Abstract
Background/Aims
The diagnosis of hyperplastic polyps (HPs) may involve a conglomeration of subgroups of serrated polyps. The diagnosis of HPs may therefore be revisited if this is sessile serrated adenoma (SSA). The aim of this study was to determine clinically and endoscopically relevant information associated with reclassification to SSA.
Methods
After reviewing the data from 1,372 patients who underwent colonoscopic polypectomy, 49 HPs larger than 10 mm were analyzed in this study. Two gastrointestinal pathologists reclassified each of the original 49 HPs as conventional HPs, SSAs, and others.
Results
Among the 49 initially diagnosed HPs, 18.4% were reclassified into SSAs or mixed polyps. Overall architectural features were useful for the diagnosis of SSA, but cytological features were less useful. The patient and polyp characteristics did not differ between HPs with and without reclassification of the initial pathological diagnosis.
Conclusions
A significant number of SSAs might not be accurately diagnosed in daily clinical practice without any predilection for size, shape, and location. Therefore, when large HPs are diagnosed in clinical practice, it is necessary for physicians to have greater awareness of the diagnosis of SSA and to individualize subsequent surveillance.
Keywords: Colorectal polyp, Hyperplastic polyp, Sessile serrated adenoma
INTRODUCTION
It is well established that colorectal adenomas are precursors of most colorectal cancers.1,2 In contrast, hyperplastic polyps (HPs) have been traditionally considered as harmless lesions with little or no malignant potential.3 However, this traditional view may be too simplistic because conventional HPs may have considerable heterogeneity with regard to histology and some of the lesions may be precursors of colorectal cancer. Serrated polyps, which are histologically characterized by their saw-tooth architecture, include conventional HPs, sessile serrated adenomas (SSAs), traditional serrated adenomas (TSAs) and mixed polyps.4-6 Serrated polyps were previously classified as HPs before the histological differences between subgroups of serrated polyps were fully appreciated.
In daily clinical practice, the diagnosed HPs may comprise a conglomeration of subgroups of serrated polyps. Furthermore, the diagnosis of a HP larger than 10 mm may be revisited by the endoscopists and pathologists to determine if this is SSA. In an effort to explore this issue, in this study, we microscopically reclassified 49 HPs larger than 10 mm diagnosed by colonoscopic polypectomy.
MATERIALS AND METHODS
1. Patients
Data from 1,372 patients who underwent colonoscopic polypectomy in our hospital between June 1, 2006, and March 30, 2009, were retrospectively reviewed, and a group of 49 HPs larger than 10 mm was chosen for further analysis. Only HPs larger than 10 mm were included in this analysis based on their size, because HPs larger than 10 mm were routinely polypectomized in our hospital. Patients whose colorectal polyps were removed by hot or cold biopsy only were excluded from this analysis. Data for all subjects were obtained from endoscopy reports, electronic medical records and laboratory results. The institutional review board of our hospital approved the analysis of the data and reporting of the results of this study.
2. Colonoscopic polypectomy
Colonoscopic polypectomies were performed in a standard manner using a standard video colonoscope (EC-590ZW; Fujinon Inc., Saitama, Japan). Colonoscopic polypectomies were not performed if patients did not agree to the procedure, or if they were actively anticoagulated, or if they were in poor general conditions (greater than Grade III in the classification provided by the American Society of Anesthesiologists) or if they had lesions with malignant polyps. For conscious sedative endoscopy, an individualized dose of midazolam and/or propofol was administered by the endoscopist based on the age, weight and general condition of the patients. An electrocautery ERBE ICC 200 (ERBE Electromedizin, Tübingen, Germany) or an argon plasma coagulation ERBE APC 200 (ERBE Electromedizin) were used as the electrosurgical device. Standard sclerosis needles and endoscopic snares were used during the polypectomy procedures.
3. Review of medical records
Cases originally reported as HPs were reviewed to record their age, sex, indications for colonoscopic polypectomy; the location, size, and number of HPs; and presence of synchronous adenomas or carcinomas. The size of a polyp was recorded by review of all endoscopy reports and images. The shape of the polyp was classified according to the Paris classification system.7 The right colon was defined as the colonic region from the cecum to the splenic flexure and the left colon was defined as the colonic region distal to the splenic flexure.
