Abstract
Pancreatic cancer is considered an aggressive malignancy that responds poorly to current treatments and therefore has a dismal survival rate. This disease is usually not diagnosed until a late stage, at which point palliative chemotherapy with the purine analogue gemcitabine and/or a fluoropyrimidine or a platinum agent is the standard approach. There are some new data on the molecular and genetic changes that take place in pancreatic cancer, which may facilitate the accuracy of diagnosis and efficacy of treatments. However, translational efforts in clinical practice have increased clinicians' options with a targeted agent, erlotinib, in combination with the standard gemcitabine chemotherapy. Many other novel drugs currently being tested in the field of pharmaco-oncology target various altered biological pathways and molecules. Nevertheless, the lack of clinically significant improvements in treatments is rendering efforts to develop methods of early diagnosis both more urgent and promising. The aim of this review was to summarize the molecular basis of pancreatic carcinogenesis and the latest developments in diagnosis by molecular means, focusing on the results of clinical research into targeted and personalized treatments.
Keywords: Pancreatic ductal carcinoma, Molecular targets, Pharmacogenetics, Novel agents
INTRODUCTION
Pancreatic cancer (PC) is the 4th commonest cause of cancer related deaths according to statistics for 2008 by the American Cancer Society. The mortality rate of pancreatic cancer is very high (99%) and the 5-year survival rate for all stages equal or less to 5%. The incidence of this lethal disease is fortunately much lower, representing only 2% of all cancers (10th commonest cause) in United States and rather the same in the rest of western world.
There are few risk factors that have been identified in the sporadic form of pancreatic cancer which accounts for the 90% of all cases (genetic syndromes are accountable for the rest 10%). Such risk factors are cigarette smoking, age >55 years, obesity, lack of exercise, male gender and possibly but less certainly chronic pancreatitis and diabetes type II.1 The fact that most of the above factors have showed an increasing tendency during the last decades may explain why the mortality rate is not slowing down despite improvements in treatment.
The gold standard treatment for early stage pancreatic cancer is radical surgery (Whipple's operation) which is actually the sole curative option in this aggressive tumor. Chemotherapy can be used as adjuvant to surgery or in advanced stage pancreatic cancer where, in a small group of patients, it offers real benefit in terms of survival and quality of life. In addition, radiotherapy may offer in selected cases local control in advanced nonmetastatic disease when surgery is either not feasible or incomplete.
Due to poor results of the conventional treatments, a labor effort in translational science is taking place over the last decade aiming to an earlier diagnosis and a more effective treatment. Below, we will focus on the aberrant biological pathways involved in the pathogenesis of pancreatic cancer and the deranged molecules or genes that are attracting diagnostic or therapeutic interest. Finally, we will present the current status of novel treatments produced in drug development units which may allow applying a more rational patient's management.
GENETIC AND MOLECULAR BACKGROUND OF PANCREATIC CANCER
There are many different histological subtypes of pancreatic cancer, with variable natural history, management and outcome. Pancreatic ductal adenocarcinoma (PDAC) is the commonest subtype followed by cystic neoplasms (serous cystadenocarcinoma, intraductal papillary mucinous neoplasm-IPMN), neuroendocrine tumors, sarcoma, acinar cell carcinoma and lymphoma. Though there is evidence that PDAC may also develop on the background of mucinous neoplasms (IPMN or mucinous cystic), we will not deal with the molecular aspects of those rather rare cases in this review. In the majority of published works, the term pancreatic cancer refers exclusively to PDAC.
The carcinogenesis of pancreatic neoplasms entails transformation of a normal cell to a benign or premalignant cell, as those seen in pancreatic intraepithelial neoplasia (PanIN). Various genetic mutations, progressive nuclear alterations, such as increasing atypia and loss of polarity, as well as morphological cellular changes do occur and mount up during the malignant process from the early PanIN1to the more advanced PanIN3 or carcinoma in situ and finally pancreatic cancer.2,3
Therefore, the observed genetic mutations in this disease involve the oncogenes KRAS in the majority of cases (74-100%), HER-2/neu (in about 65%), notch1, Akt-2 and COX-2, and the tumor suppressor genes p16INK4a (in up to 98%), p53 (43 to 76%), DPC4 (about 50%), FHIT (found in 70% of cases) and BRCA2 in familial cases.4-10
Apart from single genetic changes there are specific chromosomal abnormalities involved in pancreatic carcinogenesis. Thus, we may see allelic loss mainly in chromosomes 17p (95%), 18q (88%), 9p (76%), 12q (67%) and less often in 1p, 6p, 6q, 8p, 10p, 10q, 12p, 21q, and 22q (from 50% to 60%). There are also cases where chromosomal additions do happen, such as in chromosomes 7 and 20.11 What might happen in reality is a mixture of chromosomal and genetic changes as many tumor suppressor genes are positioned in the aforementioned locations for example p53 at chromosome 17p, DPC4 gene at chromosome 18q and p16INK4a (MTS1) gene at chromosome 9p.
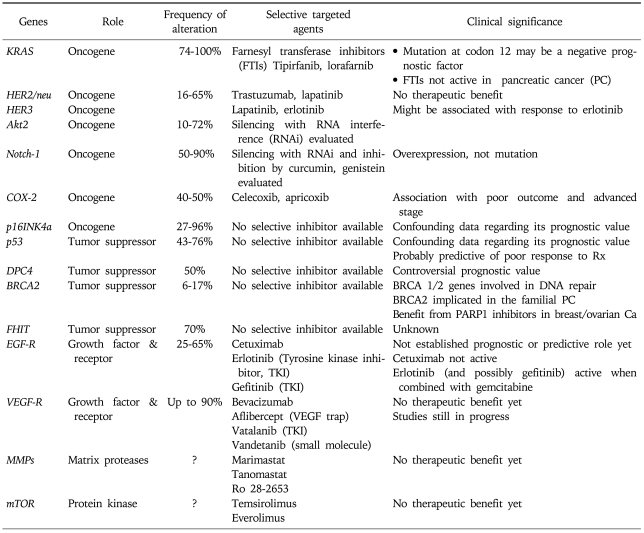
1. Altered genes and clinical significance (Table 1)
Table 1.
Most Common Molecular Alterations in Pancreatic Cancer and Applicable Targeted Agents
1) KRAS
The Kirsten Rat sarcoma virus proto-oncogene (KRAS) is found in chromosome 12p at the position 12.1. The significance of RAS pathway in signal transduction from the cell surface receptors to the nucleus, affecting the production and regulation of other key proteins has been established in numerous published works. The main action of the three proto-oncogenes of the RAS family (H-RAS, K-RAS, and N-RAS), which are located in the inner plasma membrane, is the binding of GDP and GTP. RAS proteins possess and confer intrinsic GTPase activity which cleaves the GTP to GDP and leaves it in a "switch off" position. KRAS protein is active and transmits signals by binding to GTP (turn on), but it is inactive (turn off) when GTP is converted to GDP.
KRAS mutations are associated with inactivity of GTPase which subsequently leaves GTP at the "switch on" position. Increasing role of KRAS mutations has been recognized in many gastrointestinal tumors, mainly in colorectal adenocarcinomas. In pancreatic adenocarcinoma, the vast majority of tumors harbor KRAS mutations (from 74% up to 100% in various series).12-16 The most frequent mutations observed are those in codon 12 followed by point mutations in codons 13 and 67.14 The data about the prognostic and predictive significance of the above mutations of KRAS is rather limited and conflicting.5,17 The high frequency of KRAS mutations in PC may in part explain the lack of response to epidermal growth factor receptor (EGFR) inhibitors, similarly to colorectal cancer patients.18,19
2) p16/INK4
Gene p16 is a tumor suppressor gene, located in chromosome 9p21. This gene is also named as INK4a, CDKN2 or MTS1-multiple tumor suppressor 1. Gene p16 encodes for a protein (p16INK4a) which inhibits the interaction of cyclin D with the kinases CDK4 and CDK6 and thus inhibits cell cycle progression at the G1→S step. The cyclin D-CDK4 complex phosphorylates the retinoblastoma protein (Rb1), preventing thus the formation of the E2F-Rb1 complex and leaving E2F available to act as a transcription factor facilitating cell cycle progression. In pancreatic cancer cells inactivation of p16INK4a results in uncontrolled cell cycle progression due to absence of inhibition of the cyclin D-CDK4 complex.
In PC, inactivation of p16 is caused by various means such as point mutation, hypermethylation or homozygous deletion of the gene, and is observed in the majority of these patients according to various published works.16,20,21
The prognostic significance of p16 is not established as there are conflicting data and therefore more evidence is needed before any clinical application.22-24
3) p53
This is the most known and studied tumor suppressor gene as it is frequently mutated in various neoplasms. In normal conditions, p53 is usually inactive and bound to the mdm protein (HDM2 in humans), which promotes its ubiquitination (binding with ubiquitin and degradation by proteasome) preventing its action. Triggered by damaged DNA (e.g., in ageing or ionizing radiation conditions), p53 promotes a programmed cell death by arresting cell cycle at the G1 to S point and thus inhibits cellular proliferation and growth.
