Abstract
Background/Aims
Serum YKL-40 has been linked to several human cancers. We investigated the potential role of serum YKL-40 as a marker of hepatobiliary malignancies.
Methods
Archived serum samples of patients undergoing liver transplantation evaluation at the Mayo Clinic Rochester were used to measure YKL-40 levels. Patients were divided into three groups: hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and end-stage liver disease (ESLD) without malignancies. The Model for ESLD (MELD) score was used to quantify the severity of liver disease.
Results
The median serum YKL-40 level was highest in the ESLD group at 296 ng/mL, compared to 259 ng/mL in the HCC group and 80 ng/mL in the CCA group (p<0.01). There was a significant correlation between the MELD score and serum YKL-40 level (r=0.50, p<0.01). In a multivariate analysis, there was no significant difference in serum YKL-40 level between ESLD and HCC. CCA was associated with lower YKL-40 levels, a finding that was attributable to a lower prevalence of cirrhosis.
Conclusions
The serum YKL-40 level has little utility as a cross-sectional screening tool for hepatobiliary malignancies, namely HCC and CCA. The role of YKL-40 as a surveillance marker in the follow-up of individual patients remains to be determined.
Keywords: Hepatocellular carcinoma, Cholangiocarcinoma, End stage liver disease, Model for end-stage liver disease, YKL-40
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most lethal malignancies in humans. As the sixth most common cancer and the third-leading cause of cancer deaths in the world, HCC has a high global public health burden.1 As HCC occurs almost exclusively among patients with underlying liver disease, patients at risk of developing HCC may be potentially identified. However, timely diagnosis and accurate staging in patients with HCC remain a clinical challenge.
Cholangiocarcinoma (CCA) is also a highly lethal cancer occurring in the intra- and extra-hepatic bile ducts. Although CCA is not as prevalent as HCC, it is rarely diagnosed early and most patients who presents with symptomatic disease rarely achieve long term survival. Serum markers for screening and early diagnosis of CCA are also lacking.
YKL-40, also known as human cartilage glycoprotein 39, is a glycoprotein expressed in association with inflammatory process, extracellular matrix degradation and angiogenesis.2 It is mainly secreted by human macrophages, synovial cells, chondrocytes and neutrophils, and hepatic stellate cells.2 Elevated serum YKL-40 levels is associated with wide range of inflammatory diseases including cardiovascular disease,3,4 diabetes mellitus,5,6 chronic obstructive pulmonary disease,7 asthma,8 inflammatory bowel disease,9 and osteoarthritis.10 High serum concentrations of YKL-40 have also been found in several types of malignancies and are known to be associated with poor survival. Biologically YKL-40 was shown to activate cancer signaling pathways and promote tumor angiogenesis.11,12
Although oncogenic effect of YKL-40 has not been well studied in hepatobiliary malignancy, YKL-40 is located on human chromosome 1q31-q32, a region frequently amplified in HCC.13 Currently, the potential utility of YKL-40 in the screening, diagnosis and staging of hepatobiliary malignancies has not been investigated. In this study, we explore whether serum concentration of YKL-40 may represent a marker of hepatobiliary malignancies, including HCC and CCA.
MATERIALS AND METHODS
This study is conducted utilizing serum samples collected from patients evaluated for liver transplantation at Mayo Clinic Rochester between March 2002 and December 2005. It was approved by the Mayo Foundation Institutional Review Board. Based on availability of serum samples, 107 subjects were included in this analysis consisting of 28 patients with HCC, 28 with CCA, and 51 with end stage liver disease.
Relevant clinical information at the time of listing for liver transplantation was extracted from medical records, such as patient demographics, smoking and alcohol history (past or current consumption of cigarettes or alcoholic beverages), etiology of liver disease, presence of liver cirrhosis, tumor characteristic and laboratory data, including serum concentrations of total bilirubin and creatinine and international normalized ratio (INR) for prothrombin time for the purpose of this analysis.
Based on these data, we calculated the model for end-stage liver Disease (MELD) score according to the following equation: MELD=11.2 log (INR)+9.57 log (creatinine)+3.78 log (bilirubin)+6.43. Thus, in this analysis, MELD was used as an indicator of severity of liver disease.
Serum concentrations of YKL-40 were measured by the enzyme-linked immunosorbent assay technique. The assay was conducted using a commercial assay kit (METRA®; Quidel Co., San Diego, CA, USA) and following the manufacturer's instruction.