4. Pathologic assessment
A group of 49 HPs larger than 10 mm originally reported were reclassified by two gastrointestinal pathologists (SJL, GYK). The two pathologists reviewed each hematoxylin-eosin-stained slide with multi-head light microscopes. They were not aware of the original diagnosis of HP but, to mimic routine clinical practice as closely as possible, age and sex of the patient as well as the location and size of polyps were provided for each case. The diagnostic criteria for TSA and SSA were based on the guidelines outlined by Bariol et al.8 and Torlakovic et al.9 Prior to distribution of the slide collection, the pathologists had reviewed their original papers8,9 and had also received a summary sheet of the diagnostic criteria for SSA, TSA, and mixed polyps. The pathologists were asked to reclassify each of the original 49 HPs into conventional HPs, SSAs, TSAs, mixed polyps. After completion of the independent assessment of each polyp by two pathologists, the diagnoses of all lesions were compared. When there was disagreement, the slide was jointly re-assessed, a consensus reached and a final diagnosis was made.
To reclassify the histological diagnosis, each slide was reevaluated for the following histological parameters: association with conventional adenoma or carcinoma, presence of cytologic dysplasia and its grade, amount of serration, presence of eosinophilic cytoplasm, presence of basal crypt branching, presence of basal crypt dilatation and horizontal crypts. For the histological reclassification, TSA was defined by the presence of serrations in ≥20% of the lesion crypts in association with surface epithelial dysplasia. SSA was defined by a serrated pattern throughout the entire length of the crypts and the absence or rarity of undifferentiated cells in the lower third of the crypts (Fig. 1). Architectural abnormalities including dilatation, branching or broad bases in basal crypts were considered as a minor criterion for the diagnosis of SSA. Mixed polyp was defined by foci of dysplastic but non-serrated epithelium in immediate proximity to serrated but non-dysplastic epithelium when the histology harbor characteristics of two or more morphological subtypes depending on the area. Cytologic dysplasia was graded as low- or high-grade;10 low-grade dysplasia was composed of minimal to moderate epithelial atypia and high-grade dysplasia (HGD) was composed of only severe epithelial atypia without invasion.
Fig. 1.
Histopathological features of sessile serrated adenomas (SSAs). (A) SSAs were defined by a base serration (arrows) and the absence or rarity of undifferentiated cells in the basal crypts (H&E stain, ×400). (B) Other characteristic architectural abnormalities include crypt horizontalization (arrowheads) and branching (H&E stain, ×400).
5. Statistical analysis
All data are presented as percentage (number) of patients or mean (standard deviation, SD) of variables. Continuous variables were compared using the Mann-Whitney test, if data were not normally distributed. Categorical variables were compared with the χ2 tests or Fisher's exact test, when appropriate. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software, version 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Out of 1,372 patients who underwent colonoscopic polypectomy during the study period, 49 original pathological diagnoses of HPs from 46 (3.4%) patients were analyzed. The clinical and endoscopic data of the 49 initial pathological diagnoses of HPs are listed in Table 1. In per-patient analysis, the average age of the patients was 56.9 years (SD, 12.4) and there was a slight male preponderance (58.7%). In per-polyp analysis, the mean number of HPs larger than 10 mm per patient was 1.1 and their mean size was 11.7 mm (SD, 3.5). HPs larger than 10 mm were associated with synchronous conventional adenomas in 65.2% of the patients and were associated with synchronous HGD or carcinoma in 15.2% of the patients. Hyperplastic polyposis syndrome by WHO classification11 was not diagnosed in any patient.
Table 1.
Clinical and Endoscopic Characteristics in Patients with Hyperplastic Polyps Larger than 10 mm
SD, standard deviation; FOBT, fecal occult blood test; HGD, high-grade dysplasia; HP, hyperplastic polyp; IDA, iron deficiency anemia.