Mutations or loss of p53 are a rather early event in pancreatic carcinogenesis and occur sporadically in most patients.5,16,25,26 Specific mutation of p53 (R172P) has recently been associated with increased metastatic potential in pancreatic cancer models in vitro.27 Additionally, p53 mutations have been associated with reduced chemotherapy efficacy due to impaired p53-induced apoptosis.28-30 Nevertheless, the prognostic significance of p53 alterations remains unclear. The data is conflicting as few researchers have suggested a short survival in pancreatic cancer patients with p53 mutations while others have found no association at all.4,6,31-34
4) DPC4
The tumor suppressor gene DPC4 (deleted in pancreatic cancer, locus 4) or commonly called SMAD4 has been long associated with pancreatic cancer. Genes of the SMAD family encode for proteins that participate in tissue growth factor-beta (TGF-β) mediated signal transduction and thus regulate gene transcription and growth arrest. In particular, TGF-β binds to TGF-βRII receptor which subsequently activates TGF-βRI by phosphorylation. The signal transduction cascade also involves activation of TGF-βRI, phosphorylation and activation of SMAD2 and 3 and finally formation intracellularly of a heterodimer complex with SMAD4.35,36 This SMAD complex translocates to the nucleus and interacts with DNA where it controls transcription of genes, such as c-myc, p21, and p15 which regulate cellular proliferation. The ultimate effect of SMAD4 in the normal cells will be growth arrest, apoptosis and cell differentiation by inhibition of the cell cycle at G1 point.
In pancreatic cancer, inactivation of SMAD4 by point mutations or loss of heterozygosity (LOH) allows uncontrolled cellular growth and proliferation. This is a likely late event of pancreatic carcinogenesis as the gene is expressed normally in the early PanIN1 and 2 stages but only in a third of PanIN3 cases.10,37
Up to half of pancreatic cancer patients carry the inactivated DPC/SMAD4 gene.9,37 According to a recent published work, DPC4 immunolabelling may be of diagnostic value as it can possibly differentiate pancreatic metastatic disease from primary liver, lung or ovarian neoplasms.38 Whether the DPC4 status has a prognostic value remains subject of debate. In a few studies, presence of DPC4 status was associated with better outcome and survival post resection,10,39 but in other studies DPC4 expression was associated with worse outcome after surgery or adjuvant chemotherapy.40,41 Recent data has suggested that DPC4 loss is associated with presence of widespread metastases but it is not as frequently found in locally advanced pancreatic cancer, therefore may have a role in patients' selection for systemic rather than local treatment.42
Another effect of DPC4/SMAD4 is reduction of angiogenesis by decreasing vascular endothelial growth factor (VEGF) and increasing thrombospondin (an anti-angiogenetic factor) expression. It was found that restoration of SMAD4 loss in pancreatic cancer cells resulted in slowly growing tumors with reduced vascular density, suggesting a possible tumor suppression mechanism.43
5) BRCA2
BRCA2 (breast cancer type 2) is a tumor suppressor gene, mutations of which are often associated with familial breast and ovarian cancer, and less often with other neoplasias and hematological diseases. It is located in chromosome 13q and its main function is normally the repair of damaged DNA.
Specific germline mutations of BRCA2 gene, mainly at locations 6174delT and 6158insT, have been found in 6-17% of familial pancreatic cancer.44-47 The same germline mutations were seen only in 10% of sporadic cases.48,49
The prognostic or predictive value of BRCA2 in pancreatic cancer is still unknown.
6) Erb family genes (HER-2/neu - EGF)
The proto-oncogene HER-2/neu or ErbB2 with its protein HER-2/neu is one of the four members of the epidermal growth factor receptor family (ErbB protein family) which regulate signal transduction from extracellular stimuli to the nuclear level. The relevant pathway is primarily the phosphatidylinositol-3 kinase and mitogen-activated protein kinase (PI3/Akt-MAPK) pathway though interference and cross-talk with other pathways is often taking place. Overexpression of proto-oncogenes or loss of tumor suppressor genes of the aforementioned pathways lead to unbalanced signal transduction and uncontrolled proliferation often seen in many neoplasias including pancreas.
In pancreatic adenocarcinoma, the rate of amplification or overexpression of the HER-2/neu gene varies in different studies from 16% to 65%.50-53
Most importantly, it seems that HER-2/neu gene alterations bear no prognostic or predictive significance.8,54 Targeting HER-2/neu with monoclonal antibodies has been studied in preclinical and clinical setting with unclear results as we will see later in this paper.
7) Notch1 and Hedgehog
Notch1 is a gene located at chromosome 9q which is normally involved in cell differentiation, proliferation and apoptosis. Notch signaling pathway seems to play role in embryogenesis and to regulate epithelial stem cells differentiation, survival and cell fate. Additionally, Notch interacts with the molecular pathways of Wtn and Hedgehog (Hh) in order to control proliferation of stem cells and cellular differentiation. Recent studies have suggested that sustained activation of these pathways might be related to cancer stem cells initiation and carcinogenesis. Likely, this is achieved through induction of nuclear factor-kappa B (NF-κB) and its signaling pathway by Notch. Persistent activation of NF-κB is very often found in pancreatic cancers and its role is increasingly recognized. Down-regulation of notch-1 and inactivation of NF-κB by natural products and phytochemicals such as curcumin and genistein or small interfering RNA resulted in inhibition of cancer progression and metastases.55-58 Currently, these natural compounds are tested in clinical trials in combination with conventional chemotherapy in patients with advanced pancreatic cancer. Increased expression of notch-1 was also noted in the intratumoral nerves of pancreatic cancer cell lines and it was associated with an invasive and angiogenic phenotype of pancreatic cancer in vitro. These findings suggest that notch pathway may regulate the neurovascular development of pancreatic cancer, but most importantly may be a therapeutic target in future.59 As far as sonic Hh is concerned, while it normally promotes pancreatic cells differentiation, in pancreatic cancer SHH signaling pathway is often dysregulated, promoting tumour progression by increasing desmoplasia and facilitating recruitment of fibroblasts which in turn contribute to tumour-stromal cells interaction. Targeting of SHH pathway and the associated desmoplasia, e.g., with neutralizing antibodies or by blocking the SHH receptor Smoothened (SMO) with small interfering RNA, may be a valuable treatment option in future and needs to be further explored.60,61
8) COX pathway
Cyclooxygenase (COX) pathway has long been investigated and targeted in pancreatic cancer patients. COX is an enzyme which converts arachidonic acid to thromboxanes and prostaglandins. We find COX in two isoforms, COX-1 and COX-2. COX-1 is constitutively and naturally expressed in most tissues. On the contrary, COX-2 is mainly induced by cytokines and inflammatory stimuli but also by growth factors and oncogenes. Overexpression of COX-2 has been observed and implicated in the carcinogenesis of most solid tumors, including pancreas.62
Overexpression of COX-2 is a poor prognostic factor in pancreatic cancers.63-66 The development of specific COX-2 inhibitors (celecoxib, apricoxib) has led to their investigation in clinical trials in advanced pancreatic cancer in combination with cytotoxic treatment and in the chemoprevention of pancreatic cancer.
9) Other genes
Akt-2 oncogene is implicated in pancreatic carcinogenesis and is often amplified and overexpressed in pancreatic tumors.67,68 It has been proposed that inhibition of Akt-2 results to decreased activity of NF-κB, lower levels of the anti-apoptotic gene bcl-2 and increased levels of the pro-apoptotic gene Bax. Similarly, inhibition of Akt-2 rendered cancer cells more sensitive to chemotherapy-induced apoptosis.69
Other genes involved in development of pancreatic cancer include cyclins D1 and D3, which are regulating cell cycle at the G1/S point. These genes, which are often overexpressed in pancreatic cancer, have also been associated with poor prognosis.70-72
Finally, a possible role in pancreatic carcinogenesis, tumor progression and metastasis, but with limited evidence, may be played by genes MUC4, Scr, Bcl-6, mdm2 and S100P.26,73,74
10) Latest identified altered molecules
(1) Palladin
This is an actin-associated protein that was found mutated in familial cases of pancreatic cancer and overexpressed in many sporadic pancreatic tumors and premalignant stages. There are two isoforms of this protein (65 kDa and 85 kDa), each of which is associated with specific properties and behaviour of tumor cells. Though some recent data suggests that this protein isoforms may be candidate biomarkers for early diagnosis and prediction of metastatic potential, other studies provided inconsistent conclusions about its role. Therefore, more research on this molecule is needed prior to any clinical application.75,76
(2) Micro-RNAs
These small non-coding RNA molecules control the activity of one third of all protein-coding genes. Altered expressions of miRNAs are implicated in carcinogenesis of various cancers by affecting apoptosis and cell growth. Deregulation of miRNA has been studied in pancreatic cancer in terms of cancer development and progression. There is preclinical evidence that specific miRNAs, including miR-196a, miR-190, miR-186, miR-221, miR-222, miR-200b, miR-15b, and miR-95 are upregulated in pancreatic cancer cells and involved in its pathogenesis.77 Few of these mi-RNAs, such as miR-210, miR-200a and miR-200b which promote carcinogenesis through expression of E-cadherin, may be found elevated in the serum of patients with pancreatic cancer and may therefore be used in future for diagnostic purposes.78-80
2. Molecular pathways involved
Similarly to other solid tumors, some of the complex molecular and signaling pathways that are altered in pancreatic cancer have been recognized and efforts to repair identified abnormalities are mounting in drug development units. One of the central molecules in transduction pathways is NF-κB. NF-κB represents a family of molecules in the cytoplasm which upon binding to proteins IkBa and p100 becomes inactive. The NF-κB family contains five members, p50, p52, p65, c-Rel and RelB, which form and appear in heterodimers. Activation of NF-κB is achieved by phosphorylation of its binding proteins IκBα by IKKβ and/or p100 by IKKα which causes degradation of IκBα and transformation of p100 into the small form p52. Consequently, the active heterodimers of NF-κB (the p50/p65 and p52/RelB) translocate to the nucleus where they bind to NF-κB-specific DNA-binding sites and to gene promoters regulating their transcription and expression. Known genes regulated by NF-κB include survivin, VEGF, EGF, and MMP-9 which in turn affect cellular survival and apoptosis but also tumor progression, invasion and metastasis.
