Summary
Skin, the largest organ of the human body, is organized into an elaborate layered structure consisting mainly of the outermost epidermis and the underlying dermis. A subcutaneous adipose-storing hypodermis layer and various appendages such as hair follicles, sweat glands, sebaceous glands, nerves, lymphatics and blood vessels are also present in the skin. These multiple components of the skin ensure survival by providing critical functions in protection, thermoregulation, excretion, absorption, metabolic functions, sensation, evaporation management and aesthetics. The study of how these biological functions are performed is critical in our understanding of basic skin biology, such as regulation of pigmentation and wound repair. Impairment of any of these functions may lead to pathogenic alterations, including skin cancers. Therefore, the development of genetically controlled and well-characterized skin models can have important implications, not only for scientists and physicians, but also for manufacturers, consumers, governing regulatory boards and animal welfare organizations. Since cells making up human skin tissue grow within an organized three dimensional (3D) matrix continually surrounded by neighboring cells, standard monolayer (2D) cell cultures do not recapitulate the physiological architecture of the skin. Several types of human skin recombinants, also called artificial skin, that provide this critical 3-D structure, have now been reconstructed in vitro. This review contemplates the use of these organotypic skin models in different applications, including substitutes to animal testing.
Keywords: artificial skin, skin reconstructs, skin equivalents, rafts, organotypical cultures, 3D models
GENERAL ARCHICTECTURE OF THE SKIN
I) Epidermal/dermal layers
The organization of the skin into epidermal and dermal layers that differ in thickness, strength, and flexibility allow for a structured architecture that provides a variety of functions of the skin. The outermost layer of the skin, known as the epidermis, serves as an impermeable boundary between the environment and the body. In turn, the underlying dermis is formed of strong connective tissue, which is rich in collagen and confers the characteristic flexibility to the skin. Epidermis and dermis are separated by the extracellular matrix (ECM), known as the basal lamina (Balasubramani et al., 2001; Horch et al., 2005; Godin and Touitou, 2007; Ajani et al., 2007).
The epidermal layer, derived from embryonic ectoderm, is made up of cells generated by proliferating keratinocytes of the stratum basale that move upwards while they differentiate (see Figure 1). The continuous process of proliferation, differentiation, and ultimately, cell death and shedding, allows compartmentalization into a number of strata representing different stages in keratinocyte maturation (Schulz et al., 2000; Balasubramani et al., 2001; Stark et al., 2004a). Besides keratinocytes, which account for about 80% of epidermal cells, the epidermis is also composed of the pigment-producing melanocytes, Merkel cells which are thought to play a sensory role (Feliciani et al., 1996; Boyce and Warden, 2002), and specialized dendritic Langerhans cells, which have an essential role in the skin immune defense system (Phillips, 1998; Régnier et al., 1998; Régnier et al., 1999).
Figure 1.
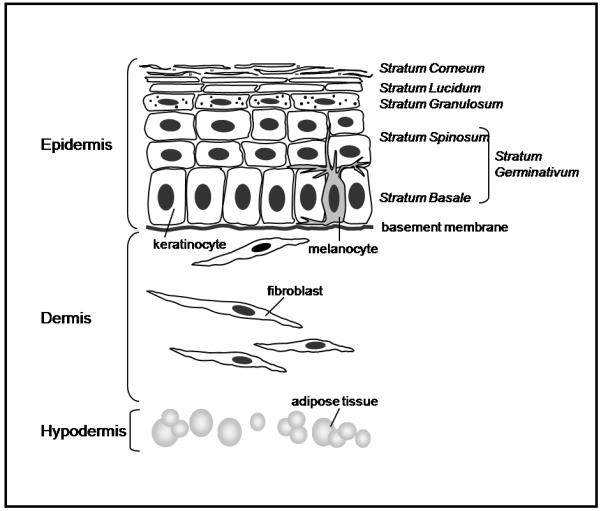
Skin draw showing skin components and layers. Epidermis: containing melanocytes and keratinocytes that are able to differentiate and form the different strata (Corneum, Lucidum, Granulosum and Germinativum). Dermis: formed by fibroblasts embedded in a matrix. Hypodermis: containing the adipose tissue.
As shown in Figure 1, ongoing keratinocyte cell division begins in the innermost stratum basal layer of the stratum germinativum and pushes daughter cells apically upwards toward the next spinous stratum, where cells become dense. As they progress toward the interface with the environment, they move into the granulosum stratum where they accumulate lipid granules, critical in maintenance of water barrier. The loss of the nucleus in differentiating keratinocytes now leads to a flattened or horny morphology, with only keratin remaining. Pigmentation is imparted by the addition of melanin, produced by melanocytes and transferred to keratinocytes in the final sublayer of the stratum lucidum. The most external layer, known as the stratum corneum, represents the final result of keratinocyte differentiation that is formed by completely differentiated dead keratinocytes interspersed with intercellular lipids (mainly ceramides and sphingolipids) (Ajani et al., 2007).
II) Basement membrane and other constituents of the skin
Epidermal development during embryogenesis, wound healing, regeneration, as well as normal homeostatic maintenance depends on interactions between the epithelia and the connective dermal tissue (Schulz et al., 2000; Balasubramani et al., 2001; Stark et al., 2004a; Ajani et al., 2007). The basal stratum of the epidermis is intimately related to the underlying dermis through a system of protein fiber connections along a micro-architecture network of rete ridges. These constitute a well-characterized basement membrane, which anchors the epidermal layer to the loose connective tissue of the underlying dermis. This basement membrane also stabilizes the overlying epidermis mechanically by its hemi-desmosomal structure (Marinkovich et al., 1992; Moll and Moll, 1998; El Ghalbzouri et al., 2005; Balasubramani et al., 2001; Schulz et al., 2000).
Basement membrane is made up mainly of collagen IV, Laminin, proteoglycans and glycosaminoglycans, as well as growth factors which have a wide variety of biological activities. This dermo-epidermal basement membrane strictly controls the traffic of bioactive molecules in both directions and is able to bind a variety of cytokines and growth factors, thus representing a reservoir for controlled release during physiological remodeling or repair processes (Iozzo, 2005). The dermal matrix, which lies underneath the basement membrane, provides energy and nutrition to the overlying epidermis and imparts considerable strength to skin by virtue of the arrangement of collagen fibers. The collagenous mesh work is interlaced with contents of elastin fibers, fibronectin, proteoglycans being GAG the predominantly hyaluronic acid and other components (Balasubramani et al., 2001). In addition to the dense matrix are found dermal fibroblasts, cells of the immune system, nerve endings, sweat and sebaceous glands, hair follicles, blood vessels and endothelial cells. The dermal fibroblasts are known to have numerous functions in synthesis and deposit of ECM components. Moreover, fibroblasts also play an important role in proliferation and migration, as well as autocrine and paracrine interactions with neighboring cells (Wong et al., 2007).
THE NEED FOR IN VITRO 3D SKIN MODELS
Interactions between an individual cell, its immediate neighbors and the ECM are responsible for the control of cell behavior (Grinnell, 1976; Bissell et al., 1982; Yang et al., 1986; Lin and Bissel, 1993; Smalley et al., 2006; Grinnell, 2008). Therefore, cells grown in 2D monolayers cannot capture the relevant complexity of the in vivo microenvironment (Mazzoleni et al., 2009). For example, it has long been suggested that cells cultured on 2D substrates such as culture plates, lose a myriad of important signals, key regulators, and tissue phenotypes. Cells growing in 3D have different cell surface receptor expression, proliferative capacity, extracellular matrix synthesis, cell density, and metabolic functions (Grinnell, 1976; Bissell et al., 1982; Yang et al., 1987; Lin and Bissel, 1993; Smalley et al., 2006; Grinnell, 2008; Horning et al., 2008; Mazzoleni et al., 2009). Thus, 2D monolayer models not only fail in the reproduction of complex and dynamic environments of in vivo tissues, but can also trigger false findings to some degree by forcing cells to adapt to an artificial, flat and rigid surface. Growing numbers of studies report differences in phenotype, cellular signaling, cell migration, and drug responses when the same cells are grown under 2D or 3D culture conditions (reviewed by Mazzoleni et al., 2009).