The diagnoses of 47 polyps were agreed between each assessment by two pathologists, and the diagnoses of the remained 2 polyps were reached by a joint re-assessment because of disagreement between two pathologists. Among 49 initial pathological diagnoses of HPs, 40 polyps (81.6%) were reclassified into conventional HPs, 8 polyps (16.4%) were reclassified into SSA and 1 (2.0%) into mixed polyp (Table 2). No polyp was reclassified into TSA or conventional adenoma. Table 3 lists the histopathological features used for the identification of SSA in 8 polyps that were reclassified into SSA. As SSA was defined by base serration and the absence of dysplasia in the basal crypts in this study, base serration of the histopathological parameters was identified in all cases. Overall architectural features (branching, dilatation, horizontal alignment of the basal crypt, and base serration) were useful for the diagnosis of SSA (Fig. 1), but cytological features (eosinophilic cytoplasm and mitosis at upper portion of crypt) were less useful.
Table 2.
Reclassified Diagnosis of Hyperplastic Polyps Larger than 10 mm
SSA, sessile serrated adenoma; TSA, traditional serrated adenoma.
Table 3.
Histopathological Features for the Identification of Sessile Serrated Adenomas in Eight Cases
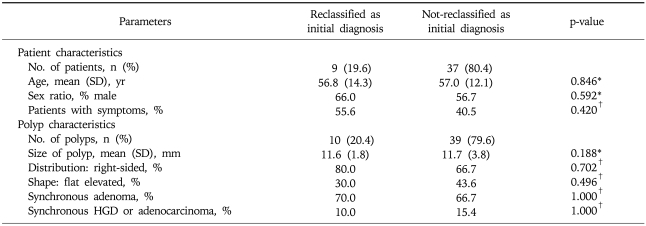
The clinical and endoscopic features of polyps with and without reclassification as initial pathological diagnoses are summarized in Table 4. There was no difference in the patient characteristics (age, sex, and symptoms) between the two groups in a per-patient analysis. There was also no difference in the polyp characteristics (size, location, shape, association with adenoma, and synchronous HGD/adenocarcinoma) between the two groups in a per-polyp analysis. There is no HGD or adenocarcinoma component in HP or SSA per se in both groups.
Table 4.
Clinical and Endoscopic Characteristics of Polyps That Were or Were Not Reclassified as Initial Pathological Diagnoses
SD, standard deviation; HGD, high grade dysplasia.
*p-value by Mann-Whitney test; †p-value by Fisher's exact test.
DISCUSSION
The concept of serrated polyps has rapidly evolved in recent years,12 and an accurate diagnosis of their subgroup is important not only for pathologists but also for endoscopists in order to optimize patient management.13 However, as the correct diagnosis of SSA may not be easy in daily clinical practice, the diagnosis of HPs larger than 10 mm may be revisited to determine if this is SSA. In this study, 18.4% of polyps which were initially diagnosed as HPs were reclassified into SSAs or mixed polyps after careful pathological reviews. This result means that a significant number of SSAs may not be accurately diagnosed in daily clinical practice, which results in an incorrect assessment of colorectal cancer risk in some patients. In an internet-based study of the variability among 168 Western pathologists in the diagnosis of HPs and SSAs, SSAs were most likely to be misdiagnosed as HPs;14 this result is consistent with our findings. Therefore, when large HPs are diagnosed, attention should be focused on the reliable identification of SSA and subsequent surveillance needs to be individualized based on the endoscopist's judgement and patient risk factors, such as size and number of HPs and personal or family history of colorectal cancer.13
In this study, histopathological features for the identification of SSA included characteristic architectural abnormalities such as base serration, crypt branching, crypt dilatation, and crypt horizontalization. Overall architectural features were useful for the diagnosis of SSA, but cytological features were less useful; these findings were consistent with those reported in the literature.14 As the most diagnostic histologic features were largely architectural and presented at the base of the crypts, a well-oriented and abundant tissue section is essential for the accurate diagnosis of SSA. Therefore, it may be difficult to distinguish SSAs from conventional HPs from small, superficial biopsies, for this reason, colorectal polyps removed by hot or cold biopsy only were excluded from this analysis. An en bloc excision of a lesion was easier to orient and diagnose SSA,14 however, even polypectomized SSAs may be confused as HPs in daily clinical practice. Considering this scenario, endoscopists must be careful in obtaining the best possible tissue specimen as possible and pathologists should attend on the correct diagnosis of SSAs. In contrast to SSAs, which may be difficult to distinguish from conventional HPs, it is not difficult to distinguish TSAs from conventional HPs because they may possess villiform architectures and their epithelial cells typically display more prominent nuclear hyperchromasia and more basophilic cytoplasm.9 Therefore, there was no HP case that was reclassified to TSA in this study.
