Abstract
Background/Aims
Elderly patients with advanced gastric cancer (AGC) have generally been excluded from clinical trials, and there are few data available on the treatment of these patients. The efficacy of palliative S-1 monotherapy as a first-line treatment regimen for elderly patients has not been well elucidated.
Methods
For this study, 25 AGC patients were enrolled between January 1, 2007 and March 31, 2009; 4 cases were recurrent AGC and 21 cases were metastatic AGC at the time of diagnosis. These patients received S-1 therapy at a dose of 40 mg/m2 twice daily for 14 days every 3 weeks. All of the patients were older than 70 years.
Results
The median follow-up duration, the median progression-free survival, and the overall survival time were 8.7 months (range, 4.9 to 12.5 months), 4.9 months (range, 3.5 to 6.3 months), and 10.8 months (range, 6.6 to 15.0 months), respectively. Grade 3/4 nonhematologic toxicities were rare. Grade 3/4 neutropenia was noted in two patients. The partial response rate was 21.7% and stable disease was observed in 34.8% of the patients. Two patients (8%) died due to chemotherapy-associated toxicity during treatment (septic shock/intracranial hemorrhage).
Conclusions
Oral S-1 chemotherapy seems to be effective as a first-line treatment regimen for elderly patients with metastatic or recurrent AGC. However, elderly patients receiving S-1 treatment should undergo continuous toxicity monitoring, since they are highly susceptible to adverse effects.
Keywords: S-1, Elderly, Gastric cancer
INTRODUCTION
Although the incidence and mortality rates of gastric cancer have been decreasing, gastric cancer is currently the second most common cancer after lung cancer.1 In Korea, gastric cancer is the most commonly occurring cancer, and it is also the second leading cause of cancer-related deaths after lung cancer.2 The incidence of gastric cancer increases rapidly in the sixth and seventh decades of life.3 Approximately 60% of all cancers occur in individuals aged ≥65 years, and this percentage is expected to increase with the aging of the population.4,5 Palliation of symptoms is generally achieved by chemotherapy, which confers considerable benefit for the overall survival.6,7 Providing adequate health care for elderly people is becoming an increasingly important issue in industrialized nations. Large randomized clinical trials have generally not included elderly patients with advanced gastric cancer (AGC) and so the data on the treatment of these patients are limited. The prognostic value of age for patients with gastric cancer remains controversial.3 Only a few studies have administered chemotherapeutic regimens to elderly patients, although chemotherapy has been confirmed to improve the survival and quality of life in patients with AGC. There is currently no standard chemotherapeutic regimen for elderly patients.
S-1 is a newly developed oral fluoropyrimidine, and this is composed of a mixture of tegafur, 5-chloro-2,4-dehydroxypyridine, and potassium oxonate in a molar ratio of 1:0.4:1.8 S-1 is an oral anticancer drug that has been shown to be well tolerable and effective for the treatment of many solid tumors. Although the toxicity of S-1 is known to be acceptable and manageable, the information on this with respect to elderly patients is limited. In addition, the S-1 regimen for 2-week treatment followed by 1-week rest showed to reduce the adverse events and increase patient compliance.9,10 We conducted a retrospective study on the safety and efficacy of three weekly oral S-1 mono-therapy as a first-line treatment regimen for elderly patients with metastatic AGC.
MATERIALS AND METHODS
1. Patient eligibility
From January 1, 2007 to March 31, 2009, 25 AGC patients were enrolled in this study. The inclusion criteria of our study included the diagnosis of metastatic or recurrent gastric adenocarcinoma that was confirmed by histological examination in patients aged ≥70 years. Patients with the presence of at least one measurable lesion were enrolled.
The patients who had undergone prior chemotherapy for metastatic disease were not enrolled. Patients with a poor performance status (Eastern Cooperative Oncology Group [ECOG] performance status ≥3) and the patients with poor renal and liver function were excluded in the present analysis. Patients who had undergone prior adjuvant chemotherapy were enrolled provided that the therapy had been completed more than 6 months prior to the development of metastatic disease. Four cases were recurrent AGC and 21 were metastatic AGC at the time of diagnosis.