In vitro studies have shown that dermal fibroblasts secrete soluble factors that diffuse to the overlying epidermis and can influence keratinocytes to induce the production of basement membrane proteins or melanogenic factors (Balasubramani et al., 2001; El Ghalbzouri et al., 2002a; Wong et al., 2007). Keratinocytes in monoculture produce only a thin epidermal layer and without mesenchymal support undergo apoptosis after about 2 weeks in culture (Wong et al., 2007). Dermal fibroblasts promote not only keratinocyte proliferation, but also the development of identifiable keratinocyte layers. Consequently, properly stratified epithelia fails to form in simple 2D feeder-layer co-cultures, upon combination of postmitotic dermal fibroblasts (feeder cells) and epidermal keratinocytes. Only in advanced 3D in vitro systems do keratinocytes develop well-ordered epithelia (El Ghalbzouri et al., 2002a; Stark et al., 2004a; Wong et al., 2007), thus offering an opportunity to more closely recapitulate artificial human skin.
Therefore, strategies that allow the reconstruction of artificial human skin equivalents in a 3D setting, including both dermal and epidermal components, will provide versatility and answers to physiological questions that cannot be solved solely in the context of monolayer tissue culture. Models that recapitulate the basic architecture of the human skin will supply sites for studying cell-cell interactions and effects of the stromal environment in the regulation of melanogenesis, proliferation and differentiation of keratinocytes, as well as re-epithelialization processes after wounding. These skin models also present a relevant platform for the creation of cosmetic, photoaging and cancer models, as well as an excellent system for pharmacological analyses. Furthermore, the human skin equivalents can easily be engineered with specific genetic alterations in either dermal or epidermal compartments to determine how the outcome of these modifications may be modulated by the stromal environment. These skin models also present time- and cost-effective alternatives to the use of laboratory animals that are currently being used for such studies.
While murine animals have routinely been used as experimental models for skin biology and skin cancer (Mancuso et al, 2009; Youssef et al, 2010; Paulitschke et al, 2010), the dissimilar architecture of mouse and human skin presents severe limitations to this approach. To begin, the epithelium of fur-covered mouse models is densely packed with hair follicles that are synchronized for the first few months of life. Instead, humans possess larger inter-follicular regions with sparse hair follicles that are asynchronous in nature. Furthermore, adult murine dermis is thin and the epidermis is comprised of typically only 3 layers, with a high rate of turnover, while human dermis is quite thick and epidermis is generally 6-10 layers thick (as shown in Figure 2). Human skin melanoctyes reside in the basal layer of the epidermis, while they are located mainly in dermal hair follicles in mice. Additionally, a cutaneous muscle layer, the panniculous carnosus that is present in mice, but non-existant in humans, has led to data that is not consistent among species (Donahue et al., 1999).
Figure 2.
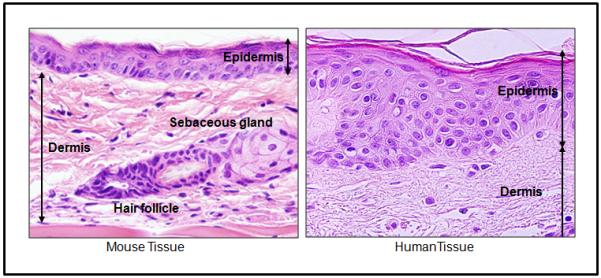
Optical microscopy showing the differences between human and mouse skin. Note that the murine dermis is thin and the epidermis is comprised of typically only 3 layers, while human dermis is quite thick and epidermis is generally 6-10 layers thick. HE staining. 60x magnification.
Skin responsiveness and functionality are also distinct among mouse and humans. For instance, wounding responses in mouse skin allow effective regeneration of tissue. However, damage response in human skin leads to scar tissue or hypertrophic dermal skin lesions known as keloids, which are absent in mouse skin (Khorshid, 2005). The function of epithelium is also dissimilar between mouse and human skin. Mouse skin provides less of a water barrier and displays higher percutaneous absorption than that of human skin, thus limiting topical drug-delivery studies (Menon, 2002).
GENERATION OF SKIN EQUIVALENTS
MULTIPLE APPROACHES
Several types of human skin equivalents that represent normal human skin tissue to a high degree have now been successfully reconstructed in vitro (Table 1). These skin equivalents consist mainly of keratinocytes that are cultured on a dermal substitute for approximately two weeks at the air–liquid interface, which allows for epidermal differentiation and development of stratified layers (Figure 3) (Prunieras et al., 1983; El Ghalbzouri et al., 2002a,b; Stark et al., 2004a; El Ghalbzouri et al., 2008). Although a major importance is given to the epidermal layer of skin equivalents, dermal substitutes are widely used in clinics for severe burns and chronic wounds (Boyce and Warden, 2002; Fimiani et al., 2005). These dermal substitutes include cell-free dermal matrices such as de-epidermized dermis (DED), inert filters, fibroblast-populated collagen matrices or lyophilized collagen-GAG membranes (Boyce et al., 2002; El Ghalbzouri et al., 2002a,b; Stark et al., 2004a; Fimiani et al., 2005; El Ghalbzouri et al., 2008).
Table 1.
Different strategies to generate artificial skin
| DERMALLAYER | EPIDERMALLAYER | SUPPLEMENTAL FACTORS | REFERENCES | |||
|---|---|---|---|---|---|---|
| Presence | Type | Cellular Components | Cellular Components | Time of Differentiation | ||
| + | DED Filter inserts Collagen gels |
NHF (in collagen matrices) |
NHK HaCaT |
1-3 weeks | 5% bovine calf serum 0,5μM hydrocortisone 0,1μM isoproterenol 0,5 μg/mL insulin 1ng/mL EGF 0,1nM cholera toxin |
Boelsma et al., 1999 |
| + | collagen type I | NHF | NHK | 7 days | Asselineau et al., 1985 Bernerd & Asselineau, 1997 Bernerd & Asselineau, 1998 Pageon & Asselineau, 2005 |
|
| + | rat tail collagen type I |
MDCK for basement membrane formation (removed before keratinocyte seeding) |
REK (neonatal rat epidermal kerationcytes) |
5 days | Ajani et al., 2007 | |
| + | NHF mouse 3T3 fibroblast |
newborn NHK | 2-3 weeks | 24,3μg/mL adenine 5% Fetalclone II 5 μg/mL insulin 0,4 μg/mL hydrocortisone 1nM cholera toxn 10 ng/mL EGF |
Dube et al., 2010 | |
| − | Filter insert (covered or not with collagen type IV) |
NHK | 17 days | 5% calf serum 1μM hydrocortisone 1μM isoproterenol 0,1μM insulin 100 μM L-serine 1 IMdL-a-tocopherol-acetate and a lipid supplement containing 25 IM palmitic acid, 15 IM linoleic acid, 7 IM arachidonic acid and 2.4 10 5 M bovine serum albumin 0,01 μM L-carnitine 1μM dL-α-tocopherol-acetate 25 μM palmitic acid 30 μM linoleic acid 7 μM arachidonic acid 50 μg/ml ascorbic acid 1 ng/ml EGF |
||
| + | calf collagen type I | NHF | NHK + NHM (20:1) |
over 1 week | Liu et al., 2007 | |
| + | collagen type I (Gen-col block collagen |
NHF | NHF + NHM (4:1) |
7 days | inserts immersed | Souto et al., 2009 |
| + | DED Collagen type I |
NHF NMF |
NHK + NHM (20:1) |
8 days | SCF hyman HGF Endotelin 1 or 3 |
Cario André et al., 2006 |
| + | Collagen type I | NHF 3T3-MEF |
NHK HaCaT |
7-10 days (human) 10-14 days (mouse) |
Calf serum Insulin EGF Cholera toxin Adenine Hydrocortisone Ascorbic acid TGF-α (only in HaCaT) |
Stark et al., 2004a |
| + | Hyalograft 3-D | NHF | NHK | up to 12 weeks | FAD medium 10% fetal bovine serum 0,1M cholera toxin 0,4 μg hydrocortisone 50 μg L-ascorbic acid 200 U/mL/ day aprotinin |
Stark et al., 2004b
Stark et al., 2006 Boehnke et al., 2007 |
| + | Collagen type I Matrigel |
NHK | 2-3 weeks | Aprotinin solution | Sobral et al., 2007 | |
Figure 3.
