Abstract
Background/Aims
With the progress of product development, single-step endoscopic ultrasound (EUS)-guided transmural drainage can overcome some disadvantages of the blind or two-step procedures used in the treatment of pancreatic pseudocysts. We therefore evaluated the technical feasibility, efficacy, and safety of single-step EUS-guided transmural drainage of pancreatic pseudocysts.
Methods
Endoscopic drainage of pancreatic pseudocysts was performed in 47 patients (median age, 46 years; range, 38 years to 59 years; 40 men) by using interventional echoendoscopes with a single-step device suitable for ballooning, bougination, and plastic-stent insertion.
Results
Endoscopic stent placement was successful in 42 patients (89%; transgastric approach, 34/38; transduodenal approach, 8/9) and failed in 5 patients because of acute angulation (n=4) or small cyst (n=1). The volume of the pseudocyst was reduced by more than 90% or it disappeared completely in all of 41 patients (100%), based on a mean follow-up period of 17 months (range, 11 months to 20 months). The overall recurrence rate was 12% (5/41) after improvement by the procedure. Minor complications (one case of bleeding, three cases of pneumoperitoneum, and one case of peritonitis) occurred after the procedure in five patients (11%), but there were no major complications.
Conclusions
Single-step EUS-guided transmural drainage can be used to treat pancreatic pseudocysts with acceptable feasibility, efficacy, and safety.
Keywords: Drainage, Endoscopic ultrasonography, Pancreatic pseudocyst
INTRODUCTION
Pancreatic pseudocyst is defined as a collection of pancreatic fluid enclosed by a wall of nonepithelialized granulation tissue arising as a consequence of acute or chronic pancreatitis, pancreatic trauma, or pancreatic obstruction.1 Pancreatic pseudocysts should initially be managed conservatively as many resolve spontaneously within 4 to 6 weeks. A surgical approach has traditionally been accepted as the treatment of choice for persistent pseudocysts. Although surgery is effective, complications can occur in up to 35% of patients, and the mortality cases are also noted.2
Therefore, a number of minimally invasive approaches such as percutaneous or endoscopic drainage have been developed. The drawbacks of percutaneous drainage include skin discomfort, the possibility of infection, and development of a cutaneous fistula after tube removal.3 Blind endoscopic drainage carries a risk of bleeding or perforation and is not preferred for nonbulging pseudocyst because avoiding major vessels and adjacent structures is difficult.4 After the development of endoscopic ultrasound (EUS), beside accurate diagnosis,5 two-step EUS-guided drainage has been tried for added safety, which is performed with a duodenoscope after marking an optimal site by EUS. However, this technique prolongs the procedure time and increases patient discomfort and the need for sedation.6 Furthermore, when switching from EUS to duodenoscope, the best site and angle of needle puncture can be changed.
Single-step EUS-guided transmural drainage overcomes some of the disadvantages of the blind and two-step procedures. This method involves placing a plastic or metal stent and/or a nasocystic catheter between the cyst and the gastric lumen or the duodenal lumen, those are named as cystogastrostomy or cystoduodenostomy, to permit continuous drainage in a single-step procedure.7-9 Recent pilot studies indicate the efficacy of this approach.7,8,10
We therefore conducted this study to evaluate the technical feasibility, efficacy, and safety of single-step EUS-guided transmural drainage of pancreatic pseudocysts.
MATERIALS AND METHODS
1. Patients
This study retrospectively reviewed the records of 47 consecutive patients who underwent single-step EUS-guided transmural drainage of pancreatic pseudocysts between November 2007 and May 2009. The inclusion criteria were symptomatic pancreatic pseudocysts, increasing at least 5 cm in diameter and persisting for more than 6 weeks of pseudocyst, infected pseudocysts including abscesses, absence of interposed vessels, and normal coagulation status. Excluded were cysts showing acute fluid collection, pancreatic necrosis, and pancreatic cystic tumors.
The study was approved by the Institutional Review Board of the Asan Medical Center, University of Ulsan College of Medicine.
2. Methods
All pseudocysts were evaluated by endoscopic retrograde cholnagiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP) to know the status of the pancreatic duct before the procedure and the size was measured using computed tomography (CT).