Statistical analyses were conducted using the SAS 9.1 statistical software package (SAS Institute, Gary, NC, USA). Comparison of the three groups was conducted by the nonparametric Kruskal Wallis test and the chi-square test. The relation between the MELD score and YKL-40 concentration was examined using the Spearman correlation. Finally, univariate and multivariable linear regression analyses were performed to evaluate the differences in YKL-40 concentration among the three groups of patients, while adjusting for relevant covariates.
RESULTS
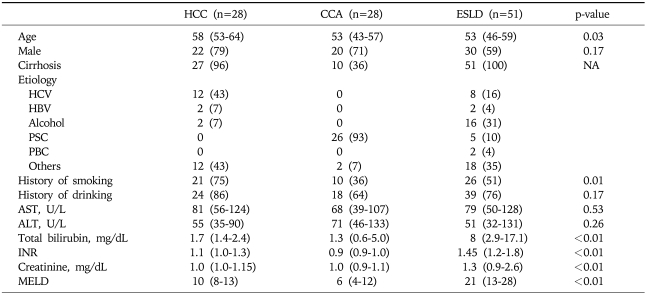
Table 1 compared the three groups of patients. The median age of HCC patients was 58 years, significantly higher than patients with end-stage liver disease (ESLD) or CCA. There appeared to be stronger male preponderance in the HCC and CCA groups, although the difference was not significant. Most patients with HCC and ESLD had cirrhotic liver disease, whereas about a third of patients with CCA had cirrhosis. Viral hepatitis and alcoholic liver disease were major etiologies for HCC. As expected, PSC was a predominant risk factor of CCA. History of smoking and alcohol consumption was more prevalent among HCC patients than the other two groups. The median MELD score was by far the highest in patients with ESLD. The MELD score was lowest among those with CCA.
Table 1.
Patients Characteristics
Data are presented as median (interquartile range) or number (proportion).
HCV, hepatitis C virus; HBV, hepatitis B virus; INR, international normalized ratio; MELD, model for end-stage liver disease.
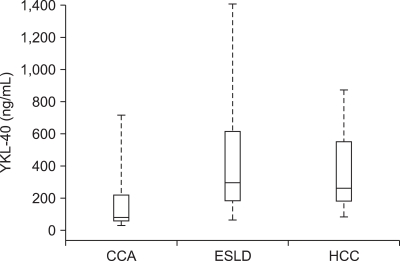
Fig. 1 displays the distribution of YKL-40 in the three groups. The median (mean) serum YKL-40 level for the ESLD group was the highest at 296 (481) ng/mL, compared to 259 (383) ng/mL in the HCC group and 80 (208) ng/mL in the CCA group. The difference among the three groups was statistically significant (p<0.01). When the groups were compared pair-wise, the difference between CCA and ESLD was significant (p<0.01), whereas that between HCC and ESLD was not (p=0.43).
Fig. 1.
Distribution of serum YKL-40 levels in the three groups of patients: CCA, cholangiocarcinoma; ESLD, end-stage liver disease; and HCC, hepatocellular carcinoma. The boxes represent interquartile ranges, while the upper and lower ends of the whiskers denote the 5th and 95th percentiles of the data, respectively.
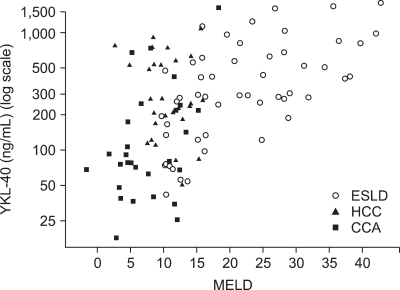
Fig. 2 depicts the correlation between the MELD score and the serum YKL-40 concentration. The latter variable was logarithmically transformed to render the distribution more symmetric. There was a significant positive correlation between MELD and YKL-40 (r=0.50, p<0.01).
Fig. 2.
Correlation between YKL-40 level and MELD score. ESLD, end-stage liver disease; HCC, hepatocellular carcinoma; CCA, cholangiocarcinoma; MELD, model for end-stage liver disease.
