Abstract
Sorafenib is an oral multikinase inhibitor that has shown a survival benefit in patients with advanced hepatocellular carcinoma, and is considered to be generally safe. We treated a patient with interstitial lung disease that was associated with sorafenib therapy for the treatment of advanced hepatocellular carcinoma. A 74-year-old man with hepatitis-C-virus-related hepatocellular carcinoma was treated with sorafenib. After 8 days of sorafenib administration, he received radiation therapy for an intrahepatic tumor located in segment eight. On the 24th day of sorafenib treatment, the patient developed acute interstitial pneumonitis that rapidly improved after the discontinuation of sorafenib and treatment with high-dose steroids. This case alerts physicians to the possibility of sorafenib-induced interstitial lung disease.
Keywords: Sorafenib, Interstitial lung disease, Hepatocellular carcinoma, Chemotherapy, Adverse effect
INTRODUCTION
Sorafenib (Nexavar™; Bayer Pharmaceuticals, West Haven, CT, USA) is an oral multikinase inhibitor that blocks tumor-cell proliferation and angiogenesis by targeting Raf-1, a member of the Raf/mitogen-activated protein extracellular kinase/extracellular signal-regulated protein kinase (Raf/MEK/ERK) signaling pathway, receptor tyrosine kinases vascular endothelial growth factor (VEGF) receptors 2 and 3 and platelet-derived growth factor receptor β, Flt-3, and c-KIT.1 It has been studied in patients with advanced stage hepatocellular carcinoma, and has shown a significant survival benefit.2
Diarrhea, weight loss, skin rash including hand-foot skin reactions, fatigue, and hypertension are reported common adverse effects of sorafenib.3,4 To date, there is no reported case of sorafenib-induced interstitial lung disease (ILD). We report a case of ILD that developed within 1 month of sorafenib treatment in a patient with advanced hepatocellular carcinoma.
CASE REPORT
A 74-year-old male with hepatitis C virus-related, multinodular hepatocellular carcinoma had progressive disease after six sessions of transarterial chemoembolization and one session of radiofrequency ablation during the past 21 months. The radiological studies showed an increased extent of the intrahepatic tumor with tumor invasion of the middle hepatic vein (Fig. 1). Although the patient was a former smoker with a 100 pack-year history of smoking, he had no respiratory symptoms and a normal chest X-ray (Fig. 2A). His performance status was good and the functional status of the liver was Child-Pugh class A. The patient was treated with sorafenib, 400 mg twice daily. Palliative radiotherapy (total radiation dose of 60 Gy in 30 fractions planned) targeted the residual hepatocellular carcinoma and the middle hepatic vein thrombosis was added 8 days after the first administration of sorafenib. The combined therapy was tolerated over 2 weeks, although the patient experienced mild nausea and diarrhea. On the 24th day of sorafenib treatment, the patient developed progressive dyspnea and fever with worsening of the nausea and general weakness, and he presented to the emergency room.
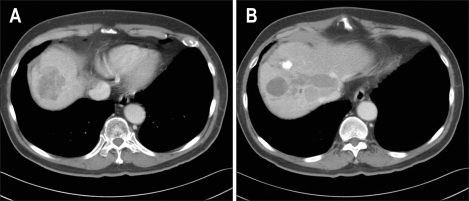
Fig. 1.
(A) Abdominal computed tomography performed before the initiation of sorafenib therapy revealed an area of low attenuation in segment eight. (B) The middle hepatic vein is dilated due to the presence of a diffuse tumor thrombus in the middle hepatic vein.
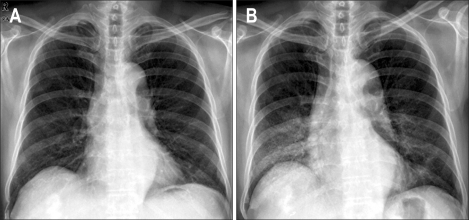
Fig. 2.
(A) Chest X-ray performed before the initiation of sorafenib therapy showing no active lung lesion. (B) Diffuse ground-glass opacity was present in both lungs on the 2nd day of hospitalization.
At the emergency room, the patient presented with dyspnea, cough, and fever. The vital signs showed a normal blood pressure of 130/80 mm Hg, respiratory rate of 22 breaths/min, pulse rate of 120 beats/min, and body temperature of 38.5℃. Inspiratory crackles were audible at the right lower lung field, and the cardiac examination was normal without cyanosis or edema. The patient was not anemic or icteric, and the abdominal examination was unremarkable, without ascites or organomegaly. The resting room air pulse oximetric saturation (SpO2) was 93.2%, and the arterial blood gas analysis showed a PaO2 of 62.5 mm Hg, a PaCO2 of 23 mm Hg, and a pH of 7.48 on ambient air. Laboratory studies were remarkable for leukocytosis (6,240 cells/uL, 79% of neutrophils) and an elevated C-reactive protein (CRP) level of 5.47 mg/dL (normal, under 0.5 mg/dL), elevated aspartate transaminase (AST) concentration of 855 IU/L (normal, 0 to 40 IU/L) and alanine transaminase (ALT) concentration of 860 IU/L (normal, 0 to 40 IU/L). The renal function test results were within normal limits.