In pancreatic cancer, NF-κB is overactivated contributing to its pathogenesis, its local progression and distal spread.55,56 Furthermore, activation of NF-κB by gemcitabine has been implicated as a potential mechanism of resistance to this drug.81 NF-κB related pathway has recently been a target of novel agents tested in cancer research.
The Hh signalling pathway has been found to play a role in pancreatic carcinogenesis as stated previously. Hh pathway is overexpressed in up to 70% of pancreatic cancers.82 Cyclopamine is a natural inhibitor of Shh able to induce apoptosis and inhibition of pancreatic cancer cell proliferation in cell lines and in vivo.83 There is evidence of cross-talk between hedgehog pathway, NF-κB and notch-1. It seems that activation of NF-κB causes overexpression of Shh and accordingly inhibition of NF-κB may cause down-regulation of Shh.84
Other altered molecular pathway involved in pancreatic carcinogenesis is the RAS/RAF/MEK/MAPK pathway. The frequently mutated KRAS gene encodes for a constitutively activated KRAS protein causing up-regulation of the downstream molecules and uncontrolled cellular proliferation and survival.
Matrix metalloproteinases (MMPs) play in general significant role in cancer progression, invasion and metastases via extracellular matrix and stroma degradation facilitating distal migration of cancer cells. The main MMPs involved in pancreatic cancer are MMP-2 and MMP-9.85 The degree of MMP-2 expression correlates with disease progression and poor prognosis.86 It seems that the level of expression of the tissue inhibitor of metalloproteinases and its ratio to MMP-2 and -9 may be a prognostic factor of this disease and its metastatic potential.85
MOLECULAR TARGETS AND DIAGNOSIS
The disappointing outcome of pancreatic cancer patients, the majority of which are diagnosed at an advanced stage, necessitates the improvement of diagnostic tools and methods in order to identify more patients as early as possible. Thus, molecular targets are sought and the most valid are presented below.
1. Glycoproteins
1) CA-19-9
Tumor-associated antigen CA19-9 is a glycoprotein often produced by gastric and pancreatobiliary tumors. Its main utility is rather treatment monitoring and detection of disease recurrence than screening and initial diagnosis. The sensitivity and specificity for pancreatic tumors are 85 and 90%, respectively.87,88 A number of other conditions including liver-biliary cirrhosis, biliary obstruction and ascites may account for increased CA19-9. For these reasons, the American Society of Clinical Oncology (ASCO) on its 2006 Update of Recommendations for the use of tumor markers in gastrointestinal cancer advised against the use of CA19-9 as a screening or diagnostic marker of pancreatic cancer.89
2) Mucins
Mucins (MUC) are the second most known glycoproteins studied in pancreatic tumors, characterized by their high molecular weight. Around twenty genes control and encode fourteen mucin proteins, which are linked individually with a particular pancreatic histological subtype.90,91 Of particular interest is MUC1 which is highly expressed in invasive ductal carcinoma, and less important for this review article is MUC2 which is expressed in Intraductal Papillary Mucinous Neoplasm (IPMN) of dark cell type and MUC6 found in clear cell type IPMN.91,92
MUC1 interferes normally to the intracellular and cell to stroma interaction, as well as inhibits the signal transduction in tumors and finally the cancer progression. There is evidence that MUC1 play a role in diagnosis and differential diagnosis of pancreatic cancer.91,93 According to a recent meta-analysis of 17 studies (1,363 patients in total) regarding the role of MUC1 as a diagnostic tool in pancreatic adenocarcinoma, the accuracy of the test showed a sensitivity of 0.83 (95% CI, 0.81 to 0.86), specificity 0.63 (95% CI, 0.59 to 0.66) and diagnostic odds ratio of 20.44 (95% CI, 9.53 to 43.85). For these reasons MUC1 could be a potential test with moderate diagnostic accuracy in pancreatic adenocarcinoma.
MUC4 is another member of the MUC genes which has been found overexpressed in pancreatic cancer but not in benign conditions and therefore may be used as a potential diagnostic marker.73 Overexpression of MUC4 is a poor prognostic factor and is associated with advanced stage of pancreatic cancer and aggressiveness.94-96 Preclinical data from pancreatic cancer mice models suggest that inhibition of MUC4 with an antisense MUC4 RNA causes significant suppression of cancer growth and metastasis.97 Therefore, MUC4 needs further exploration as both a diagnostic marker and therapeutic target.
MOLECULAR TARGETS FOR TREATMENT
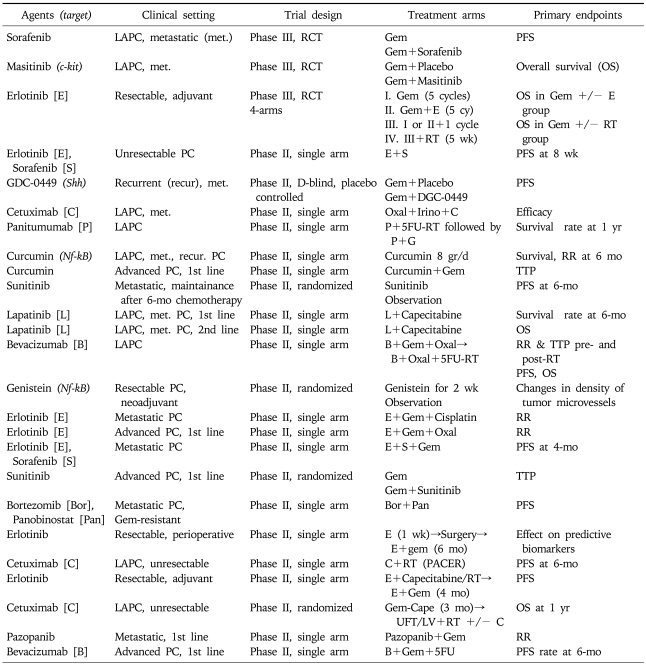
We presented above evidence on the main biological alterations implicated in pancreatic cancer pathophysiology. Treatment strategies and rationale of drug development is actually based on these molecular changes aiming to counteract the chief abnormal stimuli driving tumors (Table 1). Therefore, the main targeted agents which have been tested in pancreatic cancer include molecules against the EGFR, the HER2/neu receptor, MMP, the VEGF, the mTOR pathway, molecules against the activated KRAS protein (farsenyl transferase inhibitors [FTI]) as well as many other agents which will be discussed later in our review. Various combinations of targeted agents with each other or with chemotherapy or radiotherapy are currently under investigation, the results of which will be available over the next few years (Table 2).
Table 2.
Phase III and Some of the Phase II Clinical Trials of Targeted Agents in Pancreatic Cancer That Are Currently in Progress
Source: www.ClinicalTrials.gov.
LAPC, locally advanced pancreatic cancer; RCT, randomized controlled trial; Gem, gemcitabine; PFS, progression free survival; RT, radiotherapy; PC, pancreatic cancer; TTP, time to progression; RR, response rate; Oxal, oxaliplatin; UFT/LV; uftoral/leucovorin.
1. Targeting the EGF pathway
The EGF pathway and its molecules have been found altered quite often in pancreatic cancer. Thus, many well designed studies have been conducted trying to demonstrate some benefit from blocking the aberrant signal at various levels of the pathway.
Of particular interest are the inhibitors of EGF receptor. Two types of inhibitors against the EGFR exist. First, monoclonal antibodies (MAbs) against the extracellular part of the receptor and second small molecules against the tyrosine kinase part intracellularly (TKIs).
1) Positive studies
The only biological compound that showed positive results in a phase III study in combination with chemotherapy in the treatment of advanced pancreatic cancer is erlotinib. Erlotinib (Tarceva™) is a tyrosine kinase inhibitor (TKI), available in oral preparations, that blocks selectively the EGF receptor. Early preclinical studies had showed its potential to inhibit the EGF and the MAPK (ERK1/2) pathway in pancreatic cancer models, enhancing cancer apoptosis in combination with gemcitabine and wortmannin (a PI3K inhibitor).98
Thereafter, many clinical studies demonstrated the satisfactory safety profile of this biological agent in pancreatic cancer patients and its potential efficacy in combination with gemcitabine, the most active cytotoxic in these patients till then.
As a result, a phase III, randomized, placebo controlled, study published in 2007 tested the efficacy of the combination erlotinib plus gemcitabine versus gemcitabine monotherapy.99 This important study recruited 569 patients in total and demonstrated a statistically significant survival benefit (6.24 months vs 5.91 months, p=0.038) in patients who received the combination treatment as compared to patients on the gemcitabine arm. Though the absolute benefit was only of a few weeks, it was for a first time that a survival benefit from a novel agent was clearly demonstrated in a phase III study. Besides, the 1-year survival rate was better in the combination arm (24% vs 17%, p=0.023). In subsequent subgroup analysis of this study, a particular survival benefit was demonstrated in those patients on erlotinib arm who developed high grade skin rash (≥2 according to the NCI Common Terminology Criteria for Adverse Events, version 3.0) providing thus a clinical biomarker of efficacy.99,100 It was observed that the higher the degree of rash, the better the disease response and survival.100 The recommended dose of erlotinib in this study was 100 mg per day.