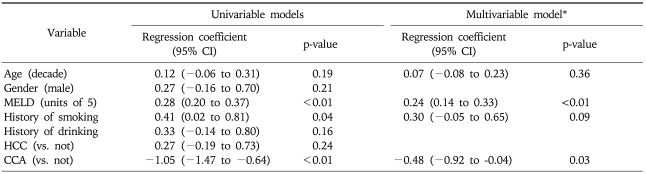
Table 2 summarizes regression models to examine associations between serum YKL-40 and diagnosis, along with age, gender, smoking, alcohol intake and the MELD score. In the univariate model, the MELD score and CCA were statistically significant. The MELD score and CCA remained significant in the multivariable model: a five-unit increase in the MELD score was associated with a 27% (e0.24-1) increase in serum YKL-40, whereas CCA was associated a 38% (e-0.48-1) decrease in serum YKL-40.
Table 2.
Univariate and Multivariate Linear Regression Models for the Prediction of YKL-40 Level
Age is included in the multivariable model because of the known association with serum YKL-40 level. YKL-40 was log transformed in the univariable and multivariable regression model.
CI, confidence interval; MELD, model for end-stage liver disease; HCC, hepatocellular carcinoma; CCA, cholangiocarcinoma.
*Variables with univariate p-value less than 0.1 were included in multivariable model.
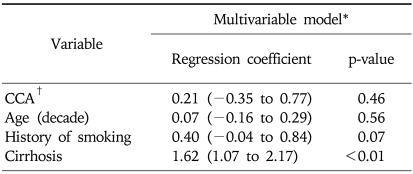
Table 3 further explores the relationship between serum YKL-40 and underlying cirrhosis in patients with HCC and CCA. Cirrhosis was associated with 5-fold (e1.621) increase in YKL-40, whereas there was no significant difference between HCC and CCA. Other covariates in the model were not significant.
Table 3.
Multivariate Linear Regression Model for the Prediction of YKL-40 Level among Patients with Hepatocellular Carcinoma or Cholangiocarcinoma
CCA, cholangiocarcinoma.
*Variables were selected based on clinical significance and result from the model in Table 2. MELD was removed due to the significant collinearity with cirrhosis. YKL-40 was log transformed in the multivariable regression model; †Hepatocellular carcinoma as reference group.
DISCUSSION
The main results of this study are two-fold: 1) serum YKL-40 concentration was not found to be useful as a marker of HCC or CCA, which was because 2) serum YKL-40 concentrations correlated with severity of liver fibrosis which is widely prevalent among individuals at risk of HCC and CCA. Of the two malignancy groups, serum YKL-40 was higher among patients with HCC than those with CCA. Our data showed (Table 3) that this is attributable to a lower prevalence of cirrhosis among CCA patients, although it is possible that there may be a minor tumor-specific difference in the expression of YKL-40 between HCC and CCA. Nonetheless, our data suggest that in this patient population, it is likely that serum YKL-40 has a limited clinical utility as a tumor marker for either type of hepatobiliary malignancies.
Our results are confirmatory for the correlation between the serum YKL-40 and hepatic fibrosis.14 Johansen et al.15 made an initial observation that serum YKL-40 increased progressively from normal subjects (median, 102 ng/mL) to patients with mild fibrosis (270 ng/mL) to those with moderate (466 ng/mL) to severe (676 ng/mL) fibrosis. Immunohistochemical analysis using the frozen liver tissues demonstrated positive staining for YKL-40 antigen in areas with fibrosis, particularly if fibrogenesis is active.15 Subsequent studies showed that serum YKL-40 decreased in patients with hepatitis C treated with interferon, particularly if the virus is cleared16 and that among patients with alcoholic liver disease, elevated serum YKL-40 was associated with shortened survival.17 Similarly, in chronic hepatitis B patients, YKL-40 was reported to be higher in patients with cirrhosis than patients without cirrhosis.18 Cirrhosis is characterized by increased tissue remodeling of extracellular matrix and accumulation of fibrosis tissue in the liver. Activated hepatic stellate cells are the key modulators of this pathologic process. Hepatic stellate cells are thought to be a potential source of YKL-40 secretion in the liver, explaining high serum concentration of YKL-40 in cirrhotic patients. Our data further showed that serum YKL-40 correlated with MELD, suggesting that even among patients with cirrhosis, serum YKL-40 may further identify patients with more advanced disease. Whether serum YKL-40 has prognostic information over and above the MELD score in these patients is not known.