2. Treatment schedule and dose modification
S-1 was administered orally twice daily according to the intermittent schedule (two weeks of treatment followed by a week rest period, every three weeks). The initial dose of S-1 was determined on the basis of the patient's body surface area (40 mg/m2). The actual doses of S-1 according to the body surface area (BSA) were: BSA<1.25 m2, 40 mg twice a day; 1.25 m2≤BSA<1.5 m2, 50 mg twice a day; and 1.5 m2≤BSA, 60 mg twice a day. Chemotherapy was continued until disease progression or unacceptable toxicity, and it was discontinued if the patient refused further treatment. The intensity adjustments to the dose of the S-1 were recorded throughout treatment.
3. Response evaluation to treatment and the adverse effects
The baseline evaluation of each patient included a complete medical history, physical examination, a complete blood count, the serum chemistry and analysis of the computed tomography (CT) scans of the measurable or nonmeasurable lesions. Physical examination, blood counts and serum chemistry were carried out before each cycle of therapy. The CT scans of the measurable lesions were assessed at baseline and CT scans were repeated for every three cycles of treatment. The tumor responses were classified according to the guidelines of the Response Evaluation Criteria in Solid Tumors (RECIST). A complete response (CR) was defined as the disappearance of all clinical evidence of the tumor. A partial response (PR) was defined as a decrease (≥30%) in the sum of the longest diameter (LD) of the target lesions. The baseline sum LD served as a reference. The patients with a CR or PR did not undergo confirmatory CT scans after four weeks if the patients' symptoms, physical examination or chest X-ray before the next chemotherapy cycle did not show evidence of disease progression. Progressive disease (PD) was defined as at least a 20% increase in the sum of the LDs of the target lesions or the appearance of new lesions. Stable disease (SD) was defined as a tumor response that did not meet the above criteria of CR, PR, or PD.
Progression free survival (PFS) and overall survival (OS) were analyzed using the Kaplan-Meier method. The date of administering S-1 was considered the starting date. The PFS was assessed by measuring the time interval from the start of the S-1 treatment until confirmation of disease progression or death as a result of any cause. The OS was determined by measuring the time interval from the beginning of the treatment to the date of death. We censored the patients who were alive or were lost during the follow-up in the data analysis. All the statistical analyses were conducted using SPSS version 12.0 statistical software (SPSS Inc., Chicago, IL, USA). Toxicity was evaluated before each treatment cycle according to the National Cancer Institute Common Toxicity Criteria, version 3.0.
RESULTS
1. Patient characteristics
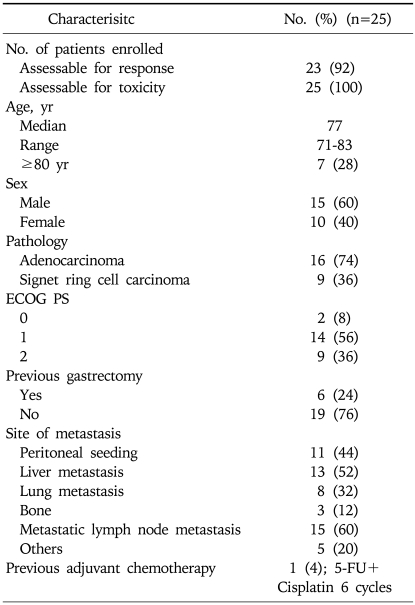
We finally analyzed 25 patients. The median age was 77 years (range, 71-83). Only one patient received prior adjuvant chemotherapy (six cycles of 5-FU and Cisplatin). The distribution of the ECOG performance status was as follows: nine patients (36%) with a performance status of 2, 14 patients (56%) with a performance status of 1 and two patients (8%) with a performance status of 0. Table 1 shows the patients' characteristics in detail.
Table 1.
Patient Characteristics
ECOG PS, Eastern Cooperative Oncology Group performance status.