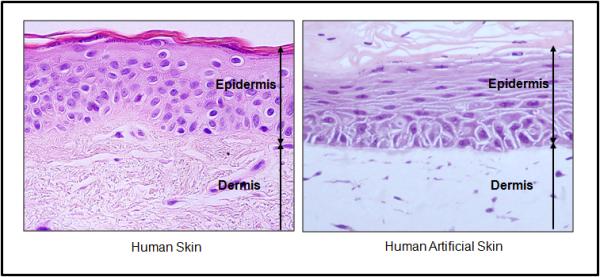
Optical microscopy of human facial skin and human artificial skin. Note that epidermis and dermis thickness is very similar. Also that human artificial skin presents all epidermal layers, showing the differentiation of this epithelium. HE staining. 60x magnification.
Engineered skin bioproducts containing biopolymers are also being used as grafts in the treatment of patients with excised burns, burn scars, and congenital skin lesions (Boyce and Warden, 2002). Additionally, materials such as collagen–glycosaminoglycan matrices, allogeneic dermis, and synthetic polymers have been used as replacements for specific skin layers (Balasubramani et al., 2001). Summarizing, there is a variety of so-called “skin models” that can be broadly categorized into (i) those containing only epidermal components, (ii) grafts consisting of dermal components alone, or (iii) full-thickness composite grafts containing both epidermal and dermal components. In the literature, these systems have been referred to as 3-D skin, reconstructed skin, skin equivalents, artificial skin, organotypic culture of skin, skin substitutes or skin grafts. To note, the last two terms are mostly commonly used for bioengineered products that make use of human or animal components such as cultured cells or collagen.
The past few years have seen an increase in the number of commercially available skin models. These include: EpiDermTM (MatTek, Ashland, MA, USA), EpiskinTM (before by Episkin, Chaponost, France now by L’Oreal, SkinEthic, Nice, France), Apligraf® (Organogenesis Inc., MA, USA) and the models engineered by SkinEthic (SkinEthic, Nice, France) (Boelsma et al., 2000; Ponec, 2002; Welss et al., 2004). Since these models are used for commercial purposes, morphological studies have been performed to demonstrate a relevant multilayered epithelium, and presence of characteristic epidermal ultrastructures and markers of epidermal differentiation, (e.g. keratins, fillagrin, involucrin, among others (El Ghalbzouri et al., 2002a, b; 2008).
In an editorial about the development of new skin equivalents, Phillips (1998) describes the historical evolution of skin substitutes. Specifically, in 1975, Rheinwald and Green described a methodology for the in vitro cultivation and serial subculture of epidermal cells that were able to produce viable keratinocyte sheets. This technique was critical in the development of tissue culture technology, and has improved the cultivation of large quantities of keratinocytes in vitro (Phillips, 1998). Once keratinocytes could be propagated in large quantities, the first step for successful epidermal differentiation was the exposure of normal human keratinocyte cultures at the air-liquid interface, to induce terminal differentiation resulting in a multilayered stratified tissue, as seen in Figure 4 (Régnier et al, 1998; 1999). Keratinocyte differentiation and consequent programmed cell death ultimately led to formation of the uppermost stratum corneum in the skin equivalent models, which is continuously formed during this process (Figure 4).
Figure 4.
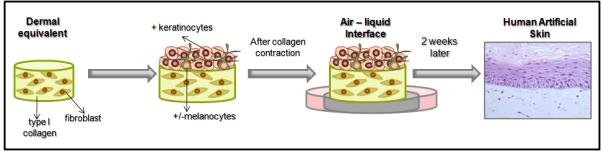
Preparation model of artificial skin. First step is the preparation of the dermal equivalent, consisting of type I collagen and fibroblasts. After polymerization of this layer, there is the plating of keratinocytes (K) and melanocytes (M). 24 hrs after plating and after the contraction of collagen gel, this whole structure is passed to a steel grid to form the air-liquid interface. After 2 weeks on the interface there is the formation of artificial skin. HE staining. 40x magnification
Studies on the effectiveness, metabolic transformation and potential pathologic effects of a huge variety of topical products have only been possible in vitro due to the presence of the stratum corneum in the reconstructed epidermis of artificial skin (Boyce et al., 2002; Ponec, 2002, Ajani et al., 2007; El Ghalbzouri et al., 2008). For safety reasons, multiple tests for topical products have been developed to detect changes in the integrity, morphology, viability, and the release of pro-inflammatory mediators in this layer (Ponec, 2002). One point to be mentioned is that after a prolonged time period in culture (6 -7 weeks), the in vitro epidermis remains viable and displays all signs of a normal differentiation program, but the thickness of the stratum corneum was gradually increased with time. This can be a critical issue in some tests, since the gradual thickening of the stratum corneum may lead to higher resistance to environmental stimuli (Ponec, 2002). Thus, these models remain imperfect, and may be intrinsically variable. Nevertheless, despite these limitations, they have already provided much information about dermal-epidermal interactions, cell-cell and cell-matrix interactions, responses of dermal and epithelial cells to biological signals and pharmacological agents, as well as effects of drugs and growth factors on skin reconstruction processes (Fimiani et al., 2005).
IMPORTANCE OF THE MICROENVIROMENT
I) ECM
The extracellular matrix (ECM), a gel-like medium produced by the surrounding fibroblasts, is the largest component of normal skin and confers the skin its unique properties of elasticity, tensile strength and compressibility. Both dermal fibroblasts and epidermal cells secrete the two critical classes of ECM molecules, fibrous proteins and proteoglycans. Strength, flexibility and resilience are imparted by fibrous structural proteins, including collagens, elastin and laminin. In turn, highly hydrated proteoglycans provide cushioning support to cells in the ECM.
The ECM can contribute to the microenvironment specifically through its mechanical features, providing support and anchorage for cells. The ECM also influences tissue segregation as well as regulation of intracellular communication via signaling pathways and its ability to bind growth factors, enzymes and other diffusible molecules. Interactions between cells and the ECM are extremely important for processes such as normal cell growth and differentiation. For example, in acute wounds, the provisional wound matrix, containing fibrin and fibronectin, provides a scaffolding to direct cells into the injury, as well as stimulating them to proliferate, differentiate and synthesize new ECM.
An increasing number of studies have identified that it is essential to maintain the composition and structural organization of the ECM (i.e. the number of cells in the dermal layer) in order to obtain normal skin tissue organization in 3D models (Chione and Grose, 2008). The cells of the underlying dermal layer act not simply as a structural passive framework, but rather influence epithelial migration, differentiation, growth, and attachment (Cooper et al., 1991; Phillips, 1998). In the case of skin reconstructs, a living dermal component can be vital to the cultured skin, and therefore the addition of a dermal element should be carefully considered in the design of any such product. For example, the composite graft (epidermal and dermal elements) had significant advantages over the epidermal sheet graft in the closure of full-thickness wounds (Cooper et al., 1991).
II) Dermal substrates
There are two types of dermal substrates that are used for the generation of reconstructed epidermis: (i) acellular structures, that can be either an inert filter or de-epidermized dermis (DED), and (ii) a cellular substrate composed of fibroblast-populated collagen matrix (Ponec et al., 1997). It was described that skin reconstructs containing only acellular dermal substrate, form a three to four viable cell layered stratified epidermis and must also be supplemented with various growth factors including epidermal growth factor (EGF), keratinocyte growth factor (KGF), and/or insulin-like growth factor (IGF). However, in the presence of fibroblasts, keratinocyte proliferation is stimulated and epidermal morphology is improved (El Ghalbzouri et al. 2002a,b; Wong et al., 2007) (Table 1). In skin reconstructs, viable fibroblasts are shown to increase epidermal growth and spreading, as well as enhance the formation of basement membranes proteins, thus improving the attachment of the epidermal layer (Cooper et al., 1991).
