Abstract
Background/Aims
Several studies have found that the frequency of colorectal polyps increases significantly from the age of 50 years. The goal of this study was to determine the differences in the clinical characteristics of colorectal polyps between patients aged 50 years and older, and younger patients.
Methods
The colonoscopy database of 3,304 patients at the Yeungnam University Medical Center between January 2009 and December 2009 was reviewed retrospectively. In total, 679 patients were divided into the younger group (n=170) and the older group (aged ≥50 years) (n=509). A matched case-control study was performed using propensity scores and 117 patients selected from each group.
Results
Compared to the younger group, the older group had a significantly higher proportion of female patients, and patients with hypertension, a smoking history, and a history of taking medications. After performing the matched case-control study, 234 patients and 679 colon polyps were included in the analysis. Compared to the younger patients, the older patients had a significantly higher proportion of multiple lesions (57.3% vs 25.6%, p<0.001), left- and right-side distribution (35.9% vs 12.0%, p<0.001), and larger polyps (mean 9.1 mm vs 6.3 mm, p<0.001). A left-sided distribution was less common in the older group than in the younger group (35.0% vs 51.3%, p=0.025).
Conclusions
The methods used to screen for colorectal cancer in older patients should include colonoscopy due to the shift to the right side as a common location for colorectal polyps in that age group.
Keywords: Colonic polyp, Colonoscopy, Korean
INTRODUCTION
A gastrointestinal polyp is a discrete mass of tissue that protrudes into the lumen of the bowel.1 A polyp may be characterized by its gross appearance. Colon polyps may be divided into two major groups: neoplastic (the adenoma and carcinoma) and nonneoplastic.1
Several studies have reported that the frequency of colorectal polyps significantly increases at 50 years of age and older.2,3 It has been generally accepted that colorectal cancers and adenomas are found more frequently in the older population.4,5 However, both genetic and nongenetic factors, such as male gender, obesity, and smoking are thought to be potentially increasing factors associated with the development of these lesions;6-8 several studies have investigated the association between co-morbidities with medications and the development of colon polyps.9-11 Therefore, we conducted a case-control study, using propensity scores to reduce the influence of these factors,12 to compare the differences in the clinical characteristics of colorectal polyps between an older group, age 50 and above, and a younger group less than 50 years of age.
MATERIALS AND METHODS
1. Endoscopic procedures
Each patient ingested four liters of polyethylene glycol electrolyte solution for bowel preparation. During the procedure, the location and size of each polyp were documented. The size of each polyp was estimated using open-biopsy forceps (diameter 8 mm). All of the polyps were removed endoscopically using biopsy forceps, endoscopic mucosal resection (EMR) or endoscopic submucosal resection (ESD).
2. Indications of colonoscopies
Colonoscopies were generally performed if the following clinical indications were present: anemia (hemoglobin below 14 g/dL in males and 13 g/dL in females), bowel habit changes, family history of colorectal cancer or inherited colorectal cancer syndromes (e.g., familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer), positive stool occult blood test, abdominal pain, abnormal abdominal mass, and hematochezia. In addition, in some cases, colonoscopy was used for colon cancer screening in asymptomatic patients aware of the availability of such screening.
3. Patient enrollments
This was a retrospective study that used information from a colonoscopy database. All colonoscopy procedures were performed at the Yeungnam University Medical Center, Daegu, South Korea from January 2009 and November 2009. Patients that underwent a colonoscopy were included in this study. Exclusion criteria included: cases with family history of colorectal cancer, family history of familial adenomatous polyposis, family history of hereditary nonpolyposis colorectal cancer, incomplete medical records, patients receiving care for a colorectal neoplasm and postcolectomy patients.
A total of 3,304 patients were identified in the colonoscopy database. The records of 1,511 patients were excluded due to: colorectal neoplasms identified on follow up, postcolectomy patients, family history of colorectal cancer and incomplete colonoscopy. A total of 839 patients (839/1,793, 46.8%) had colorectal polyps. These patients were divided into the young group (178/553, 32.2%) and the older group (661/1,240, 53.3%), (χ2 test, p<0.001). Then, 160 patients were excluded because of incomplete medical record information including heights, weights, medication history, smoking habits and co-morbidities. Each patient in the young group was matched by gender, smoking habits, comorbid conditions, medications, and body mass index (BMI) to a patient in the older group. This case control study was performed using multivariate matched sampling methods that incorporate the propensity score.
