).
An important observation for this study is the fact that while SWMC residents are exposed to significant concentrations of PM2.5 and PM-LPS, NWMC residents are exposed to higher concentrations of PM2.5 and PM associated metals (Bonner et al., 1998; Bravo & Torres-Jardón, 2002; Osornio-Vargas et al., 2003; Querol et al. 2008; Rauch et al., 2006; Rosas-Perez et al., 2007). Since elderly people are usually involved in activities around their home, exploring the inflammatory effects of air pollution in two distinctive areas within a megacity is important from a clinical point of view.
Heart dissection
The heart was removed and a representative section of the ventricular wall including the left and right ventricles and the inter-ventricular septum were fixed in 10% neutral formaldehyde for 48h and transferred to 70% alcohol for histopathology. The remaining heart tissues were quickly frozen, saved at −80°C and later processed for the RT-PCR studies. A section of the ventricular wall including the left and right ventricles and the inter-ventricular septum were selected for the RT-PCR studies.
Heart histology
Paraffin sections 8μm thick were cut and stained with hematoxylin eosin (H&E).
Estimation of mRNA abundance by RT-PCR
Total RNA was extracted from frozen hearts using Trizol Reagent (InVitrogen Corp, Carlsbad CA) according to the manufacturer’s instructions. Random-primed first-strand cDNAs were generated using the SuperScript® III First-Strand Synthesis System, InVitrogen, cat# 18080-051. Relative abundances of mRNAs encoding COX-2, IL-1β, TNF-α, IL-6, and CD14 were estimated by quantitative fluorogenic 5′ nuclease (TaqMan) assay of the first strand cDNAs as described (Calderón-Garcidueñas et al., 2004). Primers and fluorophore-labeled TaqMan probes targeting mice were designed using Primer Designer software (Scientific and Educational Software, Durham, NC).
PCR arrays
Microarray analysis was conducted with SABiosciences, Frederick, MD, USA, PCR arrays: oxidative stress and antioxidant defense (PAMM-065), and the NFκB signaling pathway (PAMM-025) following the manufacturer’s instructions using 1μg of total RNA per PCR array. Same amounts of RNA from each sample inside each group were pooled and cDNA was synthesized using the C-03 first strand kit. Relative gene expression was normalized against five housekeeping genes (GUSB, HPRT1, HSP90AB1, GAPDH, and ACTB) in each PCR array plate. The fold change for each gene was calculated as 2^ (ΔΔCt) and shown as upregulated if expression was greater than 2 or down-regulated if expression was less than -2. The myocardial gene expression profile exploring two signaling pathways were compared between the 17 month untreated control mice and matched with SWMC untreated mice and NW untreated mice, and between SW untreated and SWMC chocolate treated mice.
Statistics
Statistics were performed using Stata Statistical software (College Station, TX). To determine whether groups were statistically different, results were compared using the Student’s T-test. Significance was assumed at p<0.05. Data are expressed as mean values ± SD.
Results
Air Quality Data
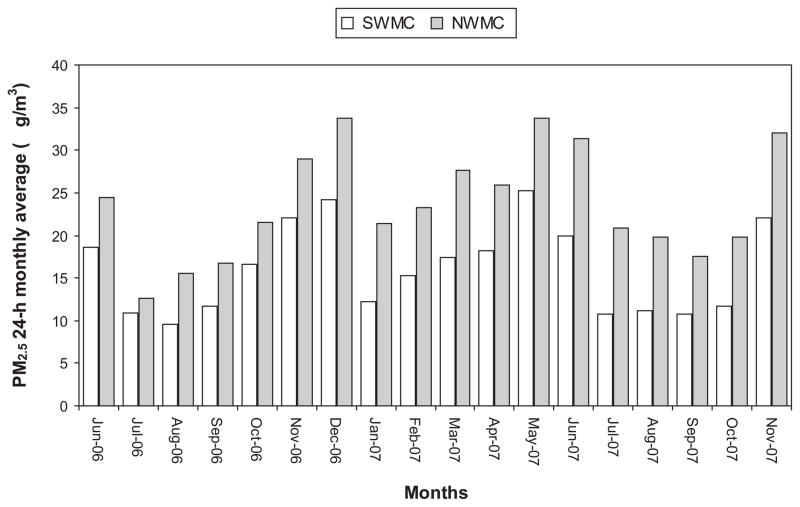
Atmospheric monitoring data from the city monitoring stations <5mi from the SWMC (INP Pedregal) and NWMC (Legaria) animal facilities were accessed (Figure 1). Historical hourly data on most of the criteria air pollutants in the study areas are available from the Government of Mexico City’s Automatic Atmospheric Monitoring Network. Even though the animal facilities were located less than 5 miles from the monitoring sites, air pollutant data for PM2.5 for the SW and NWMC animal facilities were spatially-interpolated from the nearby monitoring stations for the period June 2006 through November 2007. The latter was done in order to account for any existing gradient concentrations and for assuring the representative mice exposure PM2.5 concentration data. A pseudo linear interpolation expression was used for this purpose (US EPA, 1977). On the other hand, given that the PM2.5 monitoring sites have different time and sampling protocols (i.e., automatic monitoring performed continuously versus manual sampling performed each consecutive 6th day) several analysis of correlation were performed between the different stations in order to verify the representativeness of the data and to have an equation to estimate consecutive 24-h average concentrations. Figure 2 shows the monthly mean of 24-h PM2.5 average concentrations in the SW and NWMC obtained from the data interpolation procedure for the study period. In general, the rain season months in MC (July to October) are associated with lower PM2.5 concentration values. On the other hand, 24-h average PM2.5 concentrations as high as 60 μg/m3 were common during the dry season (November to May). Monthly average PM2.5 concentrations were always higher in the NW compared to the SWMC. Table 1 shows a comparison of the basic statistical analysis of the whole PM2.5 data for the animal facilities study areas.
Figure 1.
