Abstract
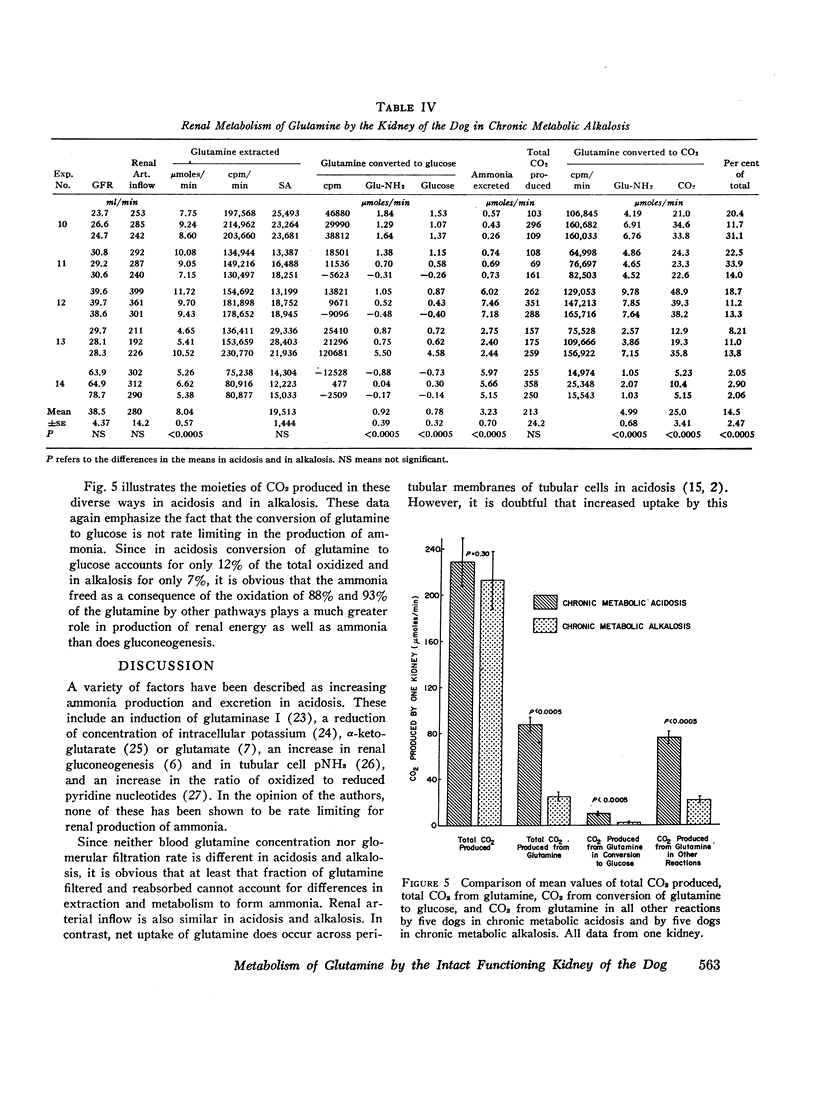
The renal conversion of glutamine to glucose and its oxidation to CO2 were compared in dogs in chronic metabolic acidosis and alkalosis. These studies were performed at normal endogenous levels of glutamine utilizing glutamine-34C (uniformly labeled) as a tracer. It was observed in five experiments in acidosis that mean renal extraction of glutamine by one kidney amounted to 27.7 μmoles/min. Of this quantity, 5.34 μmoles/min was converted to glucose, and 17.5 μmoles/min was oxidized to CO2. Acidotic animals excreted an average of 41 μmoles/min of ammonia in the urine formed by one kidney. In contrast, in five experiments in alkalosis, mean renal extraction of glutamine amounted to 8.04 μmoles/min. Of this quantity, 0.92 μmole/min was converted to glucose, and 4.99 μmoles/min was oxidized to CO2. Alkalotic animals excreted an average of 3.23 μmoles/min of ammonia in the urine. We conclude that renal gluconeogenesis is not rate limiting for the production and excretion of ammonia in either acidosis or alkalosis. Since 40% of total CO2 production is derived from oxidation of glutamine by the acidotic kidney and 14% by the alkalotic kidney, it is apparent that renal energy sources change with acid-base state and that glutamine constitutes a major metabolic fuel in acidosis.