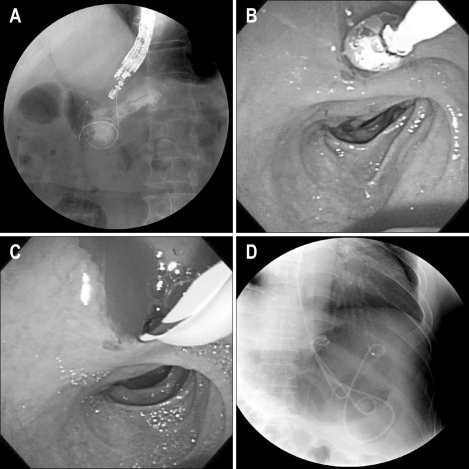
EUS-guided transmural drainage of pseudocysts such as endoscopic cystogastrostomy or cystoduodenostomy was performed using a single-step technique when there was no communication between the duct and the pseudocyst in ERCP or MRCP. EUS and endoscopic inspection of the pseudocyst, surrounding organs, and structures were performed and the best site for drainage was identified using a large-channel curvilinear-array echoendoscope (Aloka GF-UCT140-AL5; Olympus Co., Tokyo, Japan). Outer diameter of the scope was 12.6 mm and diameter of operating channel was 3.7 mm, within this channel, the needle puncture, dilation, and stent insertion could be done easily. A 19-gauge needle was used to puncture the wall under real-time EUS guidance to gain access to the cavity. The stylet was removed and a cyst fluid sample was submitted for analysis. Using simultaneous EUS and fluoroscopic guidance, a guide wire was advanced through the needle into the pseudocyst, creating a generous loop of wire inside the cavity. The needle was then removed, leaving the guidewire in place. The opening was enlarged using a 6-10 mm wire-guided Hurricane balloon dilator (Microvasive; Boston Scientific, Natick, MA, USA) or bougination (Soehendra biliary dilation catheter; Cook Endoscopy, Winston-Salem, NC, USA). Placement of the first 7 Fr double-pigtail stent was performed over the wire, with simultaneous endoscopic and fluoroscopic guidance. Where possible, two or more stents were accommodated (Fig. 1).
Fig. 1.
Technical details of single-step EUS-guided transmural drainage. (A) Puncture was performed by using a 19-gauge needle, and a guidewire was inserted into the cystic cavity. (B) The puncture site was dilated with a balloon catheter. (C) A guidewire was inserted to insert another plastic double-pigtail stent. (D) Two double-pigtail stents with nasocystic tube were inserted.
3. Definitions
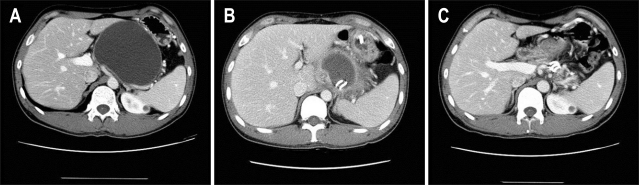
Technical success of the transmural procedure was defined as complete access and successful placement of 7 Fr stents within the pseudocyst. Clinical success was defined as complete resolution or volume reduction more than 90% of original volume which was measured by CT (Fig. 2).
Fig. 2.
An example of successful pseudocystic drainage. (A) A 10.8-cm pseudocyst was located on the pancreatic body and tail. (B) Two stents were inserted to the cyst. (C) The cyst had completely disappeared at 4 months after stent insertion.
Procedure-related complications were defined as any kind of newly developed complications after the procedure such as bleeding, pneumoperitoneum, and peritonitis. A diagnosis of an infected pseudocyst was made if pus was noted during endoscopic drainage and/or if bacteria grew in cyst fluid cultures.
4. Statistical analysis
Data represent median and range for continuous variables, and relative frequencies for categorical variables. The χ2 test or the Fisher's exact test were used to compare relative frequencies for categorical variables, and Student's t-test or the Mann-Whitney U test were used for continuous variables. A p-value of <0.05 was considered to indicate significance. All statistical analyses were performed using the SPSS program, version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
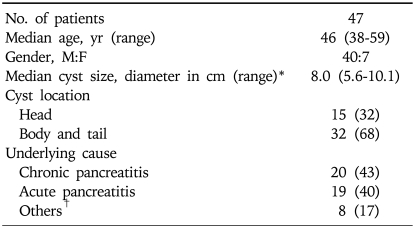
During the 19-month study period, 47 patients (40 men, 7 women) with a median age of 46 years (range, 38 years to 59 years) underwent single-step EUS-guided transmural drainage of pancreatic pseudocysts. The median pseudocyst diameter as determined by CT was 8.0 cm (range, 5.6 cm to 10.1 cm) and other characteristics were shown in Table 1. The basic causes of acute or chronic pancreatitis were alcohol ingestion (n=32), stone (n=3), drug (n=2), and others (n=10).