III) Fibroblast and its cellular interactions
Another important point to consider when manufacturing skin reconstructs for a long-time period is the contraction of the collagen matrix by the presence of fibroblasts (Bell et al., 1979). The contractility of this matrix depends on the number of fibroblasts, and the time-frame from the generation of the dermal layer to the subsequent seeding of keratinocytes (El Ghalbzouri et al., 2002b). Some groups have been trying to improve the skin model by avoiding collagen contraction, while retaining the presence of fibroblasts. El Ghalbzouri and co-workers (2002b) used a centrifugal seeding method to incorporate different numbers of fibroblasts into the DED. They observed that fibroblast initially provided a stimulatory effect on induced keratinocyte proliferation, which decreased at later time points in cell culture. They also showed that the interaction between keratinocytes and fibroblasts induced the production of growth factors by the fibroblasts that provided key signals for induction of appropriate epidermal differentiation. The factors produced by fibroblasts (influencing keratinocyte proliferation in either a negative or positive manner) were dependent on fibroblast density and culture time (El Ghalbzouri et al., 2002b).
In an attempt to preserve the epidermal homeostasis and increase the skin equivalent lifespam avoiding dermal shrinkage and increasing tissu’s stability, some authors used other matrices rather than collagen as sponges or scaffolds (Stark et al., 2004b and 2006; Boehnke et al., 2007; Auxenfans et al., 2009). In a system that used a dermal equivalent constituted by an esterified hyaluronic material (Hyalograft-3D) colonized with fibroblast (Stark et al., 2004b and 2006), improved the long-term growth and differentiation for at least 12 weeks. Differentiation and ultrastructure markers were used to show the viability of the system in studies such as skin regeneration (Boehnke et al., 2007) and homeostasis (Stark et al., 2006).
Other studies have also shown the effect of fibroblast density on keratinocyte proliferation and correct epidermal proliferation, by demonstrating that fibroblasts produce collagen types IV and VII, laminin 5 and nidogen, which all contribute to basement membrane formation. Furthermore, fibroblasts secrete cytokines such as TGF-β (transforming growth factor beta) which stimulates keratinocytes to synthesize basement membrane components, including collagen types IV and VII (Wong et al., 2007).
The importance of the role of fibroblasts in keratinocyte differentiation was also demonstrated showing that fibroblasts produce different patterns of cytokine release during their differentiation. Moreover, differentiated fibroblasts induce the production of the highest levels of keratinocyte growth factor and TGF- β1 (Nolte et al., 2008). It was also shown that fibroblasts from different body sites have different functional properties which may affect their suitability for dermal substitutes. As Nolte and co-workers, 2008 pointed out, these facts are important to consider for future in vivo human studies in tissue-engineered dermal substitutes.
IV) Additional cellular components
It is not only fibroblasts that are important in the dermal layer of a skin reconstruct. The 3D culture can also contain other cell types that will enrich the dermal microenvironment, such as myofibroblasts, endothelial cells, inflammatory cells and adipocytes. A wound healing study used human mesenchymal stem cells seeded together with dermal fibroblasts in reconstructed skin to show that human mesenchymal stem cells could contribute to the wound healing process (Chione and Grose, 2008).
ADDRESSING SKIN MODEL LIMITATIONS
I) Phototypes
Comparisons of cells in monolayer cultures versus skin reconstructs indicate that the 3D models may also address aspects of skin pigmentation. Melanocytes in skin reconstructs can be present as single cells at the basal layer of the epidermis, reflecting the distribution of human skin (Meier et al., 2000). Furthermore, melanin production is more active in melanocytes growing in 3D than in 2D (Nakazawa et al., 1998), demonstrating autocrine and paracrine signaling networks mainly between both melanocyte and keratinocyte cell-cell interactions in the skin (Costin and Hearing, 2007).
Melanocytes, however, have not been included in standard skin grafts. In these cases, even after successful transplantation, skin grafts remain depigmented and thus presented an aesthetical dilemma (Balasubramani et al., 2001; Boyce and Warden, 2002; Liu et al., 2007). Since cutaneous pigmentation is a result of not only melanin synthesis by melanocytes, but also transfer of melanosomes to neighboring keratinocytes, it is critical that melanocytes establish correct interactions with surrounding keratinocytes (Fitzpatrick and Szabo,1959; Nakazawa et al., 1998; Régnier et al., 1999; Cario-André et al., 2006).
One fact that remains to be discussed is that epidermal pigmentation is dependent upon the phototype of melanocytes. In basal conditions, intra-individual skin pigmentation varies according to the phototype of melanocytes regulated mainly by melanocortin-1 receptor (MCIR), which remains the major determinant of the pigmentation phenotype in skin (Rees, 2003). The MC1R gene encodes a seven-transmembrane G-protein-coupled receptor that regulates the quantity and quality of melanin produced via activation of adenyl cyclase and production of cyclic AMP (Mountjoy et al., 1992). Activation of this signal transduction pathways ultimately leads to activation of various genes, most notably micropthalmia transcription factor (MITF), responsible for the expression of numerous enzymes and differentiation factors of the melanogenic cascade (Levy et al, 2006) as well as survival of migrating melanoblasts (Steingrímsson et al., 2004).
The different skin phototypes (types I–VI) can be to some extent reproduced in vitro by selecting the donor for melanocyte isolation. Melanocytes derived from a darkly pigmented individual will generally give rise to a reconstructed epidermis phenotypically pigmented, independent of the origin of keratinocytes. For this reason, the use of melanocytes not only in skin grafts for clinical applications but also the modulation of melanogenesis by pro-pigmenting or de-pigmenting agents has still to be taken into consideration (Régnier et al., 1999; Cario-André et al., 2006). Also the use of pigmented skin equivalents has been adopted for the better understanding of congenital hyperpigmentary disorders (Okazaki et al, 2005).
Cario-André and co-workers (2006) have shown that there can also be a dermal modulation of human epidermal pigmentation. Epidermal reconstructs from phototypes II–III were grafted on the back of immunotolerant Swiss nu/nu mice and depending on the presence of colonizing human or mouse fibroblasts they developed a non regular pigmentation pattern. They also demonstrated that when human white Caucasoid split-thickness skin was xenografted onto the same mouse strain it phenotypically appeared black within 3 months, revealing a phototype VI pattern of melanin distribution. This demonstrates that melanocyte proliferation and melanin distribution/degradation can actually be influenced by fibroblast secretion and acellular dermal connective tissue (Cario-André et al., 2006).
II) Immortalized cell lines versus primary cells in artificial skin
Primary cells allow for the evaluation of differences in the epithelial maturation depending on the phototype of the donor, and the anatomical location of the tissue of origin. However, primary cells have a limited life span, and cannot be expanded to the large amounts that may be needed to generate multiple reconstructs needed for statistical analyses. Immortalized cell lines, can then improve the reproducibility and consistency of skin models, reducing intra- and inter-laboratory variations. Thus, established lines can allow for tight regulatory procedures for biologic and clinical studies, as well as industrial applications (Boelsma et al., 1998, Stark et al., 2004a).
The spontaneously immortalized human keratinocyte line, HaCaT, is one of the most frequently used keratinocyte cell lines because of its highly preserved differentiation capacity. HaCaT cells are able to form a differentiated, ordered, structured and functional epidermis when transplanted onto subcutaneous tissue of athymic mice (Boelsma et al., 1998; Schoop et al., 1999; Ponec, 2002). These HaCaT cells grown at the air-liquid interface initially develop a multilayered epithelium. However, during the course of culture, marked alterations in tissue architecture become apparent, with disordered tissue organization including the presence of rounded cells with abnormally shaped nuclei (Boelsma et al., 1998; Schoop et al., 1999; Stark et al., 2004a).