Full text
PDF
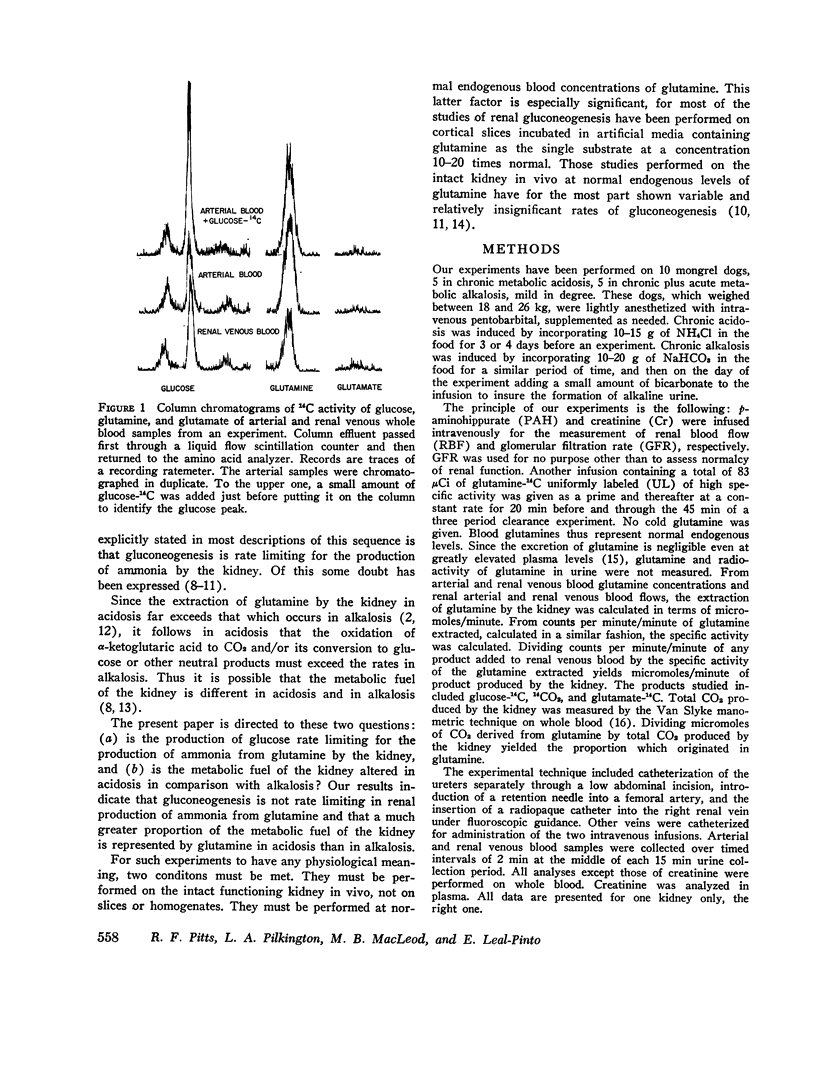
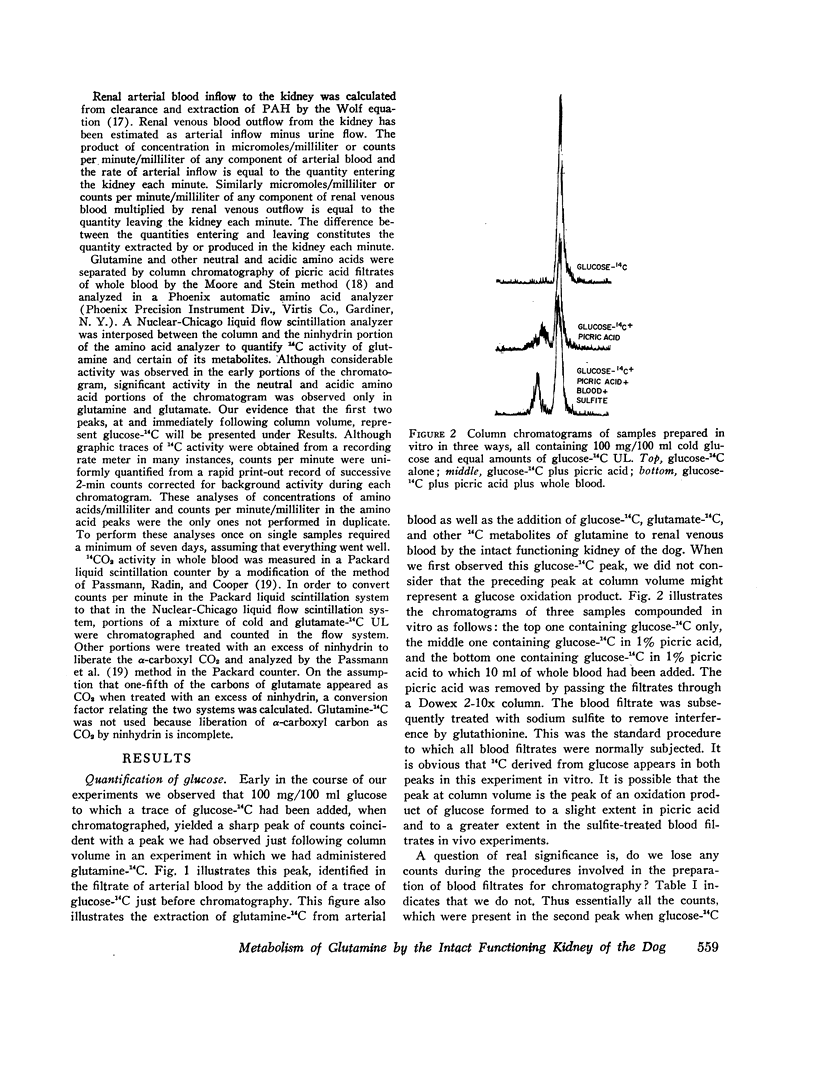

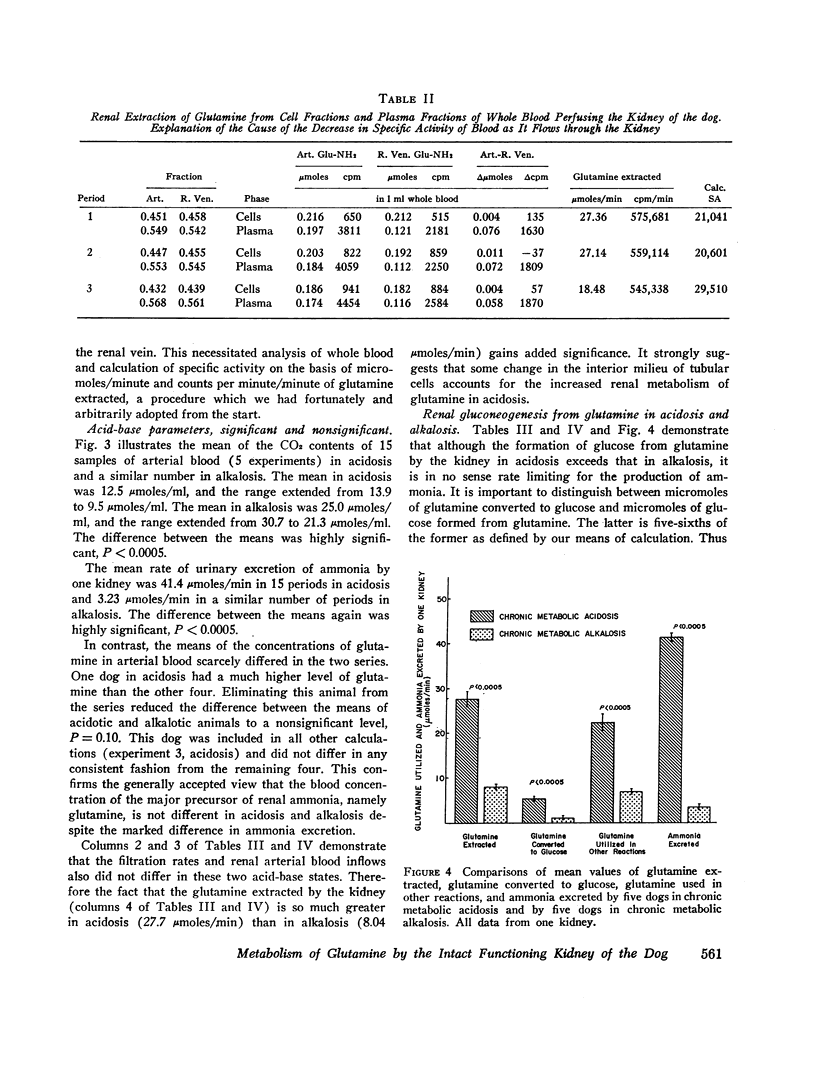
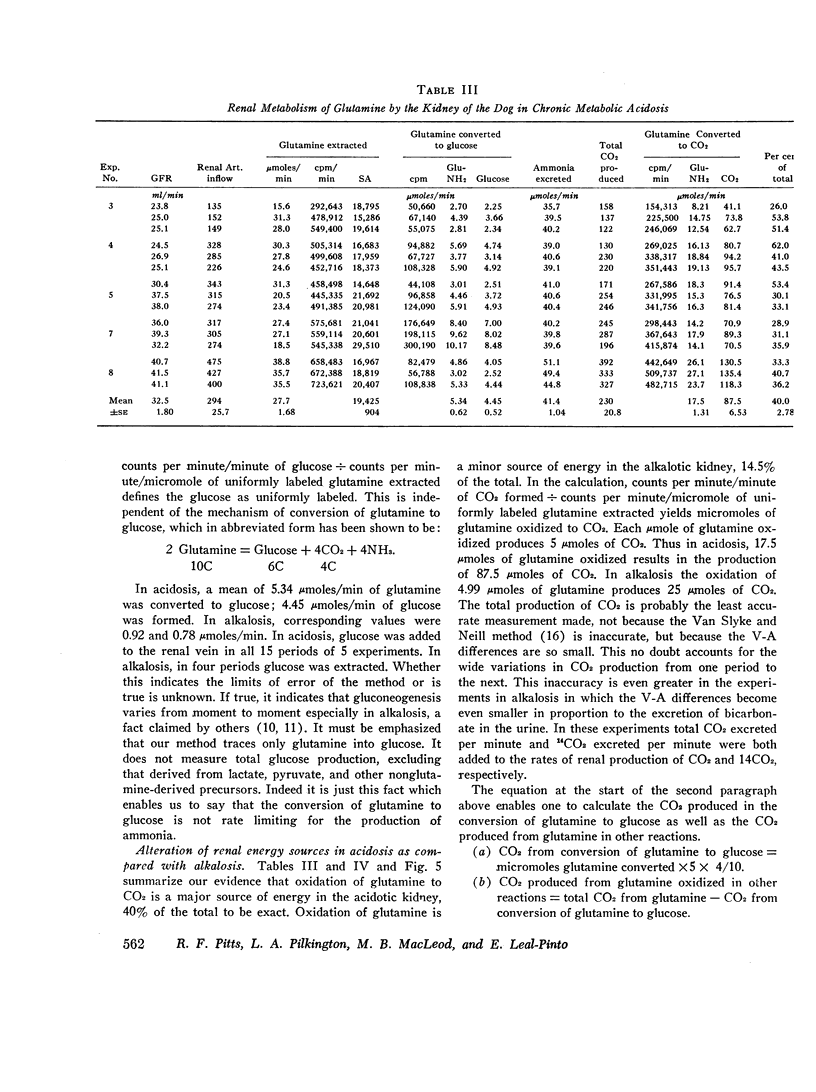


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleyne G. A., Scullard G. H. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969 Feb;48(2):364–370. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagura-Baruch S., Shurland L. M., Welbourne T. C. Effects of alpha-ketoglutarate on renal ammonia release in the intact dog. Am J Physiol. 1970 Apr;218(4):1070–1075. doi: 10.1152/ajplegacy.1970.218.4.1070. [DOI] [PubMed] [Google Scholar]
- CLARKE E., EVANS B. M., MACINTYRE I., MILNE M. D. Acidosis in experimental electrolyte depletion. Clin Sci. 1955 Aug;14(3):421–440. [PubMed] [Google Scholar]