4. Data collection of patients and polyps factors
The patients were divided into young (<50 years old) and older (≥50 years old) age groups. Demographic and clinical characteristics, including age, gender, smoking habits, BMI, past medical history and medications were investigated. The location, size, shape, and histopathological type of lesions were also evaluated. The location was classified into right-side or left-side of the colon divided at the splenic flexure, which was considered the left-side colon. Furthermore, the location was divided by cecum, ascending colon, transverse colon, descending colon, sigmoid colon and rectum. The size of the lesion was classified as <5 or ≥5 mm. The shape of the lesion was classified as a protruding type or superficial elevated type. The histopathological types included: hyperplastic polyps, adenomatous polyps, adenocarcinoma and others. Polyps were considered advanced, if they were larger than 10 mm or were tubovillous, villous, or malignant.
5. Statistical analysis
Student t-test was used to compare the mean values of continuous variables. The χ2 test and the Fisher's exact test were used as appropriate for comparison of the categorical variables. A p value less than 0.05 was accepted as statistically significant and all values are presented as the mean±standard deviation unless mentioned otherwise. The analysis was performed with a statistical software package (SPSS 17.0 version for Windows; SPSS Inc., Chicago, IL, USA). A propensity analysis was performed modeling the probability of the young. Multivariate logistic regression analysis using clinically relevant variables was used to compute a propensity score for each patient. The propensity score was then used to obtain a one-to-one match of all polyp cases by a "greedy matching" technique.12 All matching was performed with a Statistical Analysis Systems software package (Release 9.1.3; SAS Institute, Cary, NC, USA).
RESULTS
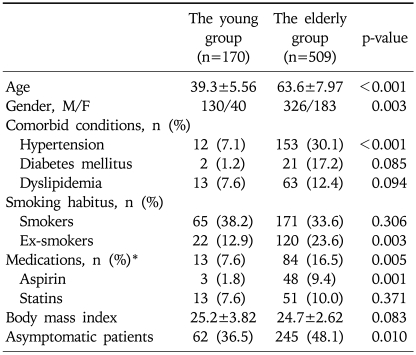
The patient characteristics of two groups are listed in Table 1. In summary, the older group had a significantly higher proportion of women, patients with hypertension, smoking history, and medication history (including COX-2 inhibitors, NSAID, Aspirin, and statins) that may influence the development of colorectal polyps, and asymptomatic patients requesting colon cancer screening compared to the young group.
Table 1.
Clinical Characteristics of the Younger versus Older Groups in 679 Unmatched Cases
*The medications included COX-2 inhibitor, NSAID, aspirin, and statins.
1. Comparison using propensity score analysis
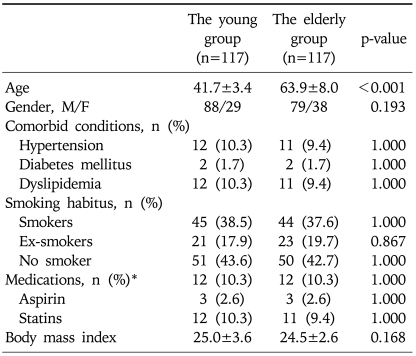
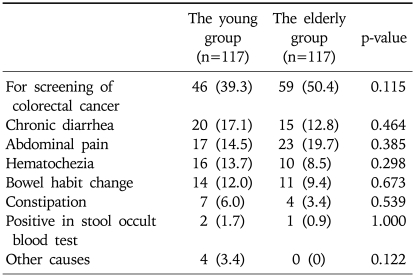
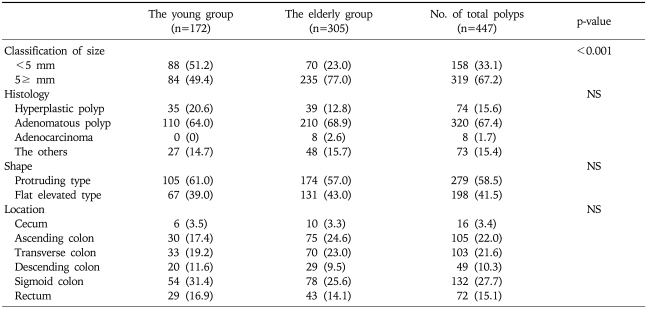
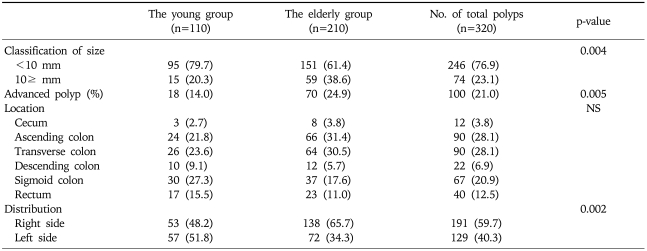
The propensity score model included 11 patient variables that are listed in the Appendix. The c statistic for this model was 0.711 (Hosmer-Lemeshow goodness-of-fit p=0.138). One hundred and seventeen young cases (68.0%) were matched to the older patients. There was no significant difference in the baseline parameters between the two groups (Table 2). Furthermore, the clinical indications for colonoscopy were not significantly different between the two groups (Table 3). When the polyps were classified by the size of the polyps into '<10 mm' and '≥10 mm', the larger polyps