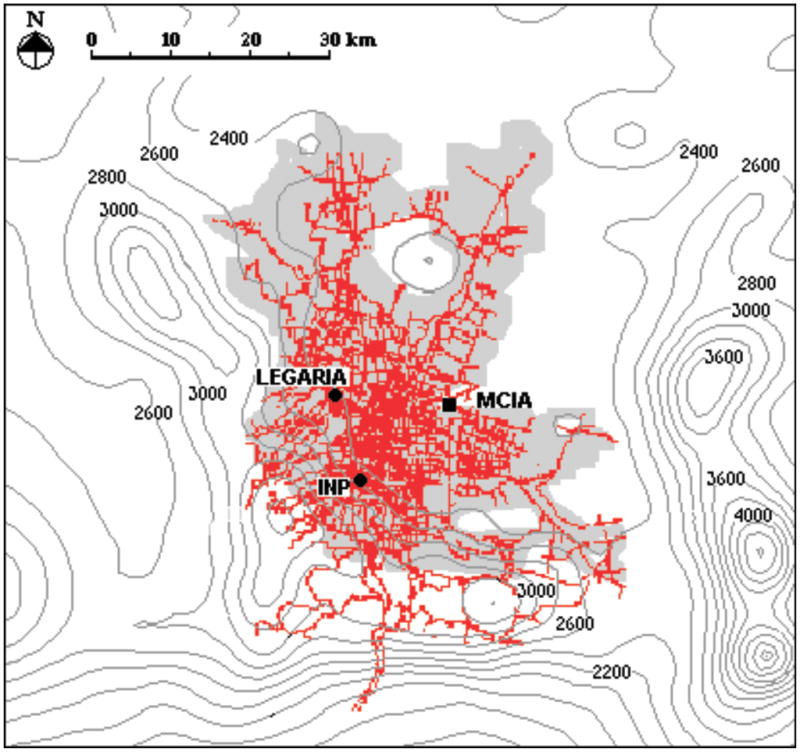
Map showing the Mexico City Metropolitan Area (MCMA) with the location of the southwest Mexico City (INP) and the northwest Mexico City (Legaria) animal facilities. The grey area shows the approximate extent of the MCMA. MCIA represents the Mexico City International Airport as a reference in the map. The red lines show the principal streets and avenues in the city and the dark-gray isolines show the elevation curves in the region.
Figure 2.
The monthly mean 24-h PM2.5 average concentrations for SW and NWMC study areas are shown. Rain season months in MC (July to October) are associated with lower PM2.5 concentration. Twenty-four hour average PM2.5 concentrations as high as 60μg/m3 are common during the dry season (November to May). Monthly average PM2.5 concentrations are always higher in the NW compared to the SW.
Table 1.
Statistical summary of the interpolated 24-h average PM2.5 concentration data for the SWMC and the NWMC animal facilities sites from June 2006 to November 2007.
| PM2.5 concentrations | SWMC INP Pedregal | NWMC Legaria |
|---|---|---|
| Average (μg/m3) | 15.9 | 23.9 |
| Maximum (μg/m3) | 36.0 | 49.4 |
| 95th Percentile (μg/m3) | 33.0 | 40.7 |
| 75th Percentile (μg/m3) | 35.3 | 44.0 |
| Median (μg/m3) | 14.0 | 23.3 |
| Standard deviation (μg/m3) | 7.8 | 9.2 |
| Interpolated days with PM2.5 levels >35 μg/m3 during the whole study perioda | 21 | 60 |
The US EPA 24-h PM2.5 average air quality standard is 35 μg/m3.
Heart histopathology
Examination of the H&E (Hematoxylin-and-Eosin) stained sections from the different cohorts showed no significant differences and specifically there was no evidence of inflammatory aggregates, myocardial necrosis or fibrosis and/or vascular changes.
Real-time PCR mRNA analysis of COX-2, IL-1β, TNF-α, IL-6, and CD14
Real-time, rapid-cycle PCR analysis of COX2, IL1β, IL6, TNF α, and CD14 in the heart samples indicated that the corresponding mRNA was present in each of the samples analyzed. Table 2 illustrates the RT-PCR results expressed as an index where the values of the target genes were normalized to the amount of GAPDH cDNA: molecules per attomole of GAPDH. There was a significant up-regulation of inflammatory genes and the LPS receptor CD14 in the hearts of Mexico City exposed mice compared to controls. Figure 3 illustrates the results of the PCR mRNA analysis with the statistical values (the p value of the SW treated chocolate is compared to the untreated SW group). IL-1β was significantly higher in the SWMC group versus controls (CTL) (p=0.012) and chocolate significantly reduced the IL-1β levels in the SWMC treated mice (p=0.02). While NWMC mice’s IL-1β were not statistically significantly different from control mice (p=0.22), TNF-α was significantly increased in both NW and SWMC mice (p<0.0001) and TNF-α was significantly reduced in the chocolate treated mice (p<0.0001). IL6 was higher in NW mice (p=0.0001), also significantly higher in SW mice (p=0.0002) and decreased expression in the chocolate treated SW animals was significant at p=0.01. CD14 was higher in the SWMC group in comparison to the CTL group (p=0.0003). CD14 was also higher in the NWMC group versus the CTL group (p=0.006). However, chocolate had a border line impact in the SW treated animals (p=0.05). Finally, COX-2 was significantly increased in all Mexico City mice relative to the control regardless of residency or treatment (NW p=0.0004 and SW p< 0.0001). There was no chocolate effect on COX-2 (p=0.78). The response to the orally administered chocolate in the SWMC mice resulted in a significant down-regulation of TNF-α (p<0.0001), IL-6 (p=0.01), and IL-1β (p=0.02).
Table 2.
RT-PCR results of target genes normalized to the amount of GADPH cDNA for Control, SWMC, SWMC chocolate treated and NWMC mice.
| Groups | IL1β | TNF α | IL6 | CD14 | COX2 |
|---|---|---|---|---|---|
| Control n:10 | 223.3±39.9 | 58.2±14.9 | 2.6±0.37 | 450.3±59.2 | 125.7±24.1 |
| SWMC n:10 | 970.4±239.7 | 1542±301.2 | 19.8±5.6 | 2110±491.5 | 581.3±96 |
| SWMC chocolate n:11 | 383.1±65.2 | 272.9±33.5 | 10.5±2.0 | 1069±98.1 | 614.3±64.3 |
| NWMC n:10 | 169.1±24 | 435.4±57.9 | 31.9±9.3 | 975.1±134.5 | 448.6±70.4 |
Figure 3.