2. Treatment and toxicity
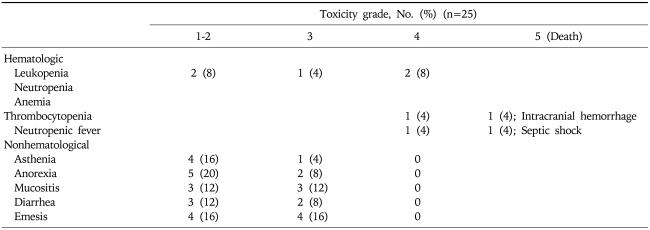
Overall, 114 cycles were administered to these 25 patients with AGC. The median duration of treatment was 3.9 months (95% confidence interval [CI], 1.5 to 6.1). The median dose intensity delivered was 86.8% of that planned for S-1. The dose of S-1 required modification (delay, dose reduction or interruption of treatment) for 60% of the patients. All patients were evaluable for toxicity. Gastrointestinal toxicity was the most frequently observed toxicity, and grade 3 or 4 nausea or vomiting occurred in 16% of the patients (n=4). Seven patients (28%) experienced anorexia. Within the treatment period, two patients died due to chemotherapy associated toxicity. One patient died due to septic shock with grade 4 neutropenia and another patient died due to intracranial hemorrhage associated grade 4 thrombocytopenia. Table 2 shows the adverse reactions observed among the 25 patients.
Table 2.
Hematological and Nonhematological Adverse Events
3. Response evaluation, PFS and OS
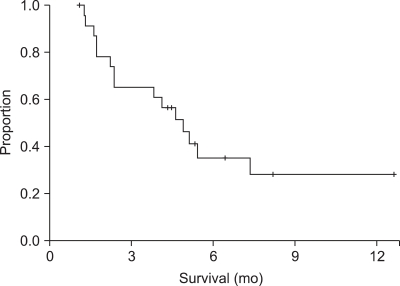
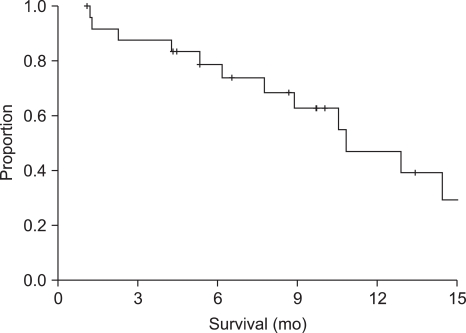
The responses of 23 patients were evaluated. Two patients could not be evaluated because of early death during the course of the chemotherapy. Five patients (21.7%) had an objective response and eight patients (34.8%) had stable disease. No patient achieved a CR. At the time of the analysis, 17 patients (73.9%) showed disease progression and 13 patients had died. Two patients exhibited chemotherapy-related mortality and another patient refused further chemotherapy after three cycles of S-1 treatment and the patient then committed suicide. However, that patient showed a PR after three cycles of S-1 treatment. The median follow-up, PFS and OS were determined to be 8.7 months (95% CI, 4.9 to 12.5), 4.9 months (95% CI, 3.5 to 6.3) (Fig. 1) and 10.8 months (95% CI, 6.6 to 15.0), respectively, by Kaplan-Meier survival analysis (Fig. 2).
Fig. 1.
Progression free survival with S-1 treatment.
Fig. 2.
Overall survival time with S-1 treatment.
DISCUSSION
Oral chemotherapy may be especially advantageous for elderly patients because of its convenience, the high acceptance rate and the potential cost savings. The few phase II trials of S-1 mono-therapy as a first-line treatment regimen for patients with AGC have been encouraging. They had a response rate of 44-49% and they showed a 1-year survival rate of 30% and a 2-year survival rate of 15%.11,12 Thus, S-1 mono-therapy seems to be very feasible and convenient. However, elderly patients or patients with a poor PS were excluded from those previous trials.
In our study, we demonstrated that oral S-1 chemotherapy has good potential as first-line chemotherapy in elderly AGC patients (older than 70 years). The overall response rate was 21.7%; the disease control rate was 56.5% with a median PFS of 4.9 months and a median OS of 10.8 months. These results seem to be analogous to the previous S-1 monotherapy results.13 Compared with other conventional chemotherapeutic regimens, this S-1 mono-therapy showed similar efficacy as the other therapies in AGC patients.14
The tolerance level of S-1 treatment was high. Apart from gastrointestinal toxicity, which was the major toxicity of S-1 monotherapy, most of the toxicities were tolerable. However, two patients died due to grade 4 hematologic toxicities (grade 4 thrombocytopenia and grade 4 febrile neutropenia). Careful toxicity monitoring and controlling the dose intensity or density may be needed in these fragile patients. On the basis of our experience, we advocate that chemotherapy-related toxicities should be assessed with caution, and especially in elderly patients. Although making comparison with other reports is difficult because of the small sample size of our study, the low percentage of grade 3-4 adverse events in this study suggests that S-1 treatment is tolerable even in elderly patients. The patients included in this study were fragile, elderly patients (36% of the patients had an ECOG performance status of 2). However, the therapeutic outcomes of the S-1 treatment in these patients were in line with those of other trials, in which younger or fitter patients were enrolled.