were detected significantly more often in the older age group (21.0% vs 12.2%, p=0.018). However, there was no statistical difference in the histology, shapes of the polyps on endoscopy, location of the polyps, between the young and the old groups (Table 4). When the only adenomatous polyps were selected, advanced polyps were detected more often in the older age group (14.0% vs 24.9%, p=0.005), and the distribution of adenomatous polyps were predominantly right-sided in the older age group (Table 5).
Table 2.
Clinical Characteristics of the Younger versus Older Groups in 234 Matched Cases Using Propensity Scores
*The medications included COX-2 inhibitor, NSAID, aspirin, and statins.
Table 3.
Clinical Indications for Colonoscopy in 234 Matched Cases
Values are presented as number (%).
Table 4.
Analysis of 447 Polyps from the Younger versus Older Groups in 234 Matched Cases
Values are presented as number (%).
NS, not significant.
Table 5.
Analysis of Adenomatous Polyps from the Younger versus Older Groups in 234 Matched Cases
Values are presented as number (%).
NS, not significant.
2. Differences in characteristics of colorectal polyps between the young and older patients
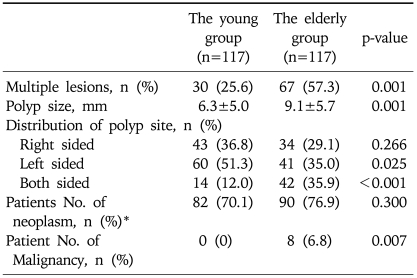
The older patients that were compared to the young patients had a significantly higher proportion of multiple lesions (57.3% vs 25.6%, p<0.001), bilateral distribution of the lesions (35.9% vs 12.0%, p<0.001), and a greater mean polyp size (9.1 mm vs 6.3 mm, p<0.001). The young patient group had a significantly higher proportion of single polyps and small sized polyps. Only a left-side distribution was detected more commonly in the young compared to the older patients (51.3% vs 35.0%, p=0.025). The number of patients with an adenoma and/or adenocarcinoma detected by colonoscopy biopsies were similar in comparisons between the young and the older patients (70.1% vs 76.9%, p=0.300). However, malignancies were more often found in the older group (6.8%) than in the young group (0%) (Fisher exact test, p=0.007) (Table 6).
Table 6.
Differences in the Characteristics of Colorectal Polyps between Younger and Older Patients in 234 Matched Cases
*Patient No. of neoplasms refers to the number of patients that had an adenoma and/or adenocarcinoma detected on the colonoscopy biopsies.
DISCUSSION
Several epidemiological studies have previously been reported regarding the analysis of colorectal polyps according to tumor location.13-16 These reports indicated that right-sided cancer was frequently found in older patients, whereas left-sided cancer was more common in younger patients. Histopathological and morphological differences have been reported between proximal and distal colon cancer.17,18 Moreover, epidemiological studies have revealed that the clinical risk factors for cancer development are also different between proximal and distal colon cancer.13,19 However, the reasons for these differences between left- and right-sided cancer remain unknown. In recent study, Yamaji et al.20 reported right-side shift of colorectal adenomas with aging, similar to cancer. Similarly, the results of this study showed that the distribution of colorectal polyps in the young was more common on the left-side of the colon, whereas the distribution of colorectal polyps among older persons tended to include both sides.