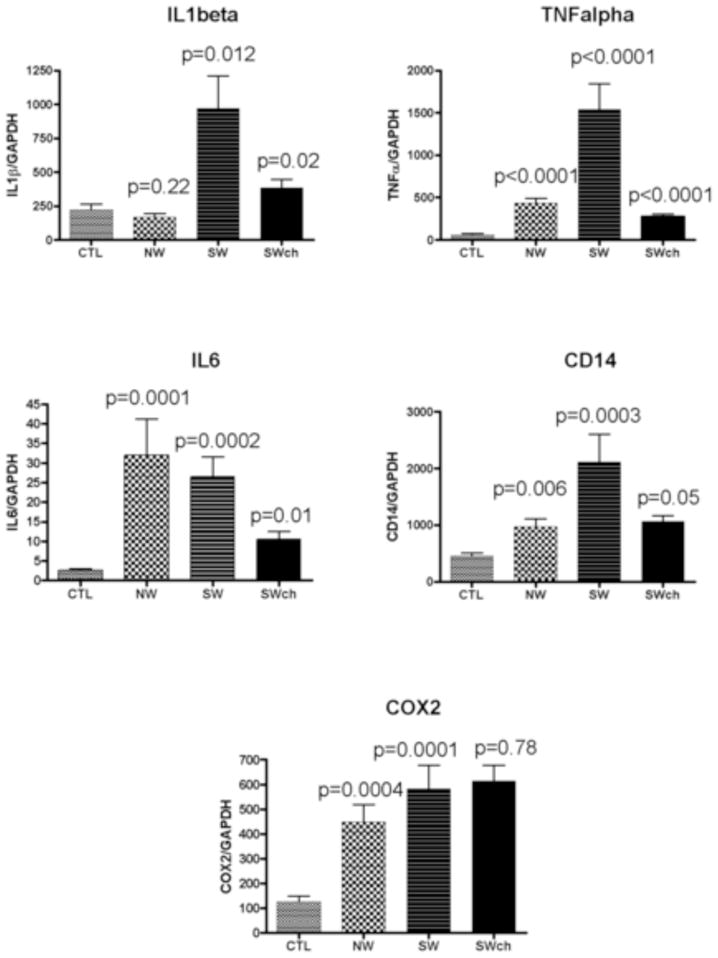
RT-PCR myocardial mRNA statistical results for IL-1β, TNF-α, IL-6, CD14, and COX-2 in Controls, NWMC and SWMC untreated and SWMC chocolate treated mice. The statistical value for the SWMC treated mice is in comparison with the untreated SW group.
PCR Arrays results: Signaling pathways related to Nuclear Factor (NF)-κ B
Of utmost interest was the gene up-regulation in the SWMC mice in comparison to the CTL in terms of apoptosis-related genes (Tnfrsf10b, Fadd, Fos, Casp-8), pro-inflammatory cytokines (TNF, IL-6), components of a cytokine-activated protein complex that is an inhibitor of the essential transcription factor NF-kappa-B complex (Chuk) and proto-oncogenes (Bcl-3) (Table 3). Up-regulation of myocardial genes essential in the regulation of inflammatory responses, oncogenes, and apoptosis was seen along with a dysbalance in the myocardial regulation of genes essential for cell proliferation, differentiation, and apoptosis (Table 3).
Table 3.
A selection of NFκB signaling pathway myocardial genes up and down regulated in Controls versus SW Mexico City mice, SWMC untreated mice versus SWMC chocolate treated mice, and Controls versus NWMC untreated mice
| Controls versus SWMC | ||
|---|---|---|
| Symbol | Gene name | Fold-up or down regulation |
| Bcl3 | B-cell CLL/lymphoma 3 | 22.88 |
| Tnfrsf10b | tumor necrosis factor receptor superfamily, member 10b | 17.24 |
| Chuk | conserved helix-loop-helix ubiquitous kinase | 15.44 |
| Tlr1 | toll-like receptor 1 | 14.89 |
| Fadd | Fas (TNFRSF6)-associated via death domain | 13.52 |
| Tnf | tumor necrosis factor (TNF superfamily, member 2) | 11.50 |
| Nfkb2 | nuclear factor of kappa light polypeptide gene enhancer in B-cells | 11.47 |
| Fos | v-fos FBJ murine osteosarcoma viral oncogene homolog | 9.85 |
| Casp8 | caspase 8, apoptosis-related cysteine peptidase | 9.38 |
| Tlr3 | toll-like receptor 3 | 5.98 |
| Jun | Jun oncogene | −108.57 |
| Slc20a1 | Solute carrier family 20 | −48.89 |
| Mapk3 | Mitogen activated protein kinase 3 | −31.76 |
| Tbk1 | TANK-binding kinase 1 | −22.32 |
| Atf1 | Activating transcription factor 1 | −17.51 |
| C3 | Complement component 3 | −15.96 |
| Stat1 | signal transducer and activator of transcription 1 | −14.61 |
| Tnfsf10 | tumor necrosis factor receptor superfamily, member 10 | −10.44 |
| Atf2 | activating transcription factor 2 | −9.95 |
| Gja1 | gap junction protein, alpha 1 | −8.82 |
| SW untreated mice versus SW chocolate treated mice | ||
|---|---|---|
| Symbol | Gene name | Fold-up or down regulation |
| Ltbr | lymphotoxin beta receptor (TNFR superfamily, member 3) | 2.44 |
| C3 | Complement component 3 | 2.17 |
| Tlr7 | Toll-like receptor 7 | −14.88 |
| Ifng | Interferon gamma | −8.72 |
| IL6 | Interleukin 6 | −8.66 |
| Tlr1 | Toll-like receptor 1 | −7.24 |
| IL1β | Interleukin 1 beta | −6.39 |
| IL1α | Interleukin 1 alpha | −5.96 |
| Tlr6 | Toll-like receptor 6 | −5.96 |
| Tlr8 | Toll-like receptor 8 | −5.30 |
| Fadd | Fas (TNFRSF6)-associated via death domain | −5.05 |
| Tnf | tumor necrosis factor (TNF superfamily, member 2) | −3.90 |
| Casp8 | Caspase 8 | −3.77 |
| Tnfsf14 | tumor necrosis factor (ligand) superfamily, member 14 | −3.52 |
| Controls versus NWMC | ||
|---|---|---|
| Symbol | Gene name | Fold-up or down regulation |
| Bcl3 | B-cell CLL/lymphoma 3 | 116.43 |
| Tlr1 | Toll-like receptor 1 | 77.59 |
| Fadd | Fas (TNFRSF6)-associated via death domain | 72.41 |
| Nlrp12 | NLR family, pyrin domain containing 12 | 65.89 |
| Chuk | conserved helix-loop-helix ubiquitous kinase | 64.23 |
| NFκB2 | nuclear factor of kappa light polypeptide gene enhancer in B-cells | 54.94 |
| IL6 | Interleukin 6 | 53.47 |
| Tnfrsf10b | tumor necrosis factor receptor superfamily, member 10b | 49.39 |
| Casp8 | caspase 8, apoptosis-related cysteine peptidase | 37.45 |
| Tnf | tumor necrosis factor (TNF superfamily, member 2) | 28.73 |
| Fos | v-fos FBJ murine osteosarcoma viral oncogene homolog | 27.82 |
| Myd88 | myeloid differentiation primary response gene | 27.48 |
| Tlr8 | toll-like receptor 8 | 23.32 |
| Ltbr | lymphotoxin beta receptor (TNFR superfamily, member 3) | 16.85 |
| Irak2 | interleukin-1 receptor-associated kinase 2 | 16.55 |
| Ikbke | inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase epsilon | 16.32 |
| Tnfrsf1b | tumor necrosis factor receptor superfamily, member 1B | 15.60 |
| Jun | Jun oncogene | −36.78 |
| Slc20a1 | solute carrier family 20 (phosphate transporter), member 1 | −5.52 |
| Tnfaip3 | tumor necrosis factor, alpha-induced protein 3 | −5.25 |
| C3 | Complement component 3 | −3.79 |
| Tbk1 | TANK-binding kinase 1 | −3.19 |
A significant down-regulation of TLR genes (TLR1, 6, 7, 8), pro-apoptotic genes (Fadd, Casp8) and pro-inflammatory genes (IL-6, IL-1β, IL-1α, TNF) was present in the chocolate treated mice (Table 3). The down-regulation of IL-6, IL-1β and TNF in the chocolate treated mice described in the RT-PCR results was confirmed in the PCR arrays. When the NFκB signaling pathway genes in SW and NW untreated mice versus the control mice were compared, it became clear that there is significant number of genes that are similarly up and down regulated in both groups in keeping with the sharing of air pollutants in both regions. Pro-inflammatory, apoptotic, and oncogenic genes predominate in the Mexico City exposed mice regardless of geographical location (Table 3).