In order to further improve the modest results, combinations of erlotinib with other agents have been under evaluation. A phase I/II study of erlotinib with bevacizumab, capecitabine and gemcitabine in chemotherapy naive patients with advanced pancreatic cancer, conducted in the United Kingdom, confirmed the safety of this combination and set the recommended dosing, according to the maximum tolerated doses, while showed promising efficacy justifying further exploration of the quadruple combination.101,102
Currently, clinical trials evaluating the combination of erlotinib/gemcitabine with MK0646 or cixutumumab (novel inhibitors of insulin growth factor receptor-1), sorafenib, GDC-0449 (hedgehog antagonist), apricoxib (selective COX-2 inhibitor), nab-paclitaxel or oxaliplatin are recruiting patients. Hopefully some of these trials will be positive providing further valuable treatment options.
2) Negative studies
Gefitinib (Iressa™) is a small molecule TKI which inhibits phosphorylation of the EGFR in the same way as erlotinib. Although, in pancreatic cancer cell lines there was evidence of antitumor activity, in a phase II on gemcitabine-resistant pancreatic cancer patients, combination of gefitinib with docetaxel, showed no actual clinical benefit.103,104 On the other hand, combination of gefitinib with gemcitabine showed some activity (response rate of 11% and disease stability 23%) in advanced PC, according to a small phase II study by the Hellenic Cooperative Oncology Group in 2007, though the results have not been replicated since.105 Lapatinib (Tyverb or Tykerb™) is a dual EGF (ErbB1) and HER2/neu (ErbB2) tyrosine kinase inhibitor. Recent phase II study of lapatinib and gemcitabine, in metastatic pancreatic patients, was terminated prematurely when an interim analysis, after 29 patients were recruited, showed that the combination was not effective.106
Apart from small molecules TKIs, quite a few monoclonal antibodies (mAbs) against the EGF family receptors have been tested in clinical trials in advanced pancreatic cancer patients, with disappointing so far results. Trastuzumab (Herceptin™) is a HER2/neu mAb known for its success in HER2/neu expressing breast cancers. Trastuzumab was also tested in PC patients as HER2/neu is often overexpressed in this disease.50,51,53 In contrast to some promising preclinical data, investigation of this agent in pancreatic cancer patients in a phase III study showed that despite its satisfactory toxicity profile and moderate activity, there was no survival benefit.107 One possible explanation for this failure could be the fact that only 12% of the enrolled patients overexpressed (+3) HER2/neu receptor. Cetuximab (Erbitux™) is a chimeric antibody that inhibits the EGF receptor (ErbB1). Cetuximab has been studied extensively in both preclinical and clinical studies in pancreatic cancer. Based on positive evidence from tumor cell lines and animal models, this EGFR inhibitor was tested in clinical trials alone or in combination with chemotherapy.
Initial results from a phase II study showed that combination of cetuximab with gemcitabine in pancreatic cancer patients was feasible, well tolerated and moderately active.108 Unfortunately, when the combination was tested in a large phase III study no survival benefit was found to justify further use.109 Combinations of cetuximab with other cytotoxics or biologicals are under evaluation and results are anticipated in the years to come. For example, a phase II study of cetuximab along with oxaliplatin and irinotecan is currently recruiting patients in the United States. The combination sounds interesting bearing in mind the positive results from a retrospective analysis of patients treated with these drugs presented at the 2007 Annual Meeting of the ASCO and the very promising activity of FOLFIRINOX combination as presented in ASCO Annual Meeting in 2010.110,111 As far as biologicals are concerned, combination of cetuximab with trastuzumab or everolimus is now under evaluation.
2. Targeting the VEGF pathway
Angiogenesis plays important role in cancer development and progression and VEGF is frequently overexpressed in pancreatic.112,113 Therefore, there is a rationale of targeting the VEGF receptor with antibodies or small molecules often concurrently with other biological or cytotoxics. The most studied VEGFR inhibitor is the mAb bevacizumab (Avastin™). This is humanized antibody that blocks both VEGF receptors 1 and 2. As most of the biological tested in this devastating disease, bevacizumab showed evidence of activity in preclinical and early clinical studies. Sadly, when a subsequent double-blind phase III placebo controlled study was conducted, the combination of gemcitabine-bevacizumab showed no survival benefit compared to gemcitabine monotherapy.114
As stated before, combinations of bevacizumab with other biologicals and conventional chemotherapy drugs are currently tested in various clinical studies. Of promise are the results of the phase II study of bevacizumab with erlotinib, capecitabine and gemcitabine conducted at the Royal Marsden Hospital, United Kingdom presented at ASCO 2010 Annual Meeting though the toxicity is of some concern for patients with less good performance status.102
More molecules against the VEGF pathway are now available for testing in cancer patients including pancreatic cancer ones. Such molecules that are evaluated in trials include aflibercept, a recombinant fusion protein which is a potent inhibitor of VEGF (known as VEGF trap) and of placental growth factor and vatalanib, a small molecule TKI targeting selectively VEGF Receptors 1, 2, and 3.
Additionally, molecules with broad spectrum of activity are available and enriching our options for clinical trials in order to identify the most effective and less toxic combination. Multi-tergeted agents with at least preclinical evidence of activity include vandetanib (Zactima™), a small molecule that inhibits the VEGFR-2, but also VEGFR-3, ErbB1 (EGF) and RET kinase115 and sorafenib (Nexavar™), an inhibitor of RAF kinase, PDGFR-beta, VEGFR-2,-3 and c-kit with antitumor activity against several cancers.
3. Targeting other pathways and molecules
1) Inhibitors of MMPs
Marimastat is the first MMP inhibitor studied in solid cancers, with broad activity against multiple MMP such as 1, 2, 3, 7 and MMP-9. Following early studies assessing activity in pancreatic cancer cells and safety in humans, two phase III studies on advanced PC patients evaluated marimastat or marimastat plus gemcitabine versus gemcitabine monotherapy and both failed to show any meaningful clinical benefit from the new agent.116,117
There are other MMP inhibitors developed in drug units, that have either failed to demonstrate activity in pancreatic cancer clinical trials, such as tanomastat (BAY 12-9566),118 or have only been tested in preclinical trials such as Ro 28-2653, a selective oral inhibitor of MMP-2 and MMP-9.119
2) Farnesyl transferase inhibitors (FTIs)
As KRAS is found altered in the majority of pancreatic cancer patients, efforts to block this molecule with selective inhibitors have been made in the last decade.
Tipifarnib (R115777, Zanestra™) is such a selective inhibitor of farnesyl transferase, one of the several enzymes required for the function of p21 (RAS), RhoB and other proteins of this pathway, involved in cell survival and apoptosis. Farnesylation of RAS is mandatory for the function of this protein and the signal transmission from the membrane receptors down to the intracellular proteins.
Contrary to many preclinical studies showing antitumor activity against pancreatic cell lines and xenograft models, tipifarnib failed to show clinical benefit either as monotherapy or in combination with gemcitabine in various phase II and a phase III study.120-122 Similarly, negative results were produced from clinical studies of a second FTI, lonafarnib (SCH66336) which despite tumor suppression in human xenografts in vivo123 and goog toxicity profile in phase I studies,124,125 no actual benefit was reported in a phase II study on patients with advanced pancreatic cancer as compared to gemcitabine treatment (Proc Am Soc Clin Oncol 2001;20:abstr 608). The many aforementioned negative studies underpin the complexity of pancreatic carcinogenesis and the need for multimodal treatment approach.
3) COX-2 inhibitors
The evidence of COX-2 overexpression in pancreatic cancer has led to their investigation in chemoprevention and treatment of this disease, often in combination with cytotoxics. Celecoxib is a COX-2 inhibitor that showed in laboratory pancreatic cancer models that it is able to induce apoptosis and to inhibit angiogenesis, tumor growth and metastasis.126,127 Celecoxib has also demonstrated synergistic effect was in combination with cytotoxics (gemcitabine or fluopyrimidines), radiotherapy128,129 or with other agents such as erlotinib and curcumin.130,131 A possible explanation of the enhanced antitumor effects is the increased down-regulation of Her2/neu, EGFR and COX-2 expression along with inactivation of NF-κB.
A number of phase II studies have tested the efficacy of celecoxib in various clinical settings in pancreatic cancer. Combination of celecoxib with protracted 5-FU infusion or capecitabine as second line chemotherapy in patients with advanced pancreatic cancer showed minimal activity (response rate, 9% to 12%) and moderate risk of gastrointestinal or haematological toxicities.132,133 Subsequent small phase II study on 18 patients with advanced inoperable pancreatic cancer showed that combination of gemcitabine, irinotecan and celecoxib was quite active (18% partial response, 70% stable disease, overall survival 13 months) but at the cost of common grade 3/4 toxicities (neutropenia [50%], anaemia [39%], diarrhoea [17%], fatigue [17%]).134 Similar results were recently reported by other researchers (20% partial response , median survival 18 months), and thus the combination merits exploration in a phase III study.135 Nevertheless, the combination of celecoxib with gemcitabine and cisplatin did not demonstrate added clinical benefit in terms of median survival in other phase II trials on advanced pancreatic cancer patients.136,137
One of the concerns regarding the use of COX-2 inhibitors is the increasing risk of cardiovascular diseases, and for that reason patients at risk for heart diseases should be considered for COX-2 inhibitors cautiously.138-141 Currently, a randomised phase III, double-blind, placebo controlled study of celecoxib, gemcitabine and curcumin is recruiting patients with advanced pancreatic cancer and the results will shed some light on the synergistic effect of this combination (www.ClinicalTrials.gov, NCT00486460).
4) NF-κB inhibitors
NF-κB is a pivotal pathway in signal transduction and its overactivation is implicated in the development of tumors. Inhibition of expression or activation of NF-κB by various agents has been studied in preclinical models and less so in clinical trials.