Several methods are used to screen for colorectal cancer. Health care providers may suggest one or more of the following tests for colorectal cancer screening: fecal occult blood testing, sigmoidoscopy, colonoscopy, virtual colonoscopy, and double contrast barium enema.21 Sigmoidoscopy is used for screening asymptomatic individuals for early cancer detection and prevention. Two large multicenter studies have shown a higher yield of advanced colorectal neoplasia (large adenomas or cancer) in subjects screened by sigmoidoscopy compared to fecal occult blood testing. Case-control studies have clearly shown that the screening sigmoidoscopy reduces by half the incidence of colorectal cancer and decreases colon cancer mortality by 60-70%.22,23 The screening for colorectal cancer using colonoscopy in asymptomatic persons with average-risk is controversial.24 However, several lines of evidence support the effectiveness of screening colonoscopy. Colonoscopy with polypectomy has been shown to reduce the expected incidence of colorectal cancer by 76-90% in cohort studies. There is direct evidence that the screening sigmoidoscopy reduces colorectal cancer mortality, and the visualization of neoplasms by colonoscopy is at least as good as by sigmoidoscopy.25-27 The results of this study suggested that colonoscopy appears to be the best screening method for the detection of colorectal neoplasms among older persons (50 and older) in Korea; this is because of the higher frequency of right-sided lesions in older persons.
The detection of neoplasms was significantly higher in the older age group among patients undergoing colonoscopies. Several reports on the findings of colonoscopy among older individuals support these findings.16,28 The results of this study showed that 32.3% of the young had colorectal polyps detected by total colonoscopy, and 53.3% of the older patients had colorectal polyps detected (χ2 test, p<0.001). However, the proportion of adenomas among the colorectal polyps in the young patients was comparable to the older patients as shown in Table 4 (64.0% vs 68.9%, p=0.310). Therefore, for persons less than 50 years of age, colorectal screening using sigmoidoscopy with polypectomy may help reduce the incidence and mortality of colorectal cancer.
The primary limitation of the study was its retrospective design. The propensity score adjustment is no substitute for a properly designed, randomized, controlled trial. The retrospective nature of the study cannot account for unknown variables affecting the outcome that might significantly correlate with the measured variables. In addition, this study was conducted at only one center, which may limit the generalization of the data collected.
In conclusion, colorectal polyps were more commonly found in older patients. Most polyps in the young patients were identified as a single lesion, were small polyps and located on the left side. However, the proportion of neoplastic polyps was similar to that of the older patients. For the older patients, colonoscopy is the best screening method for the detection of colorectal neoplasms because of the higher frequency of right-sided lesions with advanced age.
ACKNOWLEDGEMENTS
There is no conflict of interest to report regarding this article.
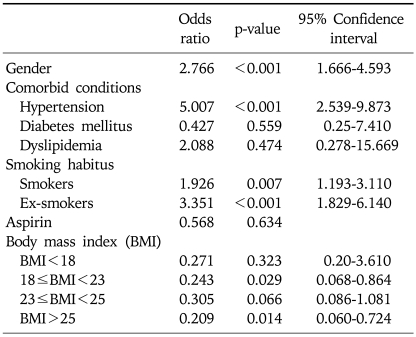
Appendix
Logistic Regression Model to Generate Propensity Scores for the Younger versus Older Groups
References
- 1.Sleisenger MH, Feldman M, Friedman LS, Brandt LJ. Sleisenger & Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. 8th ed. Volume 2. Philadelphia: Saunders Elsevier; 2006. [Google Scholar]
- 2.Khder SA, Trifan A, Danciu M, Stanciu C. Colorectal polyps: clinical, endoscopic, and histopathologic features. Rev Med Chir Soc Med Nat Iasi. 2008;112:59–65. [PubMed] [Google Scholar]
- 3.Nam JH, Yang CH. Clinical characteristics and risk factors of colon polyps in gyeongju and pohang area. Korean J Gastroenterol. 2008;52:142–149. [PubMed] [Google Scholar]