Table 1.
Characteristics of Patients Undergoing Single-Step EUS-Guided Transmural Drainage of Pancreatic Pseudocysts
Data represent as number of patients (% of total population).
*Based on the longest diameter measured by computed tomography; †Represents trauma and surgery.
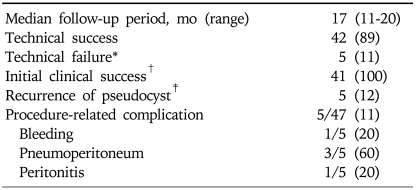
The intra- and post-procedure outcomes were summarized in Table 2. The median follow-up period was 17 months (range, 11 months to 20 months) and endoscopic stent insertion was successful in 42 patients (89%). The procedure failed in 5 patients. Stent could not be inserted or guidewire was not stayed inside the pseudocyts because of acute angulation (n=4). In one case, the size of cyst was reduced from 3.7 cm to 1.2 cm after aspiration, so stent could not be inserted due to small cystic size. In 4 of the 5 patients for whom the procedure was unsuccessful, pseudocyst fluid was aspirated and the size of pseudocyst was reduced. In one failed patient, transpapillary drainage by ERCP was performed even though we failed this procedure beforetime. Only one patient whose procedure was successful was lost to follow-up. In the other 41 patients in whom procedures were successful, cyst volume measurements showed that 5 (12%) patients had complete resolution, whereas 36 (88%) patients showed cyst volume decreases of more than 90%. There were 5 cases of recurrence following stent removal. In 2 of these cases, the recurring cyst was small and no symptoms were noted. These patients are currently being followed-up by clinical observation. Stent re-insertion was required in 2 recurrent patients, and follow-up data are not yet available. In one recurrent patient, stent re-insertion was performed, then, removal was done after complete cyst resolution.
Table 2.
Outcomes Following Single-Step EUS-Guided Transmural Drainage of Pancreatic Pseudocysts
Data represent the number of patients (% of total population).
*Failure was attributable to acute angulation in four patients and a small cyst in one patient; †Cyst volume was reduced more than 90%; ‡Represents the number of recurrences among initial clinical success patients.
For the 42 technical success patients, the median number of stents inserted was 2 (range, 1 to 4), and the median stent length was 7 cm (range, 4 cm to 10 cm). Bougination was used to dilate the tract in two patients, Hurricane ballooning in 20 patients, and both bougination and Hurricane ballooning in 20 patients.
Procedure-related complications occurred in 5 patients (11%) (Table 2). The complications were bleeding (n=1), pneumoperitoneum (n=3), and peritonitis (n=1). All complications were minor and were successfully treated with either observation alone, or antibiotics.
It was possible to collect and culture cyst fluid in 15 cases. Nine of these patients had infected pseudocysts. Bacterial species identified by culture were Streptococcus viridians (n=2), Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Escherichia coli, Klebsiella pneumoniae, and both S. viridians and E. coli (n=1, each).
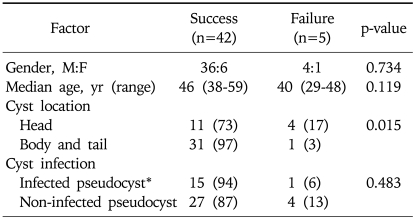
We tried to identify factors affecting clinical outcomes and compared possible factors between technical success and failure (Table 3). Cyst location was found to be a predictor of technical success; the success rate was 97% for body and tail cysts, and 73% for head cysts (p=0.015). Gender, age, or the presence of infection did not affect the technical success rate.
Table 3.
Factors Affecting the Technical Success of Single-Step EUS-Guided Transmural Drainage of Pancreatic Pseudocysts
Data represent numbers of patients (% of total population).
*Infection was defined as the presence of gross pus or the growth of bacteria from cultured aspiration fluid.