Curcumin, a natural antioxidant found in turmeric (curry), is a potent inhibitor of the overexpressed NF-κB pathway and has demonstrated activity against pancreatic cancer and other solid tumors in preclinical and clinical studies.142,143 In addition, curcumin showed it could enhance the cytotoxic effect of gemcitabine, at least in vivo.144 Likewise, the soy isoflavone genistein was able to downregulate NF-κB increasing thus, the antitumor activity of gemcitabine, cisplatin or erlotinib.145-147 Inhibition of NF-κB activation by nafamostat, a serine threonine inhibitor, was able to improve the cytotoxic effect of gemcitabine in mice models with pancreatic cancer.81
A high number of natural products or synthetic compounds may inhibit NF-κB activation, but it will take long before proper clinical studies will determine their actual impact in pancreatic cancer.
5) mTOR inhibitors
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase protein that controls cell cycle, protein translation and cell growth by regulating RNA in nucleus, but also affects angiogenesis through the hypoxia inducible factor 1 (HIF-1). This important pathway is constitutively activated in pancreatic cancer cells.148 The first inhibitor of mTOR, rapamycin, that was identified serpenditiously, was able to cause cell cycle arrest at the G1/S phase and inhibition of pancreatic cancer cell proliferation and cancer growth.149
Since then many synthetic analogues of rapamycin have been developed such as temsirolimus (Torisel™) and everolimus (Affinitor™) with similar preclinical activity on pancreatic cell lines.150 Furthermore, inhibitors of mTOR potentiated the activity of other drugs (gemcitabine or erlotinib) in pancreatic cancers in vitro and in vivo.151,152 Nevertheless, temsirolimus lacks yet clinical evidence in pancreatic cancer patients (still tested in phase I studies in combination with gemcitabine) and everolimus was found inactive in gemcitabine-resistant patients.153
Many clinical trials testing combinations of everolimus with other agents (irinotecan, capecitabine, cetuximab, sorafenib) are currently accruing patients.
6) MEK and HDAC inhibitors
The high frequency of mutations in KRAS gene has led to the development of molecules able to block the aberrant signal transduction below the level of the dominant abnormality in the RAS→RAF→MEK→MAPK (mitogen activated protein kinase)→ERK (extracellular receptor kinase)→FOS pathway.
An early study on pancreatic cancer cell lines showed that the MEK inhibitor UO126 may induce cell cycle arrest and inhibition of cancer cells proliferation by up-regulation of the cyclin-dependent kinase inhibitor p27Kip1.154
The first MEK inhibitor tested in clinical trials was CI-1040. This agent was found well tolerated and safe in a phase I clinical trial,155 but rather ineffective in pancreatic cancer patients according to subsequent phase II study on solid tumor.156 Other MEK inhibitors evaluated at present for efficacy in pancreatic cancer patients include GSK1120212 and AS703026, the former in combination with everolimus and the later with gemcitabine.
Histone deacetylases (HDAC) are enzymes that deacetylate histone, the protein which binds around DNA interfering with gene transription and suppressing their expression. Balanced function between the HDAC and histone acetyltransferases (HAT) is required for normal cellular function. Inhibition of these enzymes by HDAC inhibitors aims to modulate gene transcription and to affect cell cycle progression, apoptosis and angiogenesis.157
There is considerable preclinical data regarding the inhibitory effects of HDAC inhibitors on pancreatic cancer cells. Among those inhibitors we find Trichostatin A (TSA), the synthetic SK-7041 and FR901228.158-160 TSA also showed synergistic cytotoxic effect on pancreatic cancer lines when combined with irinotecan or gemcitabine.161,162 Another important HDAC inhibitor is Vorinostat (Zolinza™) also known as suberoylanilide hydroxamic acid (SAHA). Combination of vorinostat with gemcitabine induced apoptosis and pancreatic cancer cells inhibition in vitro.163
These studies suggest that HDAC inhibitors may sensitise cancer cells resistant previously to chemotherapy. Of interest, combination of vorinostat with the biological agents 5-Aza-2'-deoxycytidine (inhibitor of DNA methylation) or the proteasome inhibitor bortezomib (Velcade™) enhanced apoptosis and inhibition of cancer growth.164,165 There are about four phase I/II clinical trials in progress testing the safety, dosing and efficacy of vorinostat in nonmetastatic pancreatic cancer patients along with radiotherapy, fluoropyrimidines or a proteasome inhibitor. Finally, a novel HDAC inhibitor, panobinostat, is also investigated combined with bortezomib in a phase II study on gemcitabine-resistant pancreatic cancer patients.
7) Targeting SHH
Sonic hedgehog pathway is implicated in pancreatic carcinogenesis through enhanced proliferation and survival of pancreatic epithelial cells, reduced apoptosis and interactions with KRAS pathway.166 Targeting SHH by selective inhibitors such as cyclopamine, SANT1, robotnikinin, IPI-269609 and Cur-61414 have been developed and showed that may halt tumorigenesis and progression of pancreatic cancer.167,168 There is preclinical evidence that cyclopamine may augment the effect of chemotherapy, anti-EGFR therapy and radiotherapy and may suppress distal spread of pancreatic cancer cells.169,170 Similarly, combined inhibition of SHH and mTOR signaling pathways with cyclopamine and rapamycin achieved to eliminate pancreatic cancer stem cells proposing a novel therapeutic target for an aggressive disease.171 A pilot clinical study of the SHH inhibitors GDC-0449 combined with gemcitabine, in advanced pancreatic cancer patients, is now in progress planning to recruit 25 patients until 2011.
CONCLUSION
Early diagnosis and treatment of pancreatic cancer is possibly the only way to improve significantly the disappointing outcome of the majority of pancreatic cancer patients. Despite the development in technical facilities and translational research, no real progress in clinical terms has been achieved so far. What we seem to have learnt though, is the complexity of pancreatic cancer pathogenesis, the mechanisms of progression and of resistance to treatments. This knowledge allows us to focus our efforts in targeting specific abnormalities simultaneously, aiming to reduce treatment failures. The development of novel drugs is costly and time consuming. Nevertheless, new and high quality knowledge always follows the proof of concept pathway and hopefully new agents will pass successfully through the drug development pipeline and well designed clinical studies, even should be the very tight.
References
- 1.Lowenfels AB, Maisonneuve P. Epidemiology and prevention of pancreatic cancer. Jpn J Clin Oncol. 2004;34:238–244. doi: 10.1093/jjco/hyh045. [DOI] [PubMed] [Google Scholar]
- 2.Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140–2143. [PubMed] [Google Scholar]
- 3.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Dang CX, Han Y, Qin ZY, Wang YJ. Clinical significance of expression of p21 and p53 proteins and proliferating cell nuclear antigen in pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2002;1:302–305. [PubMed] [Google Scholar]
- 5.Dergham ST, Dugan MC, Kucway R, et al. Prevalence and clinical significance of combined K-ras mutation and p53 aberration in pancreatic adenocarcinoma. Int J Pancreatol. 1997;21:127–143. doi: 10.1007/BF02822384. [DOI] [PubMed] [Google Scholar]
- 6.Dong M, Dong Q, Zhang H, Zhou J, Tian Y, Dong Y. Expression of Gadd45a and p53 proteins in human pancreatic cancer: potential effects on clinical outcomes. J Surg Oncol. 2007;95:332–336. doi: 10.1002/jso.20684. [DOI] [PubMed] [Google Scholar]
- 7.Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23:74–79. doi: 10.1159/000093497. [DOI] [PubMed] [Google Scholar]
- 8.Dugan MC, Dergham ST, Kucway R, et al. HER-2/neu expression in pancreatic adenocarcinoma: relation to tumor differentiation and survival. Pancreas. 1997;14:229–236. doi: 10.1097/00006676-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Schutte M, Hruban RH, Hedrick L, et al. DPC4 gene in various tumor types. Cancer Res. 1996;56:2527–2530. [PubMed] [Google Scholar]
- 10.Hua Z, Zhang YC, Hu XM, Jia ZG. Loss of DPC4 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. World J Gastroenterol. 2003;9:2764–2767. doi: 10.3748/wjg.v9.i12.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumadue JA, Griffin CA, Osman M, Hruban RH. Familial pancreatic cancer and the genetics of pancreatic cancer. Surg Clin North Am. 1995;75:845–855. doi: 10.1016/s0039-6109(16)46731-9. [DOI] [PubMed] [Google Scholar]
- 12.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 13.Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988;16:7773–7782. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hruban RH, van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–554. [PMC free article] [PubMed] [Google Scholar]
- 15.van Heek T, Rader AE, Offerhaus GJ, et al. K-ras, p53, and DPC4 (MAD4) alterations in fine-needle aspirates of the pancreas: a molecular panel correlates with and supplements cytologic diagnosis. Am J Clin Pathol. 2002;117:755–765. doi: 10.1309/5RQ0-JCQU-5XF2-51LQ. [DOI] [PubMed] [Google Scholar]