Our results support S-1 as a first-line chemotherapy regimen for elderly patients (≥70 years) with AGC. There are some limitations in our study: we conducted a retrospective study and the sample size was small. These limitations should be considered when interpreting our data and when making inferences and recommendations based on the findings of this study.
In conclusion, oral S-1 chemotherapy seems to be effective as a first-line treatment regimen in elderly patients with metastatic or recurrent AGC. However, careful toxicity monitoring is necessary in elderly patients who are treated with S-1 to avoid chemotherapy-related toxicities since these patients are very susceptible to adverse effects. Further studies are warranted to identify the optimal management strategies for elderly patients with metastatic or recurrent AGC.
ACKNOWLEDGEMENTS
This work was supported by Inha University Research Grant.
Conflict of interest statement: none declared.
References
- 1.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Yang HK, Ahn YO. Gastric cancer in Korea. Gastric Cancer. 2002;5:177–182. doi: 10.1007/s101200200031. [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Osaki T, Murakami D, et al. Effect of age on prognosis in patients with gastric cancer. ANZ J Surg. 2006;76:458–461. doi: 10.1111/j.1445-2197.2006.03756.x. [DOI] [PubMed] [Google Scholar]
- 4.Balducci L. Supportive care of elderly patients with cancer. Support Cancer Ther. 2005;2:225–228. doi: 10.3816/SCT.2005.n.015. [DOI] [PubMed] [Google Scholar]
- 5.Liu ZF, Guo QS, Zhang XQ, et al. Biweekly oxaliplatin in combination with continuous infusional 5-fluorouracil and leucovorin (modified FOLFOX-4 regimen) as first-line chemotherapy for elderly patients with advanced gastric cancer. Am J Clin Oncol. 2008;31:259–263. doi: 10.1097/COC.0b013e31815d43ee. [DOI] [PubMed] [Google Scholar]
- 6.Abbrederis K, Lorenzen S, von Weikersthal LF, et al. Weekly docetaxel monotherapy for advanced gastric or esophagogastric junction cancer. Results of a phase II study in elderly patients or patients with impaired performance status. Crit Rev Oncol Hematol. 2008;66:84–90. doi: 10.1016/j.critrevonc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 8.Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53:4004–4009. [PubMed] [Google Scholar]
- 9.Kimura Y, Kikkawa N, Iijima S, et al. A new regimen for S-1 therapy aiming at adverse reaction mitigation and prolonged medication by introducing a 1-week drug-free interval after each 2-week dosing session: efficacy and feasibility in clinical practice. Gastric Cancer. 2003;6(Suppl 1):34–39. doi: 10.1007/s10120-003-0230-y. [DOI] [PubMed] [Google Scholar]
- 10.Lee JL, Kang HJ, Kang YK, et al. Phase I/II study of 3-week combination of S-1 and cisplatin chemotherapy for metastatic or recurrent gastric cancer. Cancer Chemother Pharmacol. 2008;61:837–845. doi: 10.1007/s00280-007-0541-5. [DOI] [PubMed] [Google Scholar]
- 11.Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–1720. doi: 10.1016/s0959-8049(98)00211-1. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191–197. doi: 10.1159/000012099. [DOI] [PubMed] [Google Scholar]
- 13.Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;99:584–590. doi: 10.1038/sj.bjc.6604536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi IS, Oh DY, Kim BS, Lee KW, Kim JH, Lee JS. Oxaliplatin, 5-FU, folinic acid as first-line palliative chemotherapy in elderly patients with metastatic or recurrent gastric cancer. Cancer Res Treat. 2007;39:99–103. doi: 10.4143/crt.2007.39.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]