Our primary interest in SSA was to evaluate clinically and endoscopically relevant information with respect to polyps with or without reclassification as initial pathological diagnoses. However, there was no difference in the patient characteristics (age, sex, and symptoms) and the polyp characteristics (size, distribution, shape, association with adenoma, and association with high-grade dysplasia/carcinoma) between the two groups. Indeed, SSAs misclassified as HPs during initial pathological diagnosis did not show any predilection for large size, flat shape, and right colon location. This result means that SSAs may be easily misdiagnosed as HPs in daily clinical practice and it has become a challenging issue that distinguishing SSAs from HPs.
Our study has some limitations. First, our findings may be limited by the fact that this is a single-center result based on a small sample size. Despite this shortcoming, our study appears to have considerable potential for the development of additional validation studies with a large number of patients. Second, our definition of HPs larger than 10 mm may be arbitrary, since the 10 mm cutoff has been used to define significant neoplasm. However, we used the 10 mm cutoff because polyps larger than 10 mm were routinely polypectomized with saline lift technique for the exact histological diagnosis in our hospital. In addition, it is a simple, easy-to-apply and widely accepted way of defining large polyps in a standard clinical practice.
In summary, a significant number of SSAs may be misdiagnosed as HPs in daily clinical practice without any predilection for size, shape, and location. Therefore, when large HPs are diagnosed in clinical practice, it is necessary for physicians to have greater awareness of the diagnosis of SSA and to individualize subsequent surveillance.
References
- 1.Jass JR. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol. 2005;2:398–405. doi: 10.1038/ncponc0248. [DOI] [PubMed] [Google Scholar]
- 2.Makinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein NS. Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification. Am J Clin Pathol. 2006;125:146–153. [PubMed] [Google Scholar]
- 4.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 5.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–537. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–755. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]
- 7.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 8.Bariol C, Hawkins NJ, Turner JJ, Meagher AP, Williams DB, Ward RL. Histopathological and clinical evaluation of serrated adenomas of the colon and rectum. Mod Pathol. 2003;16:417–423. doi: 10.1097/01.MP.0000068236.47471.DB. [DOI] [PubMed] [Google Scholar]
- 9.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Ming SC, Goldman H. Pathology of the gastrointestinal tract. 2nd ed. Baltimore: William & Wilkins; 1999. [Google Scholar]
- 11.Carvajal-Carmona LG, Howarth KM, Lockett M, et al. Molecular classification and genetic pathways in hyperplastic polyposis syndrome. J Pathol. 2007;212:378–385. doi: 10.1002/path.2187. [DOI] [PubMed] [Google Scholar]
- 12.Glatz K, Pritt B, Glatz D, Hartmann A, O'Brien MJ, Blaszyk H. A multinational, internet-based assessment of observer variability in the diagnosis of serrated colorectal polyps. Am J Clin Pathol. 2007;127:938–945. doi: 10.1309/NXDB6FMTE9X5CD6Y. [DOI] [PubMed] [Google Scholar]
- 13.Bauer VP, Papaconstantinou HT. Management of serrated adenomas and hyperplastic polyps. Clin Colon Rectal Surg. 2008;21:273–279. doi: 10.1055/s-0028-1089942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandmeier D, Seelentag W, Bouzourene H. Serrated polyps of the colorectum: is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice? Virchows Arch. 2007;450:613–618. doi: 10.1007/s00428-007-0413-8. [DOI] [PubMed] [Google Scholar]