On the chest X-ray, there was increased opacity at the right lower and mid-lung regions. The day after admission, the patient developed rapidly worsening dyspnea in spite of therapy with bronchodilator medication administered by a nebulizer and antimicrobial agents, in addition to at least 4 L/min of oxygen through nasal prongs to maintain the resting oxygen saturation levels higher than 90%. Follow-up chest X-ray showed progressive, diffuse ground-glass opacities in both lungs (Fig. 2B). Serial examination of sputum specimens did not reveal any significant bacteria or fungus. No pathogens were cultured from the blood or urine, and the result of a mycoplasma antibody test was negative. The clinical diagnosis was interstitial lung disease associated with sorafenib treatment; the sorafenib was discontinued on the 25th day of administration. Radiotherapy was also discontinued after administration of a total of 24 Gy divided in 12 fractions to mainly the segment 8 area of the liver. Treatment with prednisolone 30 mg twice a day was initiated with antibiotics (cefotaxime and metronidazole) and bronchodilator (salbutamol nebulizer) therapy. High-resolution chest computed tomography performed on the 3rd day of admission demonstrated bilateral ground-glass opacities with septal thickening throughout both lungs extending to the left upper lung field (Fig. 3A-C), which was definitely beyond the radiation field (Fig. 3D). The symptoms and radiological findings gradually improved over 11 days. The patient completely recovered from the interstitial lung disease during the 4 months of follow-up.
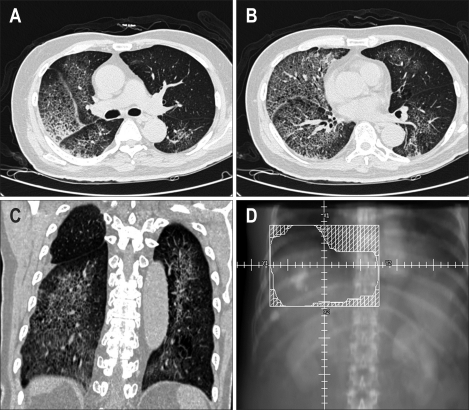
Fig. 3.
(A, B, C) Computed tomography scans of the chest performed on the 3rd day of hospitalization showing diffuse ground-glass opacity with septal thickening in both lungs. (D) Schematic illustration of the radiation field.
DISCUSSION
Here we describe a patient with advanced hepatocellular carcinoma that developed rapidly progressive interstitial lung disease after treatment with sorafenib for 24 days. In the absence of other etiologies, and given the temporal relationship between the use of sorafenib and the onset of symptoms of ILD, and improvement of the ILD after discontinuation of sorafenib, with high dose steroid therapy, this patient was considered to have sorafenib-related ILD.
Although the patient received concomitant radiotherapy with a total dose of 24 Gy divided into 12 fractions, mainly to the segment 8 area of the liver, and adjacent right lower lung field, the extent of the lung disease was far beyond the radiation fields. Moreover, the time interval from initiation of radiation to the development of ILD of 16 days was exceptionally short, considering the radiation dose administered to the lung field adjacent to the hepatic lesion. Radiation induced pneumonitis usually occurs about 4-12 weeks after the completion of treatment.5,6 Pulmonary damage rarely occurs with total doses less than 20 Gy,7 and commonly occurs with doses between 30 and 40 Gy.8 In addition, the size and number of the daily dose fractions have a direct bearing on the risk of radiation pneumonitis.9 One study showed that administration of a daily dose fraction greater than 2.67 Gy was the most significant factor associated with an increased risk of radiation pneumonitis.10 Radiation-induced lung changes are usually confined to the irradiated volume.8,11 Therefore, the ILD in this case was more likely to have been associated with the sorafenib therapy, although the possibility of the aggravation of lung disease by radiation cannot be excluded.
Although the use of many molecularly targeted agents, such as gefitinib, erlotinib, imatinib, and bortezomib has been associated with pulmonary toxicity,12-14 there has been no previous report on lung injury associated with sorafenib treatment; this is the first case report of ILD developed after administration of sorafenib. The pathogenesis of sorafenib-induced ILD is not known. The pulmonary toxicity induced by sorafenib may be related to its mechanism of the antitumor activity, that is, the inhibition of the VEGF signaling pathway. The reduction of intrapulmonary VEGF levels, in the early stages of lung injury and normalization after recovery in acute respiratory distress syndrome have been confirmed in many studies.15-19 Further studies on the molecular mechanisms responsible for sorafenib-associated lung injury are needed.