- DAVIES B. M. A., YUDKIN J. Studies in biochemical adaptation; the origin or urinary ammonia as indicated by the effect of chronic acidosis and alkalosis on some renal enzymes in the rat. Biochem J. 1952 Nov;52(3):407–412. doi: 10.1042/bj0520407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD M., SPITZER J. J. METABOLISM OF FREE FATTY ACIDS BY MYOCARDIUM AND KIDNEY. Am J Physiol. 1964 Jan;206:153–158. doi: 10.1152/ajplegacy.1964.206.1.153. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem J. 1935 Aug;29(8):1951–1969. doi: 10.1042/bj0291951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. L., Pitts R. F. Species differences in renal glutamine synthesis in vivo. Am J Physiol. 1969 Jan;216(1):117–122. doi: 10.1152/ajplegacy.1969.216.1.117. [DOI] [PubMed] [Google Scholar]
- Nieth H., Schollmeyer P. Substrate-utilization of the human kidney. Nature. 1966 Mar 19;209(5029):1244–1245. doi: 10.1038/2091244a0. [DOI] [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILKINGTON L. A., WELCH J., PITTS R. F. RELATIONSHIP OF PNH3 OF TUBULAR CELLS TO RENAL PRODUCTION OF AMMONIA. Am J Physiol. 1965 Jun;208:1100–1106. doi: 10.1152/ajplegacy.1965.208.6.1100. [DOI] [PubMed] [Google Scholar]
- Pilkington L. A., O'Donovan D. J. Metabolism of glutamine in cortex slices from dog kidney during acid-base alterations. Am J Physiol. 1971 Jun;220(6):1634–1639. doi: 10.1152/ajplegacy.1971.220.6.1634. [DOI] [PubMed] [Google Scholar]
- Pilkington L. A., Young T. K., Pitts R. F. Properties of renal luminal and antiluminal transport of plasma glutamine. Nephron. 1970;7(1):51–60. doi: 10.1159/000179807. [DOI] [PubMed] [Google Scholar]
- Pitts R. F. The role of ammonia production and excretion in regulation of acid-base balance. N Engl J Med. 1971 Jan 7;284(1):32–38. doi: 10.1056/NEJM197101072840110. [DOI] [PubMed] [Google Scholar]
- Preuss H. G. Pyridine nucleotides in renal ammonia metabolism. J Lab Clin Med. 1968 Sep;72(3):370–382. [PubMed] [Google Scholar]
- Roxe D. M., Disalvo J., Balagura-Baruch S. Renal glucose production in the intact dog. Am J Physiol. 1970 Jun;218(6):1676–1681. doi: 10.1152/ajplegacy.1970.218.6.1676. [DOI] [PubMed] [Google Scholar]
- Simpson D. P., Sherrard D. J. Regulation of glutamine metabolism in vitro by bicarbonate ion and pH. J Clin Invest. 1969 Jun;48(6):1088–1096. doi: 10.1172/JCI106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Goodman A. D., Treble D. H. Effect of metabolic acidosis on renal gluconeogenesis in vivo. Am J Physiol. 1968 Jul;215(1):211–217. doi: 10.1152/ajplegacy.1968.215.1.211. [DOI] [PubMed] [Google Scholar]