Serum YKL-40 has been shown to be a helpful diagnostic and prognostic indicator in various types of cancers, which include colorectal,19 breast,20 lung,21 head and neck,22 prostate,23 ovarian cancer,24 and malignant melanoma.25 Depending on the tumor type, the effect of serum YKL-40 on survival rates may be substantial (hazard ratio range, 1.3-4.1).26 Most recently, a Danish cohort study showed that elevated YKL-40 level predicts the increased risk of gastrointestinal cancer and decreased survival after cancer diagnosis in general population.27 Of note, median serum YKL-40 concentrations reported in patients with other cancers (57-180 ng/mL)19-22,24,28 are far lower than serum YKL-40 concentration observed in patients with advanced liver fibrosis (466-676 ng/mL).15
With regard to the role of YKL-40 in patients with hepatobiliary malignancies, previous studies have demonstrated that clusterin, a molecule closely linked with YKL-40, plays an important role in the metastasis of HCC.29 Whether serum YKL-40 has any diagnostic and/or prognostic role in patients with HCC and other hepatobiliary malignancies has not been studied. While some HCCs may produce and secrete YKL-40; however, our data showed that such signals may easily be overshadowed by the high levels of YKL-40 associated with liver fibrosis. To the extent that most of HCCs occur in the setting of advanced fibrosis, this is a significant shortcoming in using serum YKL-40 as a screening tool. Patients with CCA may or may not have underlying liver fibrosis, which explain the generally lower serum levels of YKL-40 in those patients.
In the context of assessing serum YKL-40 as a marker of hepatobiliary malignancies, some limitations to our study need to be kept in mind. This was an exploratory, cross-sectional study to evaluate inter-individual differences that may indicate that serum YKL-40 may be useful in screening and diagnosis of hepatobiliary malignancies. While the answer to that question is largely negative, it is still possible that serum YKL-40 may be useful in a given individual upon a longitudinal follow-up. This may be somewhat analogous to serum alpha-fetoprotein (AFP) in patients with active hepatitis and cirrhosis, where AFP may be a marker of inflammation or neoplasia. A longitudinal follow-up of serial AFP is often more helpful than a one-time AFP value. Similarly, the patients included in the study represent a convenient sample of individuals who were waiting for liver transplantation with easily retrievable serum specimens. Reflecting criteria for liver transplantation in patients with HCC and CCA, patients had early stage malignancies.
With regard to determinants of serum YKL-40 other than liver fibrosis and malignancies, our data were consistent with previous studies. Serum YKL-40 has been shown to correlate with age. Johansen et al shows that in both men and women, increasing age was associated with rising serum YKL-40.27 Although the exact mechanism is not known, increased inflammatory cytokines such as IL-6 in the elderly people may partly explain the elevation of YKL-40 level with age.30,31 In our data, a 10-year increase in age was associated with 10% higher level of serum YKL-40. Alcohol consumption and smoking have also been associated serum YKL-40 level. These are also consistent with the previous observations made in population based studies.27 In our analysis, there was positive, yet non-significant univariate association between these behavioral risk factors and serum YKL-40. These effects largely disappeared in the multivariable model, probably due to the modest sample size of our study. Currently, little is known how smoking and alcohol intake may independently increase the serum level of YKL-40.
In conclusion, despite previous data that YKL-40 is overexpressed in HCC tissue, we demonstrate that its serum concentrations are likely of little use as a screening or diagnostic marker of hepatobiliary malignancies. This was at least in part secondary to the correlation between serum YKL-40 and liver fibrosis/cirrhosis. It remains to be studied whether serum YKL-40 may have role as a tool for surveillance in a given individual at risk of HCC.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institute of Diabetes, Digestive and Kidney Diseases (DK-34238) and the Palumbo Foundation.