- 16.Rozenblum E, Schutte M, Goggins M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 17.Kawesha A, Ghaneh P, Andren-Sandberg A, et al. K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16(INK4A), p21(WAF-1), cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2000;89:469–474. doi: 10.1002/1097-0215(20001120)89:6<469::aid-ijc1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 19.Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–1169. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126–3130. [PubMed] [Google Scholar]
- 21.Huang L, Goodrow TL, Zhang SY, Klein-Szanto AJ, Chang H, Ruggeri BA. Deletion and mutation analyses of the P16/MTS-1 tumor suppressor gene in human ductal pancreatic cancer reveals a higher frequency of abnormalities in tumor-derived cell lines than in primary ductal adenocarcinomas. Cancer Res. 1996;56:1137–1141. [PubMed] [Google Scholar]
- 22.Hu YX, Watanabe H, Ohtsubo K, et al. Frequent loss of p16 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. Clin Cancer Res. 1997;3:1473–1477. [PubMed] [Google Scholar]
- 23.Naka T, Kobayashi M, Ashida K, Toyota N, Kaneko T, Kaibara N. Aberrant p16INK4 expression related to clinical stage and prognosis in patients with pancreatic cancer. Int J Oncol. 1998;12:1111–1116. doi: 10.3892/ijo.12.5.1111. [DOI] [PubMed] [Google Scholar]
- 24.Garcea G, Neal CP, Pattenden CJ, Steward WP, Berry DP. Molecular prognostic markers in pancreatic cancer: a systematic review. Eur J Cancer. 2005;41:2213–2236. doi: 10.1016/j.ejca.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Barton CM, Staddon SL, Hughes CM, et al. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991;64:1076–1082. doi: 10.1038/bjc.1991.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong M, Ma G, Tu W, Guo KJ, Tian YL, Dong YT. Clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer. World J Gastroenterol. 2005;11:2162–2165. doi: 10.3748/wjg.v11.i14.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton JP, Timpson P, Karim SA, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:246–251. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 29.Lowe SW. Cancer therapy and p53. Curr Opin Oncol. 1995;7:547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y. p53 and its downstream proteins as molecular targets of cancer. Mol Carcinog. 2006;45:409–415. doi: 10.1002/mc.20231. [DOI] [PubMed] [Google Scholar]
- 31.Nakamori S, Yashima K, Murakami Y, et al. Association of p53 gene mutations with short survival in pancreatic adenocarcinoma. Jpn J Cancer Res. 1995;86:174–181. doi: 10.1111/j.1349-7006.1995.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong M, Nio Y, Yamasawa K, Toga T, Yue L, Harada T. p53 alteration is not an independent prognostic indicator, but affects the efficacy of adjuvant chemotherapy in human pancreatic cancer. J Surg Oncol. 2003;82:111–120. doi: 10.1002/jso.10186. [DOI] [PubMed] [Google Scholar]
- 33.Gerdes B, Ramaswamy A, Ziegler A, et al. p16INK4a is a prognostic marker in resected ductal pancreatic cancer: an analysis of p16INK4a, p53, MDM2, an Rb. Ann Surg. 2002;235:51–59. doi: 10.1097/00000658-200201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salek C, Minarikova P, Benesova L, et al. Mutation status of K-ras, p53 and allelic losses at 9p and 18q are not prognostic markers in patients with pancreatic cancer. Anticancer Res. 2009;29:1803–1810. [PubMed] [Google Scholar]
- 35.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 36.Miyaki M, Kuroki T. Role of SMAD4 (DPC4) inactivation in human cancer. Biochem Biophys Res Commun. 2003;306:799–804. doi: 10.1016/s0006-291x(03)01066-0. [DOI] [PubMed] [Google Scholar]
- 37.Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–2006. [PubMed] [Google Scholar]
- 38.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 39.Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–4121. [PubMed] [Google Scholar]
- 40.Biankin AV, Morey AL, Lee CS, et al. DPC4/SMAD4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol. 2002;20:4531–4542. doi: 10.1200/JCO.2002.12.063. [DOI] [PubMed] [Google Scholar]
- 41.Khorana AA, Hu YC, Ryan CK, Komorowski RA, Hostetter G, Ahrendt SA. Vascular endothelial growth factor and DPC4 predict adjuvant therapy outcomes in resected pancreatic cancer. J Gastrointest Surg. 2005;9:903–911. doi: 10.1016/j.gassur.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarte-Waldhoff I, Volpert OV, Bouck NP, et al. SMAD4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:9624–9629. doi: 10.1073/pnas.97.17.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–221. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 45.Lal G, Liu G, Schmocker B, et al. Inherited predisposition to pancreatic adenocarcinoma: role of family history and germ-line p16, BRCA1, and BRCA2 mutations. Cancer Res. 2000;60:409–416. [PubMed] [Google Scholar]
- 46.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–3793. [PubMed] [Google Scholar]
- 47.Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 48.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–5364. [PubMed] [Google Scholar]
- 49.Real FX, Malats N, Lesca G, et al. Family history of cancer and germline BRCA2 mutations in sporadic exocrine pancreatic cancer. Gut. 2002;50:653–657. doi: 10.1136/gut.50.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsiambas E, Karameris A, Dervenis C, et al. HER2/neu expression and gene alterations in pancreatic ductal adenocarcinoma: a comparative immunohistochemistry and chromogenic in situ hybridization study based on tissue microarrays and computerized image analysis. JOP. 2006;7:283–294. [PubMed] [Google Scholar]
- 51.Safran H, Steinhoff M, Mangray S, et al. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol. 2001;24:496–499. doi: 10.1097/00000421-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Talar-Wojnarowska R, Gasiorowska A, Smolarz B, et al. Clinical significance of K-ras and c-erbB-2 mutations in pancreatic adenocarcinoma and chronic pancreatitis. Int J Gastrointest Cancer. 2005;35:33–41. doi: 10.1385/IJGC:35:1:033. [DOI] [PubMed] [Google Scholar]
- 53.Yamanaka Y, Friess H, Kobrin MS, et al. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol. 1993;24:1127–1134. doi: 10.1016/0046-8177(93)90194-l. [DOI] [PubMed] [Google Scholar]
- 54.Stoecklein NH, Luebke AM, Erbersdobler A, et al. Copy number of chromosome 17 but not HER2 amplification predicts clinical outcome of patients with pancreatic ductal adenocarcinoma. J Clin Oncol. 2004;22:4737–4745. doi: 10.1200/JCO.2004.05.142. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. 2006;5:483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–2513. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Inhibition of nuclear factor kappab activity by genistein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int J Cancer. 2006;118:1930–1936. doi: 10.1002/ijc.21589. [DOI] [PubMed] [Google Scholar]
- 59.Buchler P, Gazdhar A, Schubert M, et al. The Notch signaling pathway is related to neurovascular progression of pancreatic cancer. Ann Surg. 2005;242:791–800. doi: 10.1097/01.sla.0000189115.94847.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walter K, Omura N, Hong SM, et al. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res. 2010;16:1781–1789. doi: 10.1158/1078-0432.CCR-09-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 63.Albazaz R, Verbeke CS, Rahman SH, McMahon MJ. Cyclooxygenase-2 expression associated with severity of PanIN lesions: a possible link between chronic pancreatitis and pancreatic cancer. Pancreatology. 2005;5:361–369. doi: 10.1159/000086536. [DOI] [PubMed] [Google Scholar]
- 64.Juuti A, Louhimo J, Nordling S, Ristimaki A, Haglund C. Cyclooxygenase-2 expression correlates with poor prognosis in pancreatic cancer. J Clin Pathol. 2006;59:382–386. doi: 10.1136/jcp.2005.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okami J, Yamamoto H, Fujiwara Y, et al. Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res. 1999;5:2018–2024. [PubMed] [Google Scholar]
- 66.Maitra A, Ashfaq R, Gunn CR, et al. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol. 2002;118:194–201. doi: 10.1309/TPG4-CK1C-9V8V-8AWC. [DOI] [PubMed] [Google Scholar]
- 67.Cheng JQ, Ruggeri B, Klein WM, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chadha KS, Khoury T, Yu J, et al. Activated Akt and Erk expression and survival after surgery in pancreatic carcinoma. Ann Surg Oncol. 2006;13:933–939. doi: 10.1245/ASO.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Fahy BN, Schlieman M, Virudachalam S, Bold RJ. AKT inhibition is associated with chemosensitisation in the pancreatic cancer cell line MIA-PaCa-2. Br J Cancer. 2003;89:391–397. doi: 10.1038/sj.bjc.6601037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lebe B, Sagol O, Ulukus C, et al. The importance of cyclin D1 and Ki67 expression on the biological behavior of pancreatic adenocarcinomas. Pathol Res Pract. 2004;200:389–396. doi: 10.1016/j.prp.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Ebert MP, Hernberg S, Fei G, et al. Induction and expression of cyclin D3 in human pancreatic cancer. J Cancer Res Clin Oncol. 2001;127:449–454. doi: 10.1007/s004320100235. [DOI] [PubMed] [Google Scholar]
- 72.Ito Y, Takeda T, Wakasa K, Tsujimoto M, Matsuura N. Expression and possible role of cyclin D3 in human pancreatic adenocarcinoma. Anticancer Res. 2001;21:1043–1048. [PubMed] [Google Scholar]