Although many studies use HaCaT cells in skin reconstruction procedures, many studies indicate that HaCaT cells have limited ability in the production of an organized mature cornified epithelium (Boelsma et al., 1998). Despite this fact, this reconstructed epidermis is often considered functional and is used as a model for elucidation of the molecular mechanisms regulating keratinocyte growth and differentiation, making it popular for use in pharmacotoxicology studies (Schoop et al., 1999; Stark et al., 2004a). Nevertheless, the inability to generate the stratum corneum must be considered when cosmetologic applications are the main focus.
III) The Immune system: How to reconstitute this response in vitro?
Another important element to be considered in skin equivalent models is the lack of cells of the immune system. In addition to keratinocytes, supra-basal layers in the human skin contain also Langerhans cells (LC), in the proportion of approximately 30,000 cells / cm2. These cells correspond to CD34+ dendritic cells of hematopoietic origin, displaying Birkbeck granules and CD1a surface antigens (Régnier et al., 1997; Facy et al., 2004). LC cells are responsible for immune system functions in the skin, capturing allergens of low molecular-weight that bind to the skin for possible antigen processing and T cell recruitment. Allergen-induced epidermis cytokines, such as TNF-α and IL-1b are involved in the migration of these Langerhans cells, which, when stimulated, present CD86 surface markers (Facy et al., 2004; Tiznado-Orozco and Orea-Solano, 2004).
However, the integration of LC, into in vitro models, has remained a challenge since these cells, unlike keratinocytes and melanocytes cannot be subcultured and expanded in vitro (Régnier et al., 1998, 1999). A skin reconstruct composed of both dermal and epidermal layers containing not only keratinocytes, but also the pigmented and immune system constituents would be an excellent model to access not only mechanisms of epidermal and dermal cell-cell interactions, but also the role of each cell type in the skin immune response (Régnier et al., 1998).
In 1998, Régnier and collaborators demonstrated that in vitro generated dendritic cells/Langerhans cells from CD34+ haematopoietic progenitors seeded into a reconstructed epidermis gave rise to resident dendritic epidermal LC, expressing MHC class II and CD1a antigens, also containing characteristic Birbeck granules. Another feature observed by confocal laser scanning microscopy revealed cell morphology identical to that observed for LC in vivo. Interestingly, they also observed that purified CD34+ haematopoietic progenitor cells, not exposed to factors such as GM-CSF (granulocyte-macrophage colony-stimulating factor) and TNF-α, were co-seeded directly with keratinocytes and melanocytes onto the dermal equivalent, the keratinocytes were able to induce the maturation of these haematopoietic progenitors into epidermal LC (Régnier et al., 1998).
Facy and co-authors (2004) have now proposed that in a complete reconstructed epidermis, similar to Régnier and co-authors (1998), the reactivity of Langerhans cells, CD-34+ derived dendritic cells, is comparable to that in vivo. The authors also call attention to the fact that this complete model has the potential to be produced at an industrial level and may possibly be an alternative model for estimating the sensitizing potential of new chemicals. Facy and co-authors (2004) pointed out the importance for research studies to also investigate Langerhans cell biology in vitro.
APPLICATIONS
I) Alternatives to animal testing and regulations
One major concern in the production and use of novel chemical reagents that are to be applied to the skin is information regarding their capacity to cause acute skin irritation upon contact. Hence, new procedures have been established for the safe handling, packaging and transport, as well as for general safety assessment of these reagents (Fentem et al., 2001; Ponec, 2002). These reagents are often evaluated for their irritant potential by the application to animals, followed by observations of visible changes including erythema and oedema. However, testing for skin irritation in animals is not always predictive of human responses. Furthermore, these irritability tests may cause pain and discomfort to the experimental animals (Boelsma et al., 1998; Ponec, 2002; Welss et al., 2004).
The European Cosmetics Association (COLIPA) is committed to the replacement of animal testing as well as improving the prediction of irritants in the cosmetic and toiletry industry without compromising human safety or environmental protection (de Silva, 2002; Welss et al., 2004). In 1992, COLIPA coordinated the efforts of the cosmetics industry to develop alternative methods to animal testing for the safety assessment of cosmetics and hence created the Steering Committee on Alternatives to Animal Testing (SCAAT) (de Silva, 2002). In 1994, the European Centre for the Validation of Alternative Methods (ECVAM) organized a workshop on the potential use of non-invasive methods in the safety assessment of cosmetic products, and COLIPA created a specific task force to create guidelines that would reflect the protocols and practices of industry (Boelsma et al., 2000; Fentem et al., 2001; de Silva, 2002; Welss et al., 2004).
Skin reconstructs are now arising as an alternative method to animal testing for assessing irritation, corrosiveness, phototoxicity and genotoxicity of various reagents (Welss et al., 2004; Curren et al., 2006; Hu et al., 2009). These reconstructs are favored over monolayer keratinocyte cultures for multiple reasons, including the ability to test compounds with low water-solubility, as well as the fact that the concentrations inducing irritant responses in monolayer keratinocytes cell cultures are extremely different in comparison to in vivo. Furthermore typically obtained from monolayer cell tests have been difficult to correlate with the in vivo situation (de Silva, 2002; Welss et al., 2004; MacNeil,2007). Percutaneous absorption, which cannot be properly assessed in monolayer conditions, is another critical factor in considering the safety assessment of cosmetic ingredients, since this governs the margin of safety for the ingredients studied (de Silva, 2002).
Once skin reconstructs appeared as an alternative method to animal testing, various companies provided reconstituted human epidermal in vitro skin equivalents and the number of distributors is increasingly growing.
During 1999 and 2000, ECVAM commissioned a validation study of five in vitro tests for acute skin irritation. This study specifically addressed aspects of: protocol refinement (phase I), protocol transfer (phase II), and protocol performance (phase III), in accordance with the scheme defined by ECVAM (Fentem et al., 2001; Portes et al., 2002; Botham, 2004). Results indicated an acceptable intra-laboratory reproducibility. However, inter-laboratory reproducibility was of concern, and the predictive ability of all methods was also inadequate. One explanation for the insufficient reproducibility and false positives is the common formation of an imperfect barrier function in reconstructed epidermis. Furthermore, the use of various experimental protocols differing in concentrations of the applied irritants, the time of exposure and the time of incubation made it difficult to create a standardized protocol for assessing irritation in vitro (Welss et al., 2004). Protocol refinements and improvements in 2007, enabled two skin reconstruct models to be validated by ECVAM for use as alternative methods in skin irritation tests (Fentem et al., 2001; Fentem and Botham, 2002; Portes et al., 2002; Botham, 2004; Hoffman et al., 2008).
A promising new use of 3D human skin models for the evaluation of genotoxicity of topically applied compounds and formulations is also underway. Curren and co-authors (2006) developed a micronucleus assay employing the EpiDerm 3D human skin model which is now in the process of validation (Hu et al., 2009).
II) Photoaging model: the UV effect
Sun exposure causes various deleterious cutaneous effects, leading to short term responses such as erythema, sunburn, and suntan. Long term effects include skin cancers and premature photoaging (Bernerd and Asselineau, 1998; Bernerd et al., 2000). Solar UV light reaching earth is a combination of both UVB (290–320 nm) and UVA (320–400 nm) wavelengths. Although UVB irradiation has received more attention, an increasing number of studies are now emphasizing harmful effects of UVA (Bernerd and Asselineau, 1998; Bernerd and Asselineau, 2008). However, for ethical reasons, experimental chronic UV exposure assays pose a challenge in humans.
Conventional monolayer cultures do not reproduce accurate physiological conditions for studying UV exposure (Bernerd and Asselineau, 1998; 2008). Instead, the full 3D skin model composed of dermal and epidermal equivalent layers may be suitable for determining specific biological effects induced by UVB and UVA irradiation (Bernerd and Asselineau, 1998; Bernerd et al., 2000). Since the reconstructed skin model is able to differentiate epidermal horny layers, topical application of compounds or sunscreens on the skin mimicking a more realistic situation can be performed (Bernerd et al., 2000). For a proper UV-irradiation study concerning topically applied sunscreens, the use of a pigmented reconstructed epidermis containing melanocytes, is crucial for these experiments regarding UV effects on photoaging (Nakazawa et al., 1998; Régnier et al., 1999).