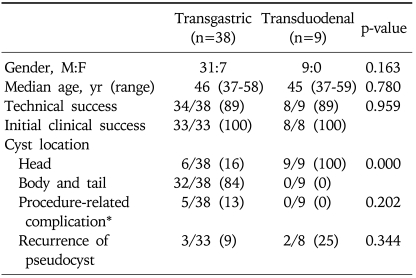
We analyzed our data with respect to the approach route (Table 4). A transgastric approach was used in 38 patients, and a transduodenal approach in 9. The type of approach employed was influenced by the location of cyst. The transgastric approach was used in patients whose cysts were located in the body and tail (n=32), and in the head of the pancreas (n=6). In contrast, the transduodenal approach was employed only in patients in whom cysts were located in the pancreatic head (n=9). Transduodenal and transgastric approach outcomes were similar in terms of technical and clinical success, complication level, and recurrence.
Table 4.
Outcomes by the Transgastric or Transduodenal Approach
Data are representative of the number of patients (% of total population).
*Bleeding, pneumoperitoneum, and peritonitis.
DISCUSSION
EUS has been reported to be useful to detect various pancreatic lesions,5,11 and in these days, many procedures has been used for diagnosis or treatment with this technique.12-16 Endoscopic drainage of pancreatic pseudocysts has become an accepted alternative to surgery when intervention is indicated.6 The advantages of endoscopic drainage compared to percutaneous drainage include the ability to place multiple internal drains through one puncture site and the avoidance of development of a pancreaticocutaneous fistula in pseudocysts that communicate with the pancreatic duct. Single-step EUS-guided pseudocyst drainage was first described by Vilmann and colleagues17 and Giovannini and associates.18 This technique showed higher technical success rate compared to conventional method in previous reports.4,19
The present study investigated retrospectively the technical and clinical success, complication, and recurrence, in 47 patients undergoing single-step EUS-guided transmural drainage of pancreatic pseudocysts in a single center. The median follow-up time was 16.5 months. Only one patient was lost to follow-up. The procedure was found to be technically successful in 89% of patients, and initial clinical success was achieved in 100% of all technical success patients. Pseudocyst recurrence after stent removal occurred in five (12%) patients, and three required stent reinsertion. These results appear to compare favorably with previous reports on single-step EUS-guided drainage.7,8,10 However, inter-study comparison is difficult as the definitions of initial clinical success and recurrence vary among reports.
In the current study, EUS-guided aspiration was performed in five patients in whom transmural drainage was failed. Only one of these patients required a further intervention, namely transpapillary drainage. Other authors have reported that single-step aspiration can be used as an alternative treatment when appropriately indicated.10 Until now, it is difficult to choose established standard treatment for recurrent pancreatic pseudocysts. We chose observation in 2 recurred patients, and EUS-guided stent reinsertion for the other three patients. Other reported treatments in recurred cases include percutaneous drainage, EUS-guided procedures, and surgical cystojejunostomy.7,8
The procedure-related complication rate in the present study was 11% including bleeding, pneumoperitoneum, and peritonitis. Often, pneumoperitoneum has been thought of as a major problem in reality and 11% complication rate is high. However, in this study, all complications including pneumoperitoneum were controlled effectively using bleeding control by epinephrine injection, or conservative management, so we dealt with these complications as minor and we think this procedure has a safety. There was little correlation between complication rate and the number of inserted stents. Cahen and colleagues20 suggested that complications could be reduced by using double pigtail stents rather than straight stents, and Norton and associates21 reported that multiple double pigtail stents were the best option for pseudocyst treatment. However, there was no association between complications and the presence of infection, number of inserted stents, or cyst size in our study, consistent with other reports.9,22
To our knowledge, any previous study did not analyze the factors affecting technical success. We found a greater success rate for the cyst which is located in the body and tail compared to that of head (97% vs 73%; p=0.015). With this result, we can think that those pseudocysts located at the head are more likely to cause a luminal compression than those in tail which was extended to splenic bed or renal fossa, so the EUS-guided procedure is difficult when the cyst was in head portion. Neither the type of endoscopic approach nor the presence of infection affected technical success, procedure-related complication rate, or recurrence rate. Two previous reports showed that infection did not affect the complication or recurrence rates.8,9
In conclusion, single-step EUS-guided transmural drainage of pancreatic pseudocysts showed a high technical success rate, a high initial clinical success frequency, a low procedure-related complication rate, and a low recurrence rate. In addition, there was no mortality. These findings suggest that single-step EUS-guided transmural drainage may be the optimal treatment for pseudocysts. However, further randomized large-scale studies are required.