Unfortunately, we didn't performed other diagnostic tests such as lung biopsy or bronchoalveolar lavage, because the patient's condition did not permit to do those tests. If we had performed such tests, the result might have helped to exclude other similar condition.
The case presented here highlights the potential for sorafenib to induce ILD. Therefore, physicians should be aware of ILD as a potential pulmonary toxicity associated with sorafenib treatment. Acute onset of respiratory symptoms in patients treated with sorafenib warrants pulmonary evaluation and appropriate treatment.
References
- 1.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 5.Gross NJ. Pulmonary effects of radiation therapy. Ann Intern Med. 1977;86:81–92. doi: 10.7326/0003-4819-86-1-81. [DOI] [PubMed] [Google Scholar]
- 6.Roswit B, White DC. Severe radiation injuries of the lung. AJR Am J Roentgenol. 1977;129:127–136. doi: 10.2214/ajr.129.1.127. [DOI] [PubMed] [Google Scholar]
- 7.Jennnings FL, Arden A. Development of radiation pneumonitis. Time and dose factors. Arch Pathol. 1962;74:351–360. [PubMed] [Google Scholar]
- 8.Libshitz HI, Southard ME. Complications of radiation therapy: the thorax. Semin Roentgenol. 1974;9:41–49. doi: 10.1016/0037-198x(74)90008-x. [DOI] [PubMed] [Google Scholar]
- 9.Movsas B, Raffin TA, Epstein AH, Link CJ., Jr Pulmonary radiation injury. Chest. 1997;111:1061–1076. doi: 10.1378/chest.111.4.1061. [DOI] [PubMed] [Google Scholar]
- 10.Roach M, 3rd, Gandara DR, Yuo HS, et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol. 1995;13:2606–2612. doi: 10.1200/JCO.1995.13.10.2606. [DOI] [PubMed] [Google Scholar]
- 11.Ikezoe J, Morimoto S, Takashima S, Takeuchi N, Arisawa J, Kozuka T. Acute radiation-induced pulmonary injury: computed tomography evaluation. Semin Ultrasound CT MR. 1990;11:409–416. [PubMed] [Google Scholar]
- 12.Gotoh A, Ohyashiki K, Oshimi K, et al. Lung injury associated with bortezomib therapy in relapsed/refractory multiple myeloma in Japan: a questionnaire-based report from the "Lung Injury by Bortezomib" Joint Committee of the Japanese Society of Hematology and the Japanese Society of Clinical Hematology. Int J Hematol. 2006;84:406–412. doi: 10.1532/IJH97.06142. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303–306. doi: 10.1634/theoncologist.8-4-303. [DOI] [PubMed] [Google Scholar]
- 14.Gemma A. Drug-induced interstitial lung diseases associated with molecular-targeted anticancer agents. J Nippon Med Sch. 2009;76:4–8. doi: 10.1272/jnms.76.4. [DOI] [PubMed] [Google Scholar]
- 15.Maitre B, Boussat S, Jean D, et al. Vascular endothelial growth factor synthesis in the acute phase of experimental and clinical lung injury. Eur Respir J. 2001;18:100–106. doi: 10.1183/09031936.01.00074701. [DOI] [PubMed] [Google Scholar]
- 16.Hanaoka M, Droma Y, Naramoto A, Honda T, Kobayashi T, Kubo K. Vascular endothelial growth factor in patients with high-altitude pulmonary edema. J Appl Physiol. 2003;94:1836–1840. doi: 10.1152/japplphysiol.00575.2002. [DOI] [PubMed] [Google Scholar]
- 17.Abadie Y, Bregeon F, Papazian L, et al. Decreased VEGF concentration in lung tissue and vascular injury during ARDS. Eur Respir J. 2005;25:139–146. doi: 10.1183/09031936.04.00065504. [DOI] [PubMed] [Google Scholar]
- 18.Meyer KC, Cardoni A, Xiang ZZ. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J Lab Clin Med. 2000;135:332–338. doi: 10.1067/mlc.2000.105618. [DOI] [PubMed] [Google Scholar]
- 19.Vasakova M, Sterclova M, Kolesar L, et al. Bronchoalveolar lavage fluid cellular characteristics, functional parameters and cytokine and chemokine levels in interstitial lung diseases. Scand J Immunol. 2009;69:268–274. doi: 10.1111/j.1365-3083.2008.02222.x. [DOI] [PubMed] [Google Scholar]