- 73.Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 74.Arumugam T, Simeone DM, Van Golen K, Logsdon CD. S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res. 2005;11:5356–5364. doi: 10.1158/1078-0432.CCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 75.Goicoechea SM, Bednarski B, Stack C, et al. Isoform-specific upregulation of palladin in human and murine pancreas tumors. PLoS One. 2010;5:e10347. doi: 10.1371/journal.pone.0010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salaria SN, Illei P, Sharma R, et al. Palladin is overexpressed in the non-neoplastic stroma of infiltrating ductal adenocarcinomas of the pancreas, but is only rarely overexpressed in neoplastic cells. Cancer Biol Ther. 2007;6:324–328. doi: 10.4161/cbt.6.3.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y, Li M, Wang H, et al. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J Surg. 2009;33:698–709. doi: 10.1007/s00268-008-9833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li A, Omura N, Hong SM, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–5237. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rachagani S, Kumar S, Batra SK. MicroRNA in pancreatic cancer: pathological, diagnostic and therapeutic implications. Cancer Lett. 2010;292:8–16. doi: 10.1016/j.canlet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ho AS, Huang X, Cao H, et al. Circulating miR-210 as a novel hypoxia marker in pancreatic cancer. Transl Oncol. 2010;3:109–113. doi: 10.1593/tlo.09256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uwagawa T, Chiao PJ, Gocho T, Hirohara S, Misawa T, Yanaga K. Combination chemotherapy of nafamostat mesilate with gemcitabine for pancreatic cancer targeting NF-kappaB activation. Anticancer Res. 2009;29:3173–3178. [PubMed] [Google Scholar]
- 82.Morton JP, Mongeau ME, Klimstra DS, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakashima H, Nakamura M, Yamaguchi H, et al. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 2006;66:7041–7049. doi: 10.1158/0008-5472.CAN-05-4588. [DOI] [PubMed] [Google Scholar]
- 85.Gong YL, Xu GM, Huang WD, Chen LB. Expression of matrix metalloproteinases and the tissue inhibitors of metalloproteinases and their local invasiveness and metastasis in Chinese human pancreatic cancer. J Surg Oncol. 2000;73:95–99. doi: 10.1002/(sici)1096-9098(200002)73:2<95::aid-jso7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 86.Juuti A, Lundin J, Nordling S, Louhimo J, Haglund C. Epithelial MMP-2 expression correlates with worse prognosis in pancreatic cancer. Oncology. 2006;71:61–68. doi: 10.1159/000100988. [DOI] [PubMed] [Google Scholar]
- 87.Jalanko H, Kuusela P, Roberts P, Sipponen P, Haglund CA, Makela O. Comparison of a new tumour marker, CA 19-9, with alpha-fetoprotein and carcinoembryonic antigen in patients with upper gastrointestinal diseases. J Clin Pathol. 1984;37:218–222. doi: 10.1136/jcp.37.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350–355. [PubMed] [Google Scholar]
- 89.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 90.Saif MW. Translational research in pancreatic cancer. Highlights from the "44th ASCO Annual Meeting". Chicago, IL, USA. May 30 - June 3, 2008. JOP. 2008;9:398–402. [PubMed] [Google Scholar]
- 91.Nagata K, Horinouchi M, Saitou M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–254. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 92.Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328–341. doi: 10.1007/s005340200037. [DOI] [PubMed] [Google Scholar]
- 93.Ueda M, Miura Y, Kunihiro O, et al. MUC1 overexpression is the most reliable marker of invasive carcinoma in intraductal papillary-mucinous tumor (IPMT) Hepatogastroenterology. 2005;52:398–403. [PubMed] [Google Scholar]
- 94.Swartz MJ, Batra SK, Varshney GC, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 95.Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 96.Saitou M, Goto M, Horinouchi M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 98.Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Ther. 2002;1:777–783. [PubMed] [Google Scholar]
- 99.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 100.Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913–3921. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 101.Starling N, Watkins D, Cunningham D, et al. Dose finding and early efficacy study of gemcitabine plus capecitabine in combination with bevacizumab plus erlotinib in advanced pancreatic cancer. J Clin Oncol. 2009;27:5499–5505. doi: 10.1200/JCO.2008.21.5384. [DOI] [PubMed] [Google Scholar]
- 102.Watkins DJ, Starling N, Chau I, et al. The combination of a chemotherapy doublet (gemcitabine plus capecitabine) with a biologic doublet (bevacizumab plus erlotinib) in patients with advanced pancreatic adenocarcinoma: The TARGET study. ASCO Meeting Abstracts. 2010;28:4036. [Google Scholar]
- 103.Ignatiadis M, Polyzos A, Stathopoulos GP, et al. A multicenter phase II study of docetaxel in combination with gefitinib in gemcitabine-pretreated patients with advanced/metastatic pancreatic cancer. Oncology. 2006;71:159–163. doi: 10.1159/000106064. [DOI] [PubMed] [Google Scholar]
- 104.Brell JM, Matin K, Evans T, et al. Phase II study of docetaxel and gefitinib as second-line therapy in gemcitabine pretreated patients with advanced pancreatic cancer. Oncology. 2009;76:270–274. doi: 10.1159/000206141. [DOI] [PubMed] [Google Scholar]
- 105.Fountzilas G, Bobos M, Kalogera-Fountzila A, et al. Gemcitabine combined with gefitinib in patients with inoperable or metastatic pancreatic cancer: a phase II Study of the Hellenic Cooperative Oncology Group with biomarker evaluation. Cancer Invest. 2008;26:784–793. doi: 10.1080/07357900801918611. [DOI] [PubMed] [Google Scholar]
- 106.Safran H, Miner T, Bahary N, et al. Lapatinib and gemcitabine for metastatic pancreatic cancer: a phase II study. ASCO Meeting Abstracts. 2009;27:e15653. doi: 10.1097/coc.0b013e3181d26b01. [DOI] [PubMed] [Google Scholar]
- 107.Safran H, Iannitti D, Ramanathan R, et al. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706–712. doi: 10.1081/cnv-200032974. [DOI] [PubMed] [Google Scholar]
- 108.Xiong HQ, Rosenberg A, LoBuglio A, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 109.Philip PA, Benedetti J, Fenoglio-Preiser C, et al. Phase III study of gemcitabine [G] plus cetuximab [C] versus gemcitabine in patients [pts] with locally advanced or metastatic pancreatic adenocarcinoma [PC]: SWOG S0205 study. ASCO Meeting Abstracts. 2007;25:LBA4509. [Google Scholar]
- 110.Lee F, Roach M, Duong L, Heywood G, Parasher G, Rasila K. Improved survival with combination oxaliplatin, irinotecan, cetuximab for metastatic pancreatic cancer. ASCO Meet Abstr. 2007;25:15180. [Google Scholar]
- 111.Conroy T, Desseigne F, Ychou M, et al. Randomized phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) versus gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. ASCO Meeting Abstracts. 2010;28:4010. [Google Scholar]
- 112.Egawa S, Tsutsumi M, Konishi Y, et al. The role of angiogenesis in the tumor growth of Syrian hamster pancreatic cancer cell line HPD-NR. Gastroenterology. 1995;108:1526–1533. doi: 10.1016/0016-5085(95)90703-3. [DOI] [PubMed] [Google Scholar]
- 113.Itakura J, Ishiwata T, Friess H, et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3:1309–1316. [PubMed] [Google Scholar]
- 114.Kindler HL, Niedzwiecki D, Hollis D, et al. A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): a preliminary analysis of Cancer and Leukemia Group B CALGB. ASCO Meeting Abstracts. 2007;25:4508. [Google Scholar]
- 115.Conrad C, Ischenko I, Kohl G, et al. Antiangiogenic and antitumor activity of a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor ZD6474 in a metastatic human pancreatic tumor model. Anticancer Drugs. 2007;18:569–579. doi: 10.1097/CAD.0b013e3280147d13. [DOI] [PubMed] [Google Scholar]
- 116.Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447–3455. doi: 10.1200/JCO.2001.19.15.3447. [DOI] [PubMed] [Google Scholar]
- 117.Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moore MJ, Hamm J, Dancey J, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296–3302. doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 119.Kilian M, Gregor JI, Heukamp I, et al. Matrix metalloproteinase inhibitor RO 28-2653 decreases liver metastasis by reduction of MMP-2 and MMP-9 concentration in BOP-induced ductal pancreatic cancer in Syrian Hamsters: inhibition of matrix metalloproteinases in pancreatic cancer. Prostaglandins Leukot Essent Fatty Acids. 2006;75:429–434. doi: 10.1016/j.plefa.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 120.Cohen SJ, Ho L, Ranganathan S, et al. Phase II and pharmacodynamic study of the farnesyltransferase inhibitor R115777 as initial therapy in patients with metastatic pancreatic adenocarcinoma. J Clin Oncol. 2003;21:1301–1306. doi: 10.1200/JCO.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 121.Macdonald JS, McCoy S, Whitehead RP, et al. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest New Drugs. 2005;23:485–487. doi: 10.1007/s10637-005-2908-y. [DOI] [PubMed] [Google Scholar]
- 122.Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 123.Liu M, Bryant MS, Chen J, et al. Antitumor activity of SCH 66336, an orally bioavailable tricyclic inhibitor of farnesyl protein transferase, in human tumor xenograft models and wap-ras transgenic mice. Cancer Res. 1998;58:4947–4956. [PubMed] [Google Scholar]
- 124.Eskens FA, Awada A, Cutler DL, et al. Phase I and pharmacokinetic study of the oral farnesyl transferase inhibitor SCH 66336 given twice daily to patients with advanced solid tumors. J Clin Oncol. 2001;19:1167–1175. doi: 10.1200/JCO.2001.19.4.1167. [DOI] [PubMed] [Google Scholar]
- 125.Awada A, Eskens FA, Piccart M, et al. Phase I and pharmacological study of the oral farnesyltransferase inhibitor SCH 66336 given once daily to patients with advanced solid tumours. Eur J Cancer. 2002;38:2272–2278. doi: 10.1016/s0959-8049(02)00379-9. [DOI] [PubMed] [Google Scholar]