Signaling pathways related to oxidative stress and antioxidant defense
In the untreated SWMC mice, up-regulation of inflammatory mediator genes (IL-19, IL-22, MPO), antioxidant enzymes, and NADPH oxidases (Nox) family of enzymes generating reactive oxygen species (ROS) supports the view that healthy mice under extreme exposure conditions have an imbalance between inflammation and production of ROS and defensive mechanisms against oxidative damage (Dominguez-Rodriguez et al 2009; Gangemi et al., 2009; Kamata, 2009; Polytarchou et al., 2008) (Table 4). The up-regulation of genes such as IL-22, a potential indicator of chronic heart failure progression is likely to further harm the myocardium in these highly exposed animals (Gangemi et al., 2009). Chocolate treated mice had a significant up-regulation of powerful antioxidant enzymes including glutathione peroxidase-3 (Gpx3) and a catalase (Cat) that protects cells from the toxic effects of hydrogen peroxide. ApoE, essential for the normal catabolism of triglyceride-rich lipoprotein constituents, was also up-regulated. Down-regulated genes included: pro-inflammatory genes such as IL-19, and superoxide dysmutases. Up-regulation of xin actin-binding repeat containing 1 gene was also interesting, given its protective actin filament role from depolymerization. Xin has been identified as filamin c binding partner in intercalated discs of the adult heart (van der Ven et al., 2006). In cultured cardiomyocytes, the proteins also localize in the non-striated part of the myofibrils where sarcomeres are assembled, and thus, their potential importance for sarcomere assembly (van der Ven et al., 2006).
Table 4.
A selection of oxidative stress and anti-oxidant defense myocardial genes up and down regulated in Controls versus SW Mexico City, SWMC untreated versus SWMC chocolate treated mice, and Controls versus NWMC untreated mice.
| Controls versus SWMC | ||
|---|---|---|
| Symbol | Gene name | Fold-up or down regulation |
| IL19 | Interleukin 19 | 77.35 |
| Rag 2 | recombination activating gene 2 | 67.54 |
| Mpo | myeloperoxidase | 63.70 |
| IL22 | Interleukin 22 | 45.76 |
| Nox1 | NADPH oxidase 1 | 45.68 |
| Nox 4 | NADPH oxidase 4 | 4.02 |
| Noxo1 | NADPH oxidase organizer 1 | 6.09 |
| Nos 2 | nitric oxide synthase 2, inducible | 4.16 |
| Recq14 | RecQ protein-like 4 | 31.48 |
| Gpx2 | glutathione peroxidase 2 | 28.82 |
| Epx | eosinophil peroxidase | 25.90 |
| Serpin 1b | serpin type 1b | 22.65 |
| Apo E | Apolipoprotein E | −46.60 |
| Gpx 3 | glutathione peroxidase 3 | −25.07 |
| Prdx3 | peroxiredoxin 3 | −9.95 |
| Tmod1 | tropomodulin 1 | −8.40 |
| Cat | catalase | −6.85 |
| Gpx4 | glutathione peroxidase 4 | −4.80 |
| Untreated SW mice versus SWMC chocolate treated | ||
|---|---|---|
| Symbol | Gene name | Fold-up or down regulation |
| Gpx3 | glutathione peroxidase 3 (plasma) | 10.98 |
| APO E | apolipoprotein E | 9.46 |
| Cat | catalase | 5.53 |
| Xirp1 | Cardiomyopathy-associated protein 1 xin actin-binding repeat containing 1 | 2.52 |
| Ngb | Neuroglobin | −20.46 |
| Rag2 | recombination activating gene 2 | −17.99 |
| Lpo | lactoperoxidase | −13.45 |
| IL-19 | Interleukin 19 | −10.37 |
| Gpx7 | glutathione peroxidase 7 | −9.46 |
| Tpo | thyroid peroxidase | −9.34 |
| Nox1 | NADPH oxidase 1 | −8.90 |
| Nox4 | NADPH oxidase 4 | −3.76 |
| Nudt15 | nudix (nucleoside diphosphate linked moiety X)-type motif 15 | −4.80 |
| Prdx1 | Peroxiredoxin 1 | −3.20 |
| Recql4 | RecQ protein 4 | −4.08 |
| Mpo | myeloperoxidase | −8.66 |
| Gpx6 | glutathione peroxidase 6 | −8.25 |
| Aass | alpha-aminoadipate semialdehyde synthase | −6.11 |
| Ccs | copper chaperone for superoxide dismutase | −4.99 |
| Controls versus NWMC mice | ||
|---|---|---|
| Symbol | Gene name | Fold-up or down regulation |
| Rag2 | recombination activating gene 2 | 71.14 |
| Nox1 | NADPH oxidase 1 | 59.97 |
| Tpo | thyroid peroxidase | 58.51 |
| Mpo | myeloperoxidase | 48.99 |
| IL19 | Interleukin 19 | 46.97 |
| Gpx2 | glutathione peroxidase 2 | 45.55 |
| Epx | eosinophil peroxidase | 28.83 |
| Serpin 1b | serpin type 1b | 24.37 |
| Gpx7 | glutathione peroxidase 7 | 20.92 |
| Il22 | Interleukin 22 | 18.86 |
| Sod2 | superoxide dismutase 2, mitochondrial | −14.26 |
| APO E | apolipoprotein E | −7.72 |
| Tmod1 | tropomodulin 1 | −5.05 |
| Zmynd17 | zinc finger, MYND-type containing 17 | −4.94 |
| Gpx3 | glutathione peroxidase 3 | −3.34 |
Discussion
Chronic exposures to urban air pollution produces myocardial up-regulation of inflammatory genes and dark chocolate down-regulates inflammation and increases antioxidant and cardioprotective genes. Key inflammatory cytokines including TNF-α, IL-6, and IL-1β, as well as cyclooxygenase-2 and the LPS receptor CD14 were up-regulated in the exposed healthy 17 month old mice. Significant changes in myocardial genes essential for inflammatory and anti-oxidant responses, ischemic tolerance modulation and apoptosis were present in urban exposed mice.