- 4.Haenszel W, Correa P. Cancer of the colon and rectum and adenomatous polyps. A review of epidemiologic findings. Cancer. 1971;28:14–24. doi: 10.1002/1097-0142(197107)28:1<14::aid-cncr2820280105>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Yamaji Y, Mitsushima T, Ikuma H, et al. Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese. Gut. 2004;53:568–572. doi: 10.1136/gut.2003.026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachem C, Morgan R, Johnson M, Kuebeler M, El-Serag H. Statins and the risk of colorectal carcinoma: a nested case-control study in veterans with diabetes. Am J Gastroenterol. 2009;104:1241–1248. doi: 10.1038/ajg.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omata F, Brown WR, Tokuda Y, et al. Modifiable risk factors for colorectal neoplasms and hyperplastic polyps. Intern Med. 2009;48:123–128. doi: 10.2169/internalmedicine.48.1562. [DOI] [PubMed] [Google Scholar]
- 8.Setoguchi S, Glynn RJ, Avorn J, Mogun H, Schneeweiss S. Statins and the risk of lung, breast, and colorectal cancer in the elderly. Circulation. 2007;115:27–33. doi: 10.1161/CIRCULATIONAHA.106.650176. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959–2967. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002;11:1012–1018. [PubMed] [Google Scholar]
- 11.Potter JD, Bigler J, Fosdick L, et al. Colorectal adenomatous and hyperplastic polyps: smoking and N-acetyltransferase 2 polymorphisms. Cancer Epidemiol Biomarkers Prev. 1999;8:69–75. [PubMed] [Google Scholar]
- 12.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Distler P, Holt PR. Are right- and left-sided colon neoplasms distinct tumors? Dig Dis. 1997;15:302–311. doi: 10.1159/000171605. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez EC, Roetzheim RG, Ferrante JM, Campbell R. Predictors of proximal vs. distal colorectal cancers. Dis Colon Rectum. 2001;44:251–258. doi: 10.1007/BF02234301. [DOI] [PubMed] [Google Scholar]
- 15.Jass JR. Subsite distribution and incidence of colorectal cancer in New Zealand, 1974-1983. Dis Colon Rectum. 1991;34:56–59. doi: 10.1007/BF02050208. [DOI] [PubMed] [Google Scholar]
- 16.McCashland TM, Brand R, Lyden E, de Garmo P. Gender differences in colorectal polyps and tumors. Am J Gastroenterol. 2001;96:882–886. doi: 10.1111/j.1572-0241.2001.3638_a.x. [DOI] [PubMed] [Google Scholar]
- 17.Ashktorab H, Smoot DT, Farzanmehr H, et al. Clinicopathological features and microsatellite instability (MSI) in colorectal cancers from African Americans. Int J Cancer. 2005;116:914–919. doi: 10.1002/ijc.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazama Y, Watanabe T, Kanazawa T, Tada T, Tanaka J, Nagawa H. Mucinous carcinomas of the colon and rectum show higher rates of microsatellite instability and lower rates of chromosomal instability: a study matched for T classification and tumor location. Cancer. 2005;103:2023–2029. doi: 10.1002/cncr.21022. [DOI] [PubMed] [Google Scholar]
- 19.Akhter M, Kuriyama S, Nakaya N, et al. Alcohol consumption is associated with an increased risk of distal colon and rectal cancer in Japanese men: the Miyagi Cohort Study. Eur J Cancer. 2007;43:383–390. doi: 10.1016/j.ejca.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Yamaji Y, Mitsushima T, Ikuma H, et al. Right-side shift of colorectal adenomas with aging. Gastrointest Endosc. 2006;63:453–458. doi: 10.1016/j.gie.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–877. doi: 10.1111/j.1572-0241.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 22.Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med. 1995;123:904–910. doi: 10.7326/0003-4819-123-12-199512150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 24.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 25.Brenner H, Arndt V, Sturmer T, Stegmaier C, Ziegler H, Dhom G. Long-lasting reduction of risk of colorectal cancer following screening endoscopy. Br J Cancer. 2001;85:972–976. doi: 10.1054/bjoc.2001.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiis-Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol. 1999;34:414–420. doi: 10.1080/003655299750026443. [DOI] [PubMed] [Google Scholar]
- 27.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 28.Ure T, Dehghan K, Vernava AM, 3rd, Longo WE, Andrus CA, Daniel GL. Colonoscopy in the elderly. Low risk, high yield. Surg Endosc. 1995;9:505–508. doi: 10.1007/BF00206836. [DOI] [PubMed] [Google Scholar]