Footnotes
No conflict of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 3.Michelsen AE, Rathcke CN, Skjelland M, et al. Increased YKL-40 expression in patients with carotid atherosclerosis. Atherosclerosis. 2010;211:589–595. doi: 10.1016/j.atherosclerosis.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Zheng JL, Lu L, Hu J, et al. Increased serum YKL-40 and C-reactive protein levels are associated with angiographic lesion progression in patients with coronary artery disease. Atherosclerosis. 2010;210:590–595. doi: 10.1016/j.atherosclerosis.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Rathcke CN, Persson F, Tarnow L, Rossing P, Vestergaard H. YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care. 2009;32:323–328. doi: 10.2337/dc08-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen AR, Erikstrup C, Johansen JS, et al. Plasma YKL-40: a BMI-independent marker of type 2 diabetes. Diabetes. 2008;57:3078–3082. doi: 10.2337/db08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letuve S, Kozhich A, Arouche N, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181:5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 8.Chupp GL, Lee CG, Jarjour N, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 9.Erzin Y, Uzun H, Karatas A, Celik AF. Serum YKL-40 as a marker of disease activity and stricture formation in patients with Crohn's disease. J Gastroenterol Hepatol. 2008;23:e357–e362. doi: 10.1111/j.1440-1746.2007.05121.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang K, Wu LD. YKL-40: a potential biomarker for osteoarthritis. J Int Med Res. 2009;37:18–24. doi: 10.1177/147323000903700102. [DOI] [PubMed] [Google Scholar]
- 11.Bi J, Lau SH, Lv ZL, et al. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum Pathol. 2009;40:1790–1797. doi: 10.1016/j.humpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Shao R, Hamel K, Petersen L, et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456–4468. doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau SH, Guan XY. Cytogenetic and molecular genetic alterations in hepatocellular carcinoma. Acta Pharmacol Sin. 2005;26:659–665. doi: 10.1111/j.1745-7254.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- 14.Mehta P, Ploutz-Snyder R, Nandi J, Rawlins SR, Sanderson SO, Levine RA. Diagnostic accuracy of serum hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2008;103:928–936. doi: 10.1111/j.1572-0241.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 15.Johansen JS, Christoffersen P, Moller S, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 16.Saitou Y, Shiraki K, Yamanaka Y, et al. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005;11:476–481. doi: 10.3748/wjg.v11.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nojgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39:179–186. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee KG, Seo YS, An H, et al. Usefulness of non-invasive markers for predicting liver cirrhosis in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2010;25:94–100. doi: 10.1111/j.1440-1746.2009.05953.x. [DOI] [PubMed] [Google Scholar]
- 19.Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, Nielsen HJ. Serum YKL-40 and colorectal cancer. Br J Cancer. 1999;79:1494–1499. doi: 10.1038/sj.bjc.6690238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen JS, Christensen IJ, Riisbro R, et al. High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res Treat. 2003;80:15–21. doi: 10.1023/A:1024431000710. [DOI] [PubMed] [Google Scholar]
- 21.Johansen JS, Drivsholm L, Price PA, Christensen IJ. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer. 2004;46:333–340. doi: 10.1016/j.lungcan.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Roslind A, Johansen JS, Christensen IJ, et al. High serum levels of YKL-40 in patients with squamous cell carcinoma of the head and neck are associated with short survival. Int J Cancer. 2008;122:857–863. doi: 10.1002/ijc.23152. [DOI] [PubMed] [Google Scholar]
- 23.Brasso K, Christensen IJ, Johansen JS, et al. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66:503–513. doi: 10.1002/pros.20311. [DOI] [PubMed] [Google Scholar]
- 24.Dehn H, Hogdall EV, Johansen JS, et al. Plasma YKL-40, as a prognostic tumor marker in recurrent ovarian cancer. Acta Obstet Gynecol Scand. 2003;82:287–293. doi: 10.1034/j.1600-0412.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt H, Johansen JS, Gehl J, Geertsen PF, Fode K, von der Maase H. Elevated serum level of YKL-40 is an independent prognostic factor for poor survival in patients with metastatic melanoma. Cancer. 2006;106:1130–1139. doi: 10.1002/cncr.21678. [DOI] [PubMed] [Google Scholar]
- 26.Johansen JS, Jensen BV, Roslind A, Price PA. Is YKL-40 a new therapeutic target in cancer? Expert Opin Ther Targets. 2007;11:219–234. doi: 10.1517/14728222.11.2.219. [DOI] [PubMed] [Google Scholar]
- 27.Johansen JS, Bojesen SE, Mylin AK, Frikke-Schmidt R, Price PA, Nordestgaard BG. Elevated plasma YKL-40 predicts increased risk of gastrointestinal cancer and decreased survival after any cancer diagnosis in the general population. J Clin Oncol. 2009;27:572–578. doi: 10.1200/JCO.2008.18.8367. [DOI] [PubMed] [Google Scholar]
- 28.Tanwar MK, Gilbert MR, Holland EC. Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res. 2002;62:4364–4368. [PubMed] [Google Scholar]
- 29.Lau SH, Sham JS, Xie D, et al. Clusterin plays an important role in hepatocellular carcinoma metastasis. Oncogene. 2006;25:1242–1250. doi: 10.1038/sj.onc.1209141. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 31.Johansen JS, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK, Bruunsgaard H. High serum YKL-40 level in a cohort of octogenarians is associated with increased risk of all-cause mortality. Clin Exp Immunol. 2008;151:260–266. doi: 10.1111/j.1365-2249.2007.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]