Pro-inflammatory mediators, such as TNF-α, IL-1β, and COX-2 have been implicated in the pathogenesis of myocardial dysfunction and cardiomyocyte death in ischaemia-reperfusion injury, sepsis, chronic heart failure, viral myocarditis, and cardiac allograft rejection (Cain et al., 1999; Hamid et al., 2009; Schulz & Heusch, 2009). TNF-α was strongly up-regulated in exposed urban mice. The importance of TNF in cardiovascular morbidity and mortality is well known (Hamid et al., 2009; Schulz & Heusch, 2009). In a healthy heart TNF-α is mainly located in the endothelium and in resident mast cells (Schulz & Heusch, 2009). Apoptosis, inflammation, and oxidative stress are pivotal TNF-mediated responses that are independently linked to pathological remodeling (Hamid et al., 2009). During myocardial ischaemia and promptly (within minutes) after myocardial infarction (MI), preformed TNF-α is released and contributes to both contractile dysfunction and irreversible myocardial injury (Schulz & Heusch, 2009). Interestingly, however, preconditioning with TNF-α reduces infarct size (Schulz, 2008). The issue of TNF-α cardio-protection basically depends on its concentration, the localization of increased TNF-α, the concentrations of the TNF receptors particularly TNFR1, and the myocardial duration of exposures to detrimental factors (Schulz & Heusch, 2009). In an infarcted myocardium, TNF-α contributes to cardiomyocyte apoptosis, yet in the peri-infarct area it possibly stimulates fibroblasts, stabilizes the infarcted area, and attracts stem cells for cardiac repair and a decrease in inflammation (Bao et al., 2008; Chen et al., 2003; Gurantz et al., 2005). Given that TNF-α has an ambivalent role in case of MI and that sustained post-infarction TNF-α contributes to chronic left ventricular dysfunction, it was hypothesized that myocardial TNF-α up-regulation in the context of severe exposures to air pollution is not beneficial to the exposed subject.
The significant increase in CD14 mRNA in MC mice, particularly in SWMC mice is a very important finding. CD14, is a surface differentiation antigen capable of binding to LPS (Panaro et al., 2008) and the high expression seen in the SW region mice is likely related to the historically high concentrations of LPS detected in SWMC PM10 samples (Bonner et al., 1998; Osornio-Vargas et al., 2003; Rosas-Pérez et al., 2007). The toxic effects of LPS include the release of cytokines, nitric oxide, and reactive oxygen species (ROS) by vascular endothelial cells (Fitzgerald et al., 2004; Pinksy 2007; Rudiger & Singer 2007; Tamion et al., 2008). In keeping with the CD14 up-regulation, SWMC mice also exhibited a significant up-regulation of potent oxidases—some of which play a role in apoptosis and lipopolysaccharide-mediated activation of NFκB. LPS-induced cardiac dysfunction may be in part due to reactive oxygen species mediated by inflammatory mediators like TNF-α (Kumar et al., 2000; Meldrum 1998; Nakamura et al., 1998; Rudiger & Singer 2007; Tamion et al., 2008). LPS acts through the CD14 receptor to release TNF-α, deregulates the intracellular calcium, and gives rise to the apoptotic death program (Comstock et al., 1998). LPS internalization depends on CD14, as demonstrated in 2008 by Panaro et al. in an in vitro model of chick embryo myocardiocytes exposed to endotoxin. The production of pro-inflammatory mediators occurs in the myocardium exposed to endotoxin, a situation that is critical in septic patients. In a model of low-grade chronic inflammation with the administration of low doses of LPS, there is a significant increase in myocardial fibrous tissue, infiltration of mononuclear cells, and changes in arteries and arterioles—a finding consistent with vascular disease (Smith et al., 2009). Given that historical concentrations of PM-LPS are higher in SWMC, the observation of a significant up-regulation of CD14, TNF-α, and IL-1β and of potent oxidant enzymes suggests a key role of environmental endotoxin on the health of the SWMC populace.
The issue is key for the understanding of how the sensing of “microbial invaders” (i.e., PM-LPS) could translate into signaling pathways that culminate in the transcriptional regulation of immune responsive genes and how the dysregulation of inflammasomes (Bryant & Fitzgerald 2009; Lamkanfi & Dixit, 2009) could be a contributing factor for myocardial damage in exposed populations. The innate immune system rapidly detects invading pathogenic microbes and eliminates them. A potential scenario in SWMC residents could include PM-LPS being detected as foreign and consequent inflammatory responses launched with detrimental effects. Toll-like receptors sense extra-cellular microbes (i.e., PM-LPS) and trigger anti-pathogen signaling cascades (Martinon et al., 2009). Heart tissues require up-regulation of several inflammasome components in order to assemble functional inflammasomes (Yin et al., 2009). Although the issue of myocardial inflammasomes has not yet been discussed in air pollution related damage, this study’s findings of increased IL-1β and the significant up-regulation of TLRs strongly suggests that inflammasome activation could be playing a role in exposed subjects. Thus, these observations in SW mice are potentially important for elderly SW residents due to the fact that IL-1β reduces cardiac muscle function and inhibits angiogenesis in cardiac endothelial cells (Mountain et al., 2008). An increase in myocardial IL-1β could translate into a reduced capacity for proliferation of microvascular endothelial cells and thus a fault in the myocardial repair after a heart attack. IL-1β is also important to myocardial remodeling (Hwang et al., 2001), and its increased levels are associated with a worsening of interstitial fibrosis (Ono et al., 1998). The role of IL-1β in atherothrombotic disease and following myocardial infarction when it critically regulates the inflammatory response (Bujak & Frangogiannis, 2009) is also potentially clinically important for urban residents. UFPM exposure of mono and co-cultures of primary cardiac cells has shown a pro-inflammatory effect involving both IL-1β and IL-6 (Totlandsdal et al., 2008). The lack of an IL-1β myocardial response in NWMC mice could be potentially related to inflammasome responses (Bryant & Fitzgerald, 2009), an issue that ought to be explored in human myocardium.