The solar protection factor (SPF) that corresponds to erythema prevention is one of first features to evaluate sunscreen protection. Following 24 hours of UVB exposure, typical sun burned cells (SBC) are formed in the epidermis, corresponding to the clinical appearance of erythema. SBC correspond to apoptotic keratinocytes which allows elimination of these cells that were strongly damaged by UVB irradiation. The evaluation of erythema was previously not an accessible parameter in vitro, but sunscreen efficiency can now be accessed in reconstructed skin in vitro by the observation and counting of UVB-induced SBC (Bernerd et al., 2000).
The use of a full skin reconstruct model in UV studies is essential since the major skin target of UVB is the epidermis, while UVA exposure mainly affects the dermis. UVB causes significant alterations in keratinocyte differentiation processes, while UVA induces apoptosis in fibroblasts located in the superficial area of the dermal equivalent (Bernerd and Asselineau, 1998; 2008).
Skin penetration is a key point in the evaluation of a potential skin sunscreen. The ability of these compounds to penetrate the skin will depend on the time and concentration required to reach the desired target site. The stratum corneum is the main barrier of the skin penetration phenomenon, and efficient penetration through this barrier depends on specific processes (Ponec, 2002).
The skin reconstructs are also an efficient model, not only to test the SFP of a sunscreen, but also to study cell and ECM modifications provoked by photoaging. Photoaged skin contains notorious changes observed in the uppermost epidermal compartment, but also in the deep dermal compartment of the skin, such as degradation of the connective tissue, decrease in collagen content and accumulation of abnormal elastic tissue characterizing solar elastosis (Bernerd and Asselineau, 1998; Bernerd et al., 2000). Moreover, photoaging is associated with the appearance of advanced glycation end products (AGEs). AGEs are new residues, created by cross-linked formations produced by a non-enzymatic glycation reaction in the extracellular matrix of the dermis. AGEs are now being pointed out as one of the factors responsible for loss of elasticity and other properties of the dermis during aging (Pageon and Asselineau, 2005). In a study that compared the histological results obtained within the reconstructed skin containing native collagen and collagen modified by glycation, no significant differences were observed in morphological structure except the reduction of dermal thicknesses in the glycated sample. The authors also concluded that this system is a promising model to observe the effects of aging on ECM elements and may provide a tool for evaluating the efficacy of AGE inhibitors (Pageon and Asselineau, 2005).
Other studies involving aging, the ECM and evaluation of new molecules are also utilizing skin reconstructs as a model of study. Sok et al., 2008, showed that a C-xylopyranoside derivative, C-β-D-xylopyranoside-2-hydroxy-propane (C-Xyloside) induced the neo-synthesis of matrix proteins such as glycosaminoglycans and heparan sulfate proteoglycans, and also restored dermal epidermal junction integrity, suggesting beneficial effects on aged skin (Sok et al., 2008).
III) Pharmacological applications
In the field of pharmacology, drug discovery is generally dependent upon the predictive capacity of cell based assays (Mazzoleni et al., 2009). Most frequently, the efficacy of anti-cancer drugs is tested in 2D monolayer cells cultured on plates during the initial drug development and discovery phase. However, huge differences are observed when these drugs are tested in vivo. This in vitro versus in vivo difference can result from different cell surface receptors, proliferation kinetics, ECM components, cellular densities and metabolic functions of 2D-maintained cells (Horning et al., 2008).
As mentioned above, pharmacological penetration is an important limitation in pharmacological compounds that should reach deep layers of the skin (Régnier et al., 1993; Godin and Toitou, 2007). Skin from cadavers has previously been used in drug transport studies, but limitations in availability and large variation between specimens, have now increased the application potential of skin reconstruct models (Pasonen-Seppänen et al., 2001). The 3D model has permeability characteristics and metabolic activity resembling that of native skin. This is critical since metabolic activity may affect the permeability of some drugs and their potential for research on irritation, toxicity, and keratinocyte differentiation (Régnier et al., 1993; Pasonen-Seppänen et al., 2001; Godin and Toitou, 2007).
One of the concerns regarding the skin reconstructs model is its deficiency in skin appendages including pilosebaceous units, hair follicles and sweat glands. For these reasons, this model provides much lower barrier properties than that found in whole skin. Consequently, while the skin reconstruct model is superior to a monolayer model, the kinetic parameters of skin permeation obtained from these studies must still be considered an overestimation when compared to the flux across human skin (Godin and Toitou, 2007).
IV) Skin cancer
Cancer is a heterogeneous disease, whose initiation and progression is tighly modulated by cell-cell and cell-matrix interactions. For these reasons, the use of 3-D culture models has been steadily increasing in studies regarding tumor biology (Chione and Grose, 2008).
In invasive skin cancers such as melanoma, skin reconstructs are very convenient for modeling not only the growth and progression of melanoma cells in a 3D microenvironment, but also to study the communication among melanoma cells and surrounding epidermal keratinocytes and dermal fibroblasts (Berking and Herlyn, 2001; Smalley et al., 2006). Artificial skin has allowed to show that surrounding fibroblasts are recruited by the primary melanoma and provide survival signals in the form of altered ECM deposition and growth factors, as well as stimulating the production of matrix metalloproteinases, promoting tumor cell invasion (Haass et al., 2005; Smalley et al., 2006). Férnandez and co-workers (2006) used human artificial skin to test the efficacy of Bortezomib, an anti-tumoral drug acting on the proteasome, in a three-dimensional model. Yu and co-authors (2009) evaluated the role of BRAF mutation and p53 inactivation during transformation of a subpopulation of primary human melanocytes, observing the formation of pigmented lesions reminiscent of in situ melanoma in artificial skin reconstructs. In addition, artificial skin has been used to screen the therapeutic potential of oncolytic adenoviruses in melanocytic cells. Organotypic 3D culture models are also used for different tumor types including breast, prostate and ovarian cancer (Chione and Grose, 2008).
Skin models have also serve for genetic and functional analyses of early stages of tumor development. Normal melanocytes in this model remained singly distributed at the basement membrane. In the radial growth phase of melanoma proliferation and migration of the cancer cells in the dermal reconstruct and tumorigenicity in vivo were observed when cells were transduced with the basic fibroblast growth factor gene. In the vertical growth phase the cells were able to invade the dermis and an irregular basement membrane was formed. Considering the metastatic melanoma, cells rapidly proliferated and aggressively invaded deep into the dermis, in a growth pattern extremely similar to those in vivo (Meier et al., 2000).
These skin cultures represent excellent models to assess melanoma-keratinocyte interactions (Hsu et al., 2000), as well as study keratinocyte-derived lesions.
A study characterized the migratory pattern of a squamous cell carcinoma cell line (HaCaT-II-4) when E-cadherin expression was suppressed. The authors were able to show that loss of cell adhesion enabled migration of single, intra epithelial tumor cells between normal keratinocytes being essential for initial stromal invasion (Alt-Holland et al., 2008). Boccardo and co-workers (2004) used organotypic cultures of human keratinocytes to evaluate the effects of TNF-alpha in cells that expressed HPV-8 oncogenes. Another example of the utilization of organotypic culture in other epithelial tumor models is described by Hoskins and co-workers (2009) that studied the Fanconi anemia (FA). They had described the growth and molecular properties of FA associated cancers (FANCA)-deficient versus FANCA-corrected HPV E6/E7 immortalized keratinocytes in monolayer and organotypic epithelial raft culture (Hoskins et al., 2009).