- 126.Raut CP, Nawrocki S, Lashinger LM, et al. Celecoxib inhibits angiogenesis by inducing endothelial cell apoptosis in human pancreatic tumor xenografts. Cancer Biol Ther. 2004;3:1217–1224. doi: 10.4161/cbt.3.12.1221. [DOI] [PubMed] [Google Scholar]
- 127.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–2038. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 128.Blanquicett C, Saif MW, Buchsbaum DJ, et al. Antitumor efficacy of capecitabine and celecoxib in irradiated and lead-shielded, contralateral human BxPC-3 pancreatic cancer xenografts: clinical implications of abscopal effects. Clin Cancer Res. 2005;11:8773–8781. doi: 10.1158/1078-0432.CCR-05-0627. [DOI] [PubMed] [Google Scholar]
- 129.El-Rayes BF, Ali S, Sarkar FH, Philip PA. Cyclooxygenase-2-dependent and -independent effects of celecoxib in pancreatic cancer cell lines. Mol Cancer Ther. 2004;3:1421–1426. [PubMed] [Google Scholar]
- 130.Ali S, El-Rayes BF, Sarkar FH, Philip PA. Simultaneous targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways for pancreatic cancer therapy. Mol Cancer Ther. 2005;4:1943–1951. doi: 10.1158/1535-7163.MCT-05-0065. [DOI] [PubMed] [Google Scholar]
- 131.Lev-Ari S, Zinger H, Kazanov D, et al. Curcumin synergistically potentiates the growth inhibitory and pro-apoptotic effects of celecoxib in pancreatic adenocarcinoma cells. Biomed Pharmacother. 2005;59(Suppl 2):S276–S280. doi: 10.1016/s0753-3322(05)80045-9. [DOI] [PubMed] [Google Scholar]
- 132.Milella M, Gelibter A, Di Cosimo S, et al. Pilot study of celecoxib and infusional 5-fluorouracil as second-line treatment for advanced pancreatic carcinoma. Cancer. 2004;101:133–138. doi: 10.1002/cncr.20338. [DOI] [PubMed] [Google Scholar]
- 133.Pino MS, Milella M, Gelibter A, et al. Capecitabine and celecoxib as second-line treatment of advanced pancreatic and biliary tract cancers. Oncology. 2009;76:254–261. doi: 10.1159/000205388. [DOI] [PubMed] [Google Scholar]
- 134.Kerr S, Campbell C, Legore K, Witters L, Harvey H, Lipton A. Phase II trial of gemcitabine and irinotecan plus celecoxib in advanced adenocarcinoma of the pancreas. ASCO Meeting Abstracts. 2005;23:4155. [Google Scholar]
- 135.Lipton A, Campbell-Baird C, Witters L, Harvey H, Ali S. Phase II trial of gemcitabine, irinotecan, and celecoxib in patients with advanced pancreatic cancer. J Clin Gastroenterol. 2010;44:286–288. doi: 10.1097/MCG.0b013e3181cda097. [DOI] [PubMed] [Google Scholar]
- 136.El-Rayes BF, Zalupski MM, Shields AF, et al. A phase II study of celecoxib, gemcitabine, and cisplatin in advanced pancreatic cancer. Invest New Drugs. 2005;23:583–590. doi: 10.1007/s10637-005-1028-z. [DOI] [PubMed] [Google Scholar]
- 137.Dragovich T, Burris H, 3rd, Loehrer P, et al. Gemcitabine plus celecoxib in patients with advanced or metastatic pancreatic adenocarcinoma: results of a phase II trial. Am J Clin Oncol. 2008;31:157–162. doi: 10.1097/COC.0b013e31815878c9. [DOI] [PubMed] [Google Scholar]
- 138.Caldwell B, Aldington S, Weatherall M, Shirtcliffe P, Beasley R. Risk of cardiovascular events and celecoxib: a systematic review and meta-analysis. J R Soc Med. 2006;99:132–140. doi: 10.1258/jrsm.99.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.White WB, West CR, Borer JS, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol. 2007;99:91–98. doi: 10.1016/j.amjcard.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 140.Chen LC, Ashcroft DM. Risk of myocardial infarction associated with selective COX-2 inhibitors: meta-analysis of randomised controlled trials. Pharmacoepidemiol Drug Saf. 2007;16:762–772. doi: 10.1002/pds.1409. [DOI] [PubMed] [Google Scholar]
- 141.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dhillon N, Wolff RA, Abbruzzese JL, et al. Phase II clinical trial of curcumin in patients with advanced pancreatic cancer. ASCO Meeting Abstracts. 2006;24:14151. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 143.Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 144.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 145.Banerjee S, Zhang Y, Ali S, et al. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 146.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 147.El-Rayes BF, Ali S, Ali IF, Philip PA, Abbruzzese J, Sarkar FH. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-kappaB. Cancer Res. 2006;66:10553–10559. doi: 10.1158/0008-5472.CAN-06-2333. [DOI] [PubMed] [Google Scholar]
- 148.Grewe M, Gansauge F, Schmid RM, Adler G, Seufferlein T. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 1999;59:3581–3587. [PubMed] [Google Scholar]
- 149.Shah SA, Potter MW, Ricciardi R, Perugini RA, Callery MP. FRAP-p70s6K signaling is required for pancreatic cancer cell proliferation. J Surg Res. 2001;97:123–130. doi: 10.1006/jsre.2001.6145. [DOI] [PubMed] [Google Scholar]
- 150.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The rapamycin analog CCI-779 is a potent inhibitor of pancreatic cancer cell proliferation. Biochem Biophys Res Commun. 2005;331:295–302. doi: 10.1016/j.bbrc.2005.03.166. [DOI] [PubMed] [Google Scholar]
- 151.Ito D, Fujimoto K, Mori T, et al. In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. Int J Cancer. 2006;118:2337–2343. doi: 10.1002/ijc.21532. [DOI] [PubMed] [Google Scholar]
- 152.Buck E, Eyzaguirre A, Brown E, et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 153.Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–198. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gysin S, Lee SH, Dean NM, McMahon M. Pharmacologic inhibition of RAF-->MEK-->ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1. Cancer Res. 2005;65:4870–4880. doi: 10.1158/0008-5472.CAN-04-2848. [DOI] [PubMed] [Google Scholar]
- 155.Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281–5293. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 156.Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 157.Moore PS, Barbi S, Donadelli M, et al. Gene expression profiling after treatment with the histone deacetylase inhibitor trichostatin A reveals altered expression of both pro- and anti-apoptotic genes in pancreatic adenocarcinoma cells. Biochim Biophys Acta. 2004;1693:167–176. doi: 10.1016/j.bbamcr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 158.Donadelli M, Costanzo C, Faggioli L, et al. Trichostatin A, an inhibitor of histone deacetylases, strongly suppresses growth of pancreatic adenocarcinoma cells. Mol Carcinog. 2003;38:59–69. doi: 10.1002/mc.10145. [DOI] [PubMed] [Google Scholar]
- 159.Ryu JK, Lee WJ, Lee KH, et al. SK-7041, a new histone deacetylase inhibitor, induces G2-M cell cycle arrest and apoptosis in pancreatic cancer cell lines. Cancer Lett. 2006;237:143–154. doi: 10.1016/j.canlet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 160.Sato N, Ohta T, Kitagawa H, et al. FR901228, a novel histone deacetylase inhibitor, induces cell cycle arrest and subsequent apoptosis in refractory human pancreatic cancer cells. Int J Oncol. 2004;24:679–685. [PubMed] [Google Scholar]
- 161.Piacentini P, Donadelli M, Costanzo C, Moore PS, Palmieri M, Scarpa A. Trichostatin A enhances the response of chemotherapeutic agents in inhibiting pancreatic cancer cell proliferation. Virchows Arch. 2006;448:797–804. doi: 10.1007/s00428-006-0173-x. [DOI] [PubMed] [Google Scholar]
- 162.Donadelli M, Costanzo C, Beghelli S, et al. Synergistic inhibition of pancreatic adenocarcinoma cell growth by trichostatin A and gemcitabine. Biochim Biophys Acta. 2007;1773:1095–1106. doi: 10.1016/j.bbamcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 163.Arnold NB, Arkus N, Gunn J, Korc M. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces growth inhibition and enhances gemcitabine-induced cell death in pancreatic cancer. Clin Cancer Res. 2007;13:18–26. doi: 10.1158/1078-0432.CCR-06-0914. [DOI] [PubMed] [Google Scholar]
- 164.Kumagai T, Wakimoto N, Yin D, et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (Vorinostat, SAHA) profoundly inhibits the growth of human pancreatic cancer cells. Int J Cancer. 2007;121:656–665. doi: 10.1002/ijc.22558. [DOI] [PubMed] [Google Scholar]
- 165.Bai J, Demirjian A, Sui J, Marasco W, Callery MP. Histone deacetylase inhibitor trichostatin A and proteasome inhibitor PS-341 synergistically induce apoptosis in pancreatic cancer cells. Biochem Biophys Res Commun. 2006;348:1245–1253. doi: 10.1016/j.bbrc.2006.07.185. [DOI] [PubMed] [Google Scholar]
- 166.Hwang JH, Lee SH, Lee KH, et al. Cathepsin B is a target of Hedgehog signaling in pancreatic cancer. Cancer Lett. 2009;273:266–272. doi: 10.1016/j.canlet.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 167.Stanton BZ, Peng LF. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst. 2010;6:44–54. doi: 10.1039/b910196a. [DOI] [PubMed] [Google Scholar]
- 168.Feldmann G, Fendrich V, McGovern K, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther. 2008;7:2725–2735. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shafaee Z, Schmidt H, Du W, Posner M, Weichselbaum R. Cyclopamine increases the cytotoxic effects of paclitaxel and radiation but not cisplatin and gemcitabine in Hedgehog expressing pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;58:765–770. doi: 10.1007/s00280-006-0227-4. [DOI] [PubMed] [Google Scholar]
- 170.Hu WG, Liu T, Xiong JX, Wang CY. Blockade of sonic hedgehog signal pathway enhances antiproliferative effect of EGFR inhibitor in pancreatic cancer cells. Acta Pharmacol Sin. 2007;28:1224–1230. doi: 10.1111/j.1745-7254.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 171.Mueller MT, Hermann PC, Witthauer J, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]