COX-2—the enzyme responsible for catalyzing the rate-limiting step in converting arachidonic acid to prostaglandin H2—was up-regulated in all Mexico City mice. The role of COX-2 in heart disease has been an intense subject of study ever since selective COX-2 inhibitors were approved (Zarraga & Schwarz, 2007). Several studies suggests that COX-2 mediate the cardioprotective effects of the late phase of ischaemic preconditioning and that PGE2 and PGI2 are the likely effectors of such protection (Birnbaum et al., 2005; Shinmura et al., 2000). Increased angiogenesis is also a cardioprotective factor accounting for a positive COX-2 cardiovascular effect (Zarraga & Schwarz, 2007). Thus, COX-2 is seen overall as a good player and its up-regulation related to air pollution exposure could be seen as a compensatory protective mechanism.
Exposed mice are up-regulating myocardial genes essential for inflammatory responses and apoptosis. Given that apoptosis is an important contributing factor in the loss and damage of cardiomyocytes when the myocardium is subjected to acute anoxia or ischaemic injury, the up-regulation of apoptotic genes in the scenario of air pollution becomes very relevant to myocardial pathology. Moreover, there is a imbalance in the myocardial regulation of genes such as those that are major components of the transcription factor complex AP-1 (ie. Jun and Fos) regulating the expression of genes essential for cell proliferation, differentiation, and apoptosis. Overt expression of Jun and Fos in cardiomyocytes reduces alpha myosin heavy chain expression and contributes to heart failure from chronic volume overload (Freire et al., 2007). Compensatory effects are seen in the highly exposed mice, including down-regulation of MAPK signaling cascade players.
The chronic administration of dark chocolate had expected positive myocardial responses including a significant down-regulation of inflammatory genes: TNF, IL-6, IL-1β and Toll-like receptors fundamental in pathogen recognition and activation of innate immunity. The up-regulation of antioxidant enzymes and the down-regulation of potent oxidases and pro-apoptotic signaling genes completed the protective profile. This study’s findings suggest that regular consumption of dark chocolate with high concentrations of polyphenols may reduce myocardial inflammation and have cardioprotective properties in the setting of air pollution exposures.
Chronic inflammation leads to an increase of cardiovascular disease. Findings in this study’s analyses of an association between myocardial up-regulation of apoptotic, inflammatory, microbial sensor, and oxidative genes and urban exposure provide potentially important mechanistic pathways to explain the higher risk of CV disease in susceptible urban populations. These findings are relevant to humans residing in the study areas, since the outcome of cardiac ischaemic events depends not only on the intensity and duration of the ischaemic stimulus but also on the myocardial intrinsic tolerance to ischaemic injury (Golomb et al., 2009), even in the absence of overt cardiovascular disease. Thus, the novel concept of occult cardiotoxicity as described by Golomb et al., in 2009 could be of vital importance in subjects exposed to significant concentrations of air pollutants.
In summary, exposure to air pollution produces myocardial up-regulation of key inflammatory genes and a significant imbalance in genes essential for cell proliferation, differentiation, and apoptosis. Hopefully this research will contribute to: i) awareness that within the same city detrimental cardiac effects depend on specific pollutants, ii) up-regulation of Toll-like receptor genes, fundamental in pathogen recognition and activation of innate immunity, likely play a key role in the myocardial inflammatory responses, and iii) chocolate has a protective effect.
Acknowledgments
This work was part of Rodolfo Villarreal’s high school independent study research science program at Big Sky High School in Missoula, Montana. The program directors are Mr. James Harkins and Mr. Brandon Honzel, to whom we are very grateful for their genuine support and helping of young scientists’ education.
We deeply appreciate Dr. Robert R. Maronpot’s critical review of the paper and the technical support of Norma Osnaya and Silvia Monroy from the INP. This work was supported in part by the NCRR Grant # P20RRO15583 and was presented in part at the 7th European Congress of Toxicological Pathology on September 16–18, 2009, in The Hague, The Netherlands as well as at the 2009 American Thoracic Society International Conference in San Diego, CA, by Rodolfo Villarreal-Calderon who received an ATS Minority Trainee Travel Award therein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RR, Carson L, Kwik-Uribe C, Evans EM, Erdman JW., Jr Daily consumption of a dark chocolate containing flavonols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. J Nutr. 2008;138:725–731. doi: 10.1093/jn/138.4.725. [DOI] [PubMed] [Google Scholar]
- Akita M, Kuwahara M, Itoh F, Nakano Y, Osakabe N, Kurosawa T, Tsubone H. Effects of cacao liquor polyphenols on cardiovascular and autonomic nervous functions in hypercholesterolemic rabbits. Bas Clin Pharmacol Toxicol. 2008;103:581–587. doi: 10.1111/j.1742-7843.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- Bahadorani S, Hilliker AJ. Cocoa confers life span extension in Drosophila melanogaster. Nutr Res. 2008;28:377–382. doi: 10.1016/j.nutres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Bao C, Guo J, Lin G, Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand Cardiovasc. 2008;42:56–62. doi: 10.1080/14017430701543556. [DOI] [PubMed] [Google Scholar]
- Bisson JF, Nejdi A, Rozan P, Hidalgo S, Lalonde R, Messaoudi M. Effects of long term administration of a cocoa polyphenolic extract (Acticoa powder) on cognitive performances in aged rats. Br J Nutr. 2008;100:94–101. doi: 10.1017/S0007114507886375. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Ye Y, Rosanio S, Tavackoli S, Hu ZY, Schwarz ER, Uretsky BF. Prostaglandin mediate the cardioprotective effects of atorvastin against ischemia-reperfusion injury. Cardiovasc Res. 2005;65:345–355. doi: 10.1016/j.cardiores.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Rice AB, Lindroos PM, O’Brien PO, Dreher KL, Rosas I, Alfaro-Moreno E, Osornio-Vargas AR. Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from Mexico City. American Journal of Respiratory Cell and Molecular Biology. 1998;19:672–680. doi: 10.1165/ajrcmb.19.4.3176. [DOI] [PubMed] [Google Scholar]