V) Skin disorders and clinical applications
Skin reconstructs are currently being tested and used in clinics for several skin pathologies. Disorders which may benefit from the development of human skin equivalents include psoriasis (Konstantinova et al., 1996; Barker et al., 2004; Gazel et al., 2006; Tjabringa et al., 2008), vitiligo (Bessou et al., 1997; Cario-André et al., 2007), keloids (Chiu et al., 2005; Butler et al., 2008), naevus (Gontier et al., 2002; 2004) and genodermatoses as Xeroderma Pigmentosum (Bernerd et al., 2001; Ergün et al., 2002; Bergoglio et al., 2008, Herlin et al., 2009) and Epidermolysis bullosa (Ferrari et al., 2006; Mavilio et al., 2006; Wong et al, 2007; De Luca et al., 2009).
With regard to clinical applications, the best temporary skin replacement for full-thickness burns, considering structure and function, is the cadaver allograft skin (Phillips, 1998; Hansen et al., 2001; Wong et al., 2007). However, difficulties associated with handling and transport of the material, as well as the possible risk for disease transmission have limited the availability and use of this model (Phillips, 1998; Hansen et al., 2001; Wong et al., 2007).Therefore, the use of split-thickness skin grafts or cultured epidermal grafts in clinical procedures has been increasing rapidly in the past few years (Phillips, 1998; Wong et al., 2007).
While the main use of homologous skin grafts, reconstructs or related bio-products has traditionally been in the treatment of severe burns and skin disorders, various new clinical and experimental approaches in which this type of skin substitute can be useful have recently emerged. The main new clinical indications for skin allografts include: skin loss, surgical wounds and genodermatoses (Bernerd et al., 2001; Ergün et al., 2002; Ferrari et al., 2006; Wong et al, 2007; Fimiani et al., 2005; Herlin et al., 2009; De Luca et al., 2009).
Two key factors were essential for the utilization of skin substitutes in clinical applications: the ability to grow keratinocytes in vitro and the increasing practice of early wound excision in the extensively burned patient (Cooper et al., 1991).
Human skin substitutes are now commercially available from different companies with different compositions; however they present a number of challenges regarding preclinical safety evaluation. In particular, for clinical use, the companies must demonstrate that the skin grafts do not carry pathogens, that they lack immunogenicity, that they show normal physiogical functions, and that they have no potential for tumorigenicity (Nemecek and Dayan, 1999).
FINAL COMMENTS
As artificial skin reconstructs are becoming robust and have been validated for many applications, there has been a push towards their use in the clinic (i.e. skin repair following wounding or burning). Besides these applications, organotypic skin provides an amenable setting for functional analyses of genetically altered cells in the context of physiological cell-cell and cell-matrix interactions. Possibilities for studies of UV-induced carcinogenesis and aging, as well as efficacy and selectivity of chemotherapeutic agents are also broad. In addition to melanoma, or frequent tumors of keratinocytes (i.e. basal cell or squamous cell carcinomas), other skin disorders such as vitiligo and psoriasis, in which cell-cell contact are preponderant factors, can also be also contemplated using three dimensional skin modes. Advances in the stem cell field offer the exciting possibility of generating large numbers of donor-matched backgrounds for autologous transplants, as well as a platform for in depth analyses of the physiological impact of various skin cell precursors in health and disease.
Acknowledgements
We are especially grateful to Dr. Monique Verhaegen (Dermatology Department, University of Michigan, USA), for the critical reading of the manuscript and her precious suggestions. This study was supported by FAPESP (2006/50479-7 and 2008/58817-4), CNPq, CAPES and PRP-USP. MS is supported by NIH R01 CA107237, CA125017; Spanish Ministry of Science and Innovation SAF 2008-1950 and institutional grants from the Spanish Association Against Cancer.
References
- 1.Alt-Holland A, Shamis Y, Riley KN, DesRochers TM, Fusenig NE, Herman IM, Garlick JA. E-cadherin suppression directs cytoskeletal rearrangement and intraepithelial tumor cell migration in 3D human skin equivalents. J Invest Dermatol. 2008;128:2498–507. doi: 10.1038/jid.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajani G, Sato N, Mack JA, Maytin EV. Cellular responses to disruption of the permeability barrier in a three-dimensional organotypic epidermal model. Exp Cell Res. 2007;313:3005–15. doi: 10.1016/j.yexcr.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auxenfans C, Fradette J, Lequeux C, Germain L, Kinikoglu B, Bechetoille N, Braye F, Auger FA, Damour O. Evolution of three dimensional skin equivalent models reconstructed in vitro by tissue engineering. Eur J Dermatol. 2008;19:107–13. doi: 10.1684/ejd.2008.0573. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramani M, Kumar TR, Babu M. Skin substitutes: a review. Burns. 2001;27:534–44. doi: 10.1016/s0305-4179(01)00018-3. [DOI] [PubMed] [Google Scholar]
- 5.Barker CL, McHale MT, Gillies AK, Waller J, Pearce DM, Osborne J, Hutchinson PE, Smith GM, Pringle JH. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol. 2004;123:892–901. doi: 10.1111/j.0022-202X.2004.23435.x. [DOI] [PubMed] [Google Scholar]
- 6.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U.S.A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergoglio V, Warrick E, Chevallier-Lagente O, Magnaldo T. Cutaneous gene therapy: the graft takes. Med Sci (Paris) 2008;24:607–614. doi: 10.1051/medsci/20082467607. [DOI] [PubMed] [Google Scholar]
- 8.Berking C, Herlyn M. Human skin reconstruct models: a new application for studies of melanocyte and melanoma biology. Histol Histopathol. 2001;16:669–74. doi: 10.14670/HH-16.669. [DOI] [PubMed] [Google Scholar]
- 9.Bernerd F, Asselineau D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev. Biol. 1997;183:123–138. doi: 10.1006/dbio.1996.8465. [DOI] [PubMed] [Google Scholar]
- 10.Bernerd F, Asselineau D. UVA exposure of human skin reconstructed in vitro induces apoptosis of dermal fibroblasts: subsequent connective tissue repair and implications in photoaging. Cell Death Differ. 1998;5:792–802. doi: 10.1038/sj.cdd.4400413. [DOI] [PubMed] [Google Scholar]
- 11.Bernerd F, Vioux C, Asselineau D. Evaluation of the protective effect of sunscreens on in vitro reconstructed human skin exposed to UVB or UVA irradiation. Photochem Photobiol. 2000;71:314–20. doi: 10.1562/0031-8655(2000)071<0314:EOTPEO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Bernerd F, Asselineau D, Vioux C, Chevallier-Lagente O, Bouadjar B, Sarasin A, Magnaldo T. Clues to epidermal cancer proneness revealed by reconstruction of DNA repair-deficient xeroderma pigmentosum skin in vitro. Proc Natl Acad Sci U S A. 2001;98:7817–22. doi: 10.1073/pnas.141221998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernerd F, Asselineau D. An organotypic model of skin to study photodamage and photoprotection in vitro. J Am Acad Dermatol. 2008;58:S155–9. doi: 10.1016/j.jaad.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Bessou S, Gauthier Y, Surlève-Bazeille JE, Pain C, Taïeb A. Epidermal reconstructs in vitiligo: an extrinsic factor is needed to trigger the disease. Br J Dermatol. 1997;137:890–7. [PubMed] [Google Scholar]