ACKNOWLEDGEMENTS
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 2.Vosoghi M, Sial S, Garrett B, et al. EUS-guided pancreatic pseudocyst drainage: review and experience at Harbor-UCLA Medical Center. MedGenMed. 2002;4:2. [PubMed] [Google Scholar]
- 3.vanSonnenberg E, Wittich GR, Casola G, et al. Percutaneous drainage of infected and noninfected pancreatic pseudocysts: experience in 101 cases. Radiology. 1989;170:757–761. doi: 10.1148/radiology.170.3.2644662. [DOI] [PubMed] [Google Scholar]
- 4.Park DH, Lee SS, Moon SH, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842–848. doi: 10.1055/s-0029-1215133. [DOI] [PubMed] [Google Scholar]
- 5.Song MH, Lee SK, Kim MH, et al. EUS in the evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2003;57:891–896. doi: 10.1016/s0016-5107(03)70026-1. [DOI] [PubMed] [Google Scholar]
- 6.Kitano M, Sakamoto H, Komaki T, Kudo M. Present status and future perspective of EUS-guided drainage. Dig Endosc. 2009;21(Suppl 1):S66–S70. doi: 10.1111/j.1443-1661.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 7.Antillon MR, Shah RJ, Stiegmann G, Chen YK. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797–803. doi: 10.1016/j.gie.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Kruger M, Schneider AS, Manns MP, Meier PN. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409–416. doi: 10.1016/j.gie.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 9.Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524–529. doi: 10.1080/00365520601065093. [DOI] [PubMed] [Google Scholar]
- 10.Ardengh JC, Coelho DE, Coelho JF, de Lima LF, dos Santos JS, Modena JL. Single-step EUS-guided endoscopic treatment for sterile pancreatic collections: a single-center experience. Dig Dis. 2008;26:370–376. doi: 10.1159/000177024. [DOI] [PubMed] [Google Scholar]
- 11.Song TJ, Lee SS, Park do H, et al. Yield of EUS-guided FNA on the diagnosis of pancreatic/peripancreatic tuberculosis. Gastrointest Endosc. 2009;69:484–491. doi: 10.1016/j.gie.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Hwang CY, Lee SS, Song TJ, et al. Endoscopic ultrasound guided fine needle aspiration biopsy in diagnosis of pancreatic and peripancreatic lesions: a single center experience in Korea. Gut Liver. 2009;3:116–121. doi: 10.5009/gnl.2009.3.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SS, Park do H, Hwang CY, et al. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: a prospective feasibility study. Gastrointest Endosc. 2007;66:1008–1012. doi: 10.1016/j.gie.2007.03.1080. [DOI] [PubMed] [Google Scholar]
- 14.Oh HC, Seo DW, Lee TY, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636–642. doi: 10.1016/j.gie.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Park do H, Koo JE, Oh J, et al. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168–2174. doi: 10.1038/ajg.2009.254. [DOI] [PubMed] [Google Scholar]
- 16.Song HJ, Kim JO, Eun SH, et al. Endoscopic ultrasonograpic findings of benign mediastinal and abdominal lymphadenopathy confirmed by EUS-guided fine needle aspiration. Gut Liver. 2007;1:68–73. doi: 10.5009/gnl.2007.1.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilmann P, Hancke S, Pless T, Schell-Hincke JD, Henriksen FW. One-step endosonography-guided drainage of a pancreatic pseudocyst: a new technique of stent delivery through the echo endoscope. Endoscopy. 1998;30:730–733. doi: 10.1055/s-2007-1001399. [DOI] [PubMed] [Google Scholar]
- 18.Giovannini M, Bernardini D, Seitz JF. Cystogastrotomy entirely performed under endosonography guidance for pancreatic pseudocyst: results in six patients. Gastrointest Endosc. 1998;48:200–203. doi: 10.1016/s0016-5107(98)70165-8. [DOI] [PubMed] [Google Scholar]
- 19.Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2008;68:1102–1111. doi: 10.1016/j.gie.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977–983. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- 21.Norton ID, Clain JE, Wiersema MJ, DiMagno EP, Petersen BT, Gostout CJ. Utility of endoscopic ultrasonography in endoscopic drainage of pancreatic pseudocysts in selected patients. Mayo Clin Proc. 2001;76:794–798. doi: 10.1016/S0025-6196(11)63223-0. [DOI] [PubMed] [Google Scholar]
- 22.Soliani P, Ziegler S, Franzini C, et al. The size of pancreatic pseudocyst does not influence the outcome of invasive treatments. Dig Liver Dis. 2004;36:135–140. doi: 10.1016/j.dld.2003.06.005. [DOI] [PubMed] [Google Scholar]