- Bravo HA, Torres RJ. Air Pollution levels and trends in the Mexico City metropolitan area. In: Fenn M, Bauer L, Hernández T, editors. Urban air pollution and forests: resources at risk in the Mexico City Air Basin. New York: Springer-Verlag; 2002. pp. 121–59. [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clinical Science. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp. 2009;57:165–176. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, Harken AH. Tumor necrosis factor-alpha and interleukin-1-beta synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–1318. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Reed W, Maronpot RR, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas P, Schwartz J. Particulate air pollution, oxidative stress genes and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ke Q, Yang Y, Rana JS, Tang J, Morgan JP, Xiao YF. Cardiomyocytes overexpressing TNF-alpha attract migration of embryonic stem cells via activation of p38 and c-Jun amino-terminal kinase. FASEB. 2003;17:2231–2239. doi: 10.1096/fj.03-0030com. [DOI] [PubMed] [Google Scholar]
- Comstock KL, Krown KA, Page MT. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol. 1998;30:2761–2775. doi: 10.1006/jmcc.1998.0851. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalez P, Kaski JC. Diurnal variation of circulating myeloperoxidase levels in patients with ST-segment elevation myocardial infarction. Int J Cardiol. 2009 April 1; doi: 10.1016/j.ijcard.2009.03.040. E Pub. [DOI] [PubMed] [Google Scholar]
- Estrada-Garcia T, Cerna JF, Thompson MR, López-Saucedo C. Fecal contamination and enterotoxigenic Escherichia coli in street-vended chili sauces in Mexico and its public relevance. Epidemiol Infect. 2002;129:223–226. doi: 10.1017/s0950268802007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004;6:1361–1367. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, Serafini M, Lüscher TF, Ruschitzka F, Noll G, Corti R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–2382. doi: 10.1161/CIRCULATIONAHA.107.713867. [DOI] [PubMed] [Google Scholar]
- Freire G, Ocampo C, Ilbawi N, Griffin AJ, Gupta M. Overt expression of AP-1 reduces alpha myosin heavy chain expression and contributes to heart failure from chronic volume overload. J Mol Cell Cardiol. 2007;43:465–478. doi: 10.1016/j.yjmcc.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Gangemi S, Parisi P, Ricciardi L, Saitta S, Minciullo PL, Cristani MT, Nicita-Mauro V, Saija A, Basile G. Is Interleukin-22 a possible indicator of chronic heart failure’s progression? Arch Gerontology Geriatrics. 2009 Jun 10; doi: 10.1016/j.archger.2009.05.003. E-pub. [DOI] [PubMed] [Google Scholar]
- Golomb E, Nyska A, Schwalb H. Occult cardiotoxicity-toxic effects on cardiac ischemic tolerance. Toxicol Pathol. 2009;37:572–593. doi: 10.1177/0192623309339503. [DOI] [PubMed] [Google Scholar]
- Grahame TJ, Schlesinger RB. Cardiovascular health and particulate vehicular emissions: a critical evaluation of the evidence. Air Qual Atmos Health. 2010;3:3–27. doi: 10.1007/s11869-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurantz D, Cowling RT, Varki N, Frikovsky E, Moore CD, Greenberg BH. IL-1β and TNF-α upregulate angiotensin II type type 1 (AT1) receptors on cardiac fibroblasts and are associated with increased AT1 density in the post-MI heart. J Mol Cell Cardiol. 2005;38:505–515. doi: 10.1016/j.yjmcc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor kappa B and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassing HC, Twickler TB, Kastelein JJ, Cramer MJ, Cassee FR. Air pollution as noxious environmental factor in the development of cardiovascular disease. Neth J Med. 2009;67:116–121. [PubMed] [Google Scholar]
- Hermann F, Spieker LE, Ruschitzka F, Sudano I, Binggeli C, Lüscher TF, Riesen W, Noll G, Corti R. Dark chocolate improves endothelial and platelet function. Heart. 2006;92:119–120. doi: 10.1136/hrt.2005.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang MW, Matsumori A, Furukawa Y, Ono Y, Okada M, Iwasaki A, Hara M, Miyamoto T, Touma M, Sasayama S. Neutralization of IL1β in the acute phase of myocardial infarction promotes the promotion of progression of left ventricular remodeling. Journal of American College of Cardiology. 2001;38:1546–1553. doi: 10.1016/s0735-1097(01)01591-1. [DOI] [PubMed] [Google Scholar]
- Jalil AM, Ismail A. Polyphenols in cocoa and cocoa products: is there a link between antioxidant properties and health? Molecules. 2008;13:2190–2219. doi: 10.3390/molecules13092190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009 May 4; doi: 10.1111/j.1349-7006.2009.01207.x. E Pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Gilmour PS, Wallenborn JG, Mandavilli BS, Ledbetter AD, et al. The role of particulate matter-associated zinc in cardiac injury in rats. Environ Health Perspect. 2008;116:13–20. doi: 10.1289/ehp.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. Crit Care Clin. 2000;16:251. doi: 10.1016/s0749-0704(05)70110-x. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- Marshall M, Anilkumar N, Layland J, Walker SJ, Kentish JC, Shah AM, Cave AC. Protein phosphatase A contributes to cardiac dysfunction induced by endotoxemia. Cardiovasc Res. 2009;82:67–76. doi: 10.1093/cvr/cvp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- McShea A, Ramiro-Puig E, Munro SB, Casadesus G, Castell M, Smith MA. Clinical benefit and preservation of flavonols in dark chocolate manufacturing. Nutrition Rev. 2008;66:630–641. doi: 10.1111/j.1753-4887.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandstorm T, Bloomberg A, Newby DE. Adverse cardiovascular effects of air pollution. Nature Clin Practice. 2009;6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- Molina LT, Kolb CE, de Foy B, Lamb BK, Brune WH, Jiménez JL, Ramos-Villegas R, Sarmiento J, Paramo-Figueroa VH, Cardenas B, Gutierrez-Avedoy V, Molina MJ. Air quality in North America’s most populous city overview of the MCMA-2003 campaign. Atmos Chem Phys. 2007;7:2447–2473. [Google Scholar]
- Mountain DJH, Singh M, Singh K. Interleukin-1β-mediated inhibition of the processes of angiogenesis in cardiac microvascular endothelial cells. Life Sciences. 2008;82:1224–1230. doi: 10.1016/j.lfs.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Anneling L, Avol E, Peters JM, Thorne PS. Ambient endotoxin concentrations in PM10 from Southern California. Environ Health Perspect. 2004;112:583–588. doi: 10.1289/ehp.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursu J, Voutilaihen S, Nurmi T, Rissanen TH, Virtanen JK, Kaikkonen J, Nyyssönen K, Salonen JT. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Rad Biol Med. 2004;37:1351–1359. doi: 10.1016/j.freeradbiomed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Fushimi K, Kouchi H, et al. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-a and angiotensin II. Circulation. 1998;98:794–780. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayuma S. Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation. 1998;98:149–156. doi: 10.1161/01.cir.98.2.149. [DOI] [PubMed] [Google Scholar]
- Osornio-Vargas AR, Bonner JC, Alfaro-Moreno E, Martinez L, Garcia-Cuellar C, Ponce-de-Leon-Rosales S, Miranda J, Rosas I. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect. 2003;111:1289–93. doi: 10.1289/ehp.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaro MA, Cianciulli A, Gagliardi N, Mitolo I, Acquafredda A, Cavallo P, Mitolo V. CD14 major role during lipopolysacchride-induced inflammation in chick embryo cardiomyocytes. Federation European Microbiological Societies Immunol Med Microbiology. 2008;53:35–45. doi: 10.1111/j.1574-695X.2008.00397.x. [DOI] [PubMed] [Google Scholar]
- Pinksy MR. Sepsis and multiple organ failure. Contrib Nephrol. 2007;156:47–63. doi: 10.1159/000102070. [DOI] [PubMed] [Google Scholar]
- Polytarchou C, Pfau R, Hatziapostolou M, Tsichlis PN. The JmjC domain histone demethylase Ndy1 regulates redox homeostasis and protects cells from oxidative stress. Mol Cell Biol. 2008;28:7451–7464. doi: 10.1128/MCB.00688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Dockery DW. Health Effects of Fine Particulate Air Pollution: Lines that Connect. Journal of the Air & Waste Management Association. 2006;26:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Querol X, Pey J, Minguillón MC, Pérez N, Alastuey A, Viana M, Moreno T, Bernabé RM, Blanco S, Cárdenas B, Vega E, Sosa G, Escalona S, Ruiz H, Artíñano B. PM speciation and sources in Mexico during the MILAGRO-2006 Campaign. Atmos Chem Phys. 2008;8:111–128. [Google Scholar]
- Ramana KV, Willis MS, White MD, Horton JW, DiMaio M, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2007;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- Rauch S, Peucker-Ehrenbrink B, Molina LT, Molina MJ, Ramos R, Hemond HF. Platinum group elements in airborne particles in Mexico City. Environ Sci Technol. 2006;40:7554–7560. doi: 10.1021/es061470h. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttman-Gibson H, Catalano PJ, Speizer FE, Gold DR, Dockery DW. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006;114:120–123. doi: 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Pérez I, Serrano J, Alfaro-Moreno E, Baumgardner D, Garcia-Cuellar C, Miranda J, Raga GB, Castillejos M, Drucker-Colin R, Osornio-Vargas AR. Relations between PM10 composition and cell toxicity: A multivariate and graphical Approach. Chemosphere. 2007;67:1218–1228. doi: 10.1016/j.chemosphere.2006.10.078. [DOI] [PubMed] [Google Scholar]
- Rozan P, Hidalgo S, Nejdi A, Bisson JF, Lalonde R, Messaoudi M. Preventive antioxidant effects of cocoa polyphenolic extract on free radical production and cognitive performance after heat exposure in Winstar rats. J Food Sci. 2007;72:S203–S206. doi: 10.1111/j.1750-3841.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- Rozenberg S, Besse S, Brisson H, Jozefowicz E, Kandoussi A, Mebazaa A, Riou B, Vallet B, Tavernier B. Endotoxin-induced myocardial dysfunction in senescent rats. Crit Care. 2006;10:R124. doi: 10.1186/cc5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599–1604. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- Scapellato ML, Lotti M. Short-Term Effects of Particulate Matter: An Inflammatory Mechanism? Critical Reviews in Toxicology. 2007;37:461–487. doi: 10.1080/10408440701385622. [DOI] [PubMed] [Google Scholar]
- Schulz R, Heusch G. Tumor necrosis factor-α and its receptors 1 and 2. Circulation. 2009;119:1355–1357. doi: 10.1161/CIRCULATIONAHA.108.846105. [DOI] [PubMed] [Google Scholar]
- Schulz R. TNF α in myocardial ischemia/reperfusion: damage vs protection. J Mol Cell Cardiol. 2008;45:712–714. doi: 10.1016/j.yjmcc.2008.09.119. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA. 2000;97:10917–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkhovich BZ, Marjoram P, Kleinman MT, Kloner RA. Direct and acute cardiotoxicity of ultrafine particles in young adult and old rat hearts. Basic Res Cardiol. 2007;102:467–475. doi: 10.1007/s00395-007-0681-0. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Lightfoot SA, Lerner MR, Denson KD, Morgan DL, Hanas JS, Bronze MS, Postier RG, Brackett DJ. Induction of cardiovascular pathology in a novel model of low-grade chronic inflammation. Cardiovascular Pathology. 2009;18:1–10. doi: 10.1016/j.carpath.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Tamion F, Bauer F, Richard V, Laude K, Renet S, Slama M, Thuillez C. Myocardial dysfunction in early state of endotoxemia. Role of Heme-Oxygenase-1. J Surg Res. 2008 Oct 24; doi: 10.1016/j.jss.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Totlandsdal AI, Skomedal T, Låg M, Osnes IB, Refsnes M. Pro-inflammatory potential of ultrafine particles in mono and co-cultures of primary cardiac cells. Toxicology. 2008;247:23–32. doi: 10.1016/j.tox.2008.01.019. [DOI] [PubMed] [Google Scholar]
- US EPA. EPA-450/2-77-024a. U.S. Environmental Protection Agency; Research Triangle Park, NC 27711: Guideline on Procedures for Constructing Air Pollution Air Pollution Isopleth Profiles and Population Exposure Analysis. [Google Scholar]
- van der Ven PF, Ehler E, Vakeel P, Eulitz S, Schenk JA, Milting H, Micheel B, Fürst DO. Unusual splicing events result in distinct Xin isoforms that associate differentially with filamin c and Mena/VASP. Exp Cell Res. 2006;312:2154–2167. doi: 10.1016/j.yexcr.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Yeh GY, Coull BA, Suh HH, Phillips RS, Mittleman MA. Effects of ambient air pollution on functional status in patients with chronic congestive heart failure: a repeated-measures study. Environmental Health. 2007;6:26–32. doi: 10.1186/1476-069X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarraga IGE, Schwarz ER. COXIBS and heart disease. J Am College Cardiology. 2007;49:1–14. doi: 10.1016/j.jacc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Zock JP, Hollander A, Heederik D, Douwes J. Acute lung function changes and low endotoxin exposures in the potato processing industry. Am J Ind Med. 1998;33:384–391. doi: 10.1002/(sici)1097-0274(199804)33:4<384::aid-ajim9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]