- 15.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 16.Boccardo E, Noya F, Broker TR, Chow LT, Villa LL. HPV-18 confers resistance to TNF-alpha in organotypic cultures of human keratinocytes. Virology. 2004;328:233–43. doi: 10.1016/j.virol.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Boehnke K, Mirancea N, Pavesio A, Fusenig NE, Boukamp P, Stark HJ. Effects of fibroblasts and microenvironment on epidermal regeneration and tissue function in long-term skin equivalents. Eur J Cell Biol. 2007;86:731–746. doi: 10.1016/j.ejcb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Boelsma E, Verhoeven MC, Ponec M. Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT) J Invest Dermatol. 1999;112:489–98. doi: 10.1046/j.1523-1747.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 19.Boelsma E, Gibbs S, Faller C, Ponec M. Characterization and comparison of reconstructed skin models: morphological and immunohistochemical evaluation. Acta Derm Venereol. 2000;80:82–8. [PubMed] [Google Scholar]
- 20.Botham PA. The validation of in vitro methods for skin irritation. Toxicol Lett. 2004;149:387–90. doi: 10.1016/j.toxlet.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 21.Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg. 2002;183:445–56. doi: 10.1016/s0002-9610(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 22.Butler PD, Ly DP, Longaker MT, Yang GP. Use of organotypic coculture to study keloid biology. Am J Surg. 2008;195:144–8. doi: 10.1016/j.amjsurg.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cario-André M, Pain C, Gauthier Y, et al. In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Res. 2006;19:434–42. doi: 10.1111/j.1600-0749.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 24.Cario-André M, Pain C, Gauthier Y, Taïeb A. The melanocytorrhagic hypothesis of vitiligo tested on pigmented, stressed, reconstructed epidermis. Pigment Cell Res. 2007;20:385–93. doi: 10.1111/j.1600-0749.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 25.Chioni AM, Grose R. Organotypic modelling as a means of investigating epithelial-stromal interactions during tumourigenesis. Fibrogenesis Tissue Repair. 2008;1:8. doi: 10.1186/1755-1536-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu LL, Sun CH, Yeh AT, Torkian B, Karamzadeh A, Tromberg B, Wong BJ. Photodynamic therapy on keloid fibroblasts in tissue-engineered keratinocyte-fibroblast co-culture. Lasers Surg Med. 2005;37:231–44. doi: 10.1002/lsm.20213. [DOI] [PubMed] [Google Scholar]
- 27.Cooper ML, Hansbrough JF, Spielvogel RL, Cohen R, Bartel RL, Naughton G. In vivo optimization of a living dermal substitute employing cultured human fibroblasts on a biodegradable polyglycolic acid or polyglactin mesh. Biomaterials. 1991;12:243–248. doi: 10.1016/0142-9612(91)90207-q. [DOI] [PubMed] [Google Scholar]
- 28.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–94. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 29.Curren RD, Mun GC, Gibson DP, Aardema MJ. Development of a method for assessing micronucleus induction in a 3D human skin model (EpiDerm) Mutat Res. 2006;607:192–204. doi: 10.1016/j.mrgentox.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 30.De Luca M, Pellegrini G, Mavilio F. Gene therapy of inherited skin adhesion disorders: a critical overview. Br J Dermatol. 2009;161:19–24. doi: 10.1111/j.1365-2133.2009.09243.x. [DOI] [PubMed] [Google Scholar]
- 31.de Silva O, European Federation of the Cosmetics Industry. Steering Committee on Alternatives to Animal Testing The contributions of the European cosmetics industry to the development of alternatives to animal testing: dialogue with ECVAM and future challenges. Altern Lab Anim. 2002;2:189–93. doi: 10.1177/026119290203002S29. [DOI] [PubMed] [Google Scholar]
- 32.Donahue BA, McArthur JG, Spratt SK, Bohl D, Lagarde C, Sanchez L, Kaspar BA, Sloan BA, Lee YL, Danos O, Snyder RO. Selective uptake and sustained expression of AAV vectors following subcutaneous delivery. J Gene Med. 1999;1:31–42. doi: 10.1002/(SICI)1521-2254(199901/02)1:1<31::AID-JGM3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 33.Dubé J, Rochette-Drouin O, Lévesque P, Gauvin R, Roberge CJ, Auger FA, Goulet D, Bourdages M, Plante M, Germain L, Moulin VJ. Restoration of the Transepithelial Potential Within Tissue-Engineered Human Skin In Vitro and During the Wound Healing Process In Vivo. Tissue Eng Part A. 2010 Aug 5; doi: 10.1089/ten.TEA.2010.0030. [DOI] [PubMed] [Google Scholar]
- 34.El Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. Effect of fibroblasts on epidermal regeneration. Br J Dermatol. 2002a;147:230–43. doi: 10.1046/j.1365-2133.2002.04871.x. [DOI] [PubMed] [Google Scholar]
- 35.El Ghalbzouri A, Lamme E, Ponec M. Crucial role of fibroblasts in regulating epidermal morphogenesis. Cell Tissue Res. 2002b;310:189–99. doi: 10.1007/s00441-002-0621-0. [DOI] [PubMed] [Google Scholar]
- 36.El Ghalbzouri A, Jonkman MF, Dijkman R, Ponec M. Basement membrane reconstruction in human skin equivalents is regulated by fibroblasts and/or exogenously activated keratinocytes. J Invest Dermatol. 2005;124:79–86. doi: 10.1111/j.0022-202X.2004.23549.x. [DOI] [PubMed] [Google Scholar]
- 37.El Ghalbzouri A, Siamari R, Willemze R, Ponec M. Leiden reconstructed human epidermal model as a tool for the evaluation of the skin corrosion and irritation potential according to the ECVAM guidelines. Toxicol In Vitro. 2008;22:1311–20. doi: 10.1016/j.tiv.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Ergün SS, Cek DI, Demirkesen C. Is facial resurfacing with monobloc full-thickness skin graft a remedy in xeroderma pigmentosum? Plast Reconstr Surg. 2002;110:1290–3. doi: 10.1097/01.PRS.0000025230.84677.C7. [DOI] [PubMed] [Google Scholar]
- 39.Facy V, Flouret V, Régnier M, Schmidt R. Langerhans cells integrated into human reconstructed epidermis respond to known sensitizers and ultraviolet exposure. J Invest Dermatol. 2004;122:552–3. doi: 10.1046/j.0022-202X.2004.22209.x. [DOI] [PubMed] [Google Scholar]
- 40.Feliciani C, Gupta AK, Sauder DN. Keratinocytes and cytokine/growth factors. Crit Rev Oral Biol Med. 1996;7:300–18. doi: 10.1177/10454411960070040101. [DOI] [PubMed] [Google Scholar]
- 41.Fentem JH, Briggs D, Chesné C, Elliott GR, Harbell JW, Heylings JR, Portes P, Roguet R, van de Sandt JJ, Botham PA. A prevalidation study on in vitro tests for acute skin irritation. Results and evaluation by the Management Team. Toxicol In Vitro. 2001;15:57–93. doi: 10.1016/s0887-2333(01)00002-9. [DOI] [PubMed] [Google Scholar]
- 42.Fentem JH, Botham PA. ECVAM’s activities in validating alternative tests for skin corrosion and irritation. Altern Lab Anim. 2002;2:61–7. doi: 10.1177/026119290203002S09. [DOI] [PubMed] [Google Scholar]
- 43.Fernández Y, Miller TP, Denoyelle C, Esteban JA, Tang WH, Bengston AL, Soengas MS. Chemical blockage of the proteasome inhibitory function of bortezomib: impact on tumor cell death. J Biol Chem. 2006;281:1107–18. doi: 10.1074/jbc.M511607200. [DOI] [PubMed] [Google Scholar]
- 44.Ferrari S, Pellegrini G, Matsui T, Mavilio F, De Luca M. Gene therapy in combination with tissue engineering to treat epidermolysis bullosa. Expert Opin Biol Ther. 2006;6:367–78. doi: 10.1517/14712598.6.4.367. [DOI] [PubMed] [Google Scholar]
- 45.Fimiani M, Pianigiani E, Di Simplicio FC, Sbano P, Cuccia A, Pompella G, De Aloe G, Petraglia F. Other uses of homologous skin grafts and skin bank bioproducts. Clin Dermatol. 2005;23:396–402. doi: 10.1016/j.clindermatol.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 46.Fitzpatrick TB, Szabo G. The melanocyte: cytology and cytochemistry. Invest Dermatol. 1959;32:197–209. [PubMed] [Google Scholar]
- 47.Gazel A, Rosdy M, Bertino B, Tornier C, Sahuc F, Blumenberg M. A characteristic subset of psoriasis-associated genes is induced by oncostatin-M in reconstituted epidermis. J Invest Dermatol. 2006;126:2647–57. doi: 10.1038/sj.jid.5700461. [DOI] [PubMed] [Google Scholar]
- 48.Godin B, Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 2007;59:1152–61. doi: 10.1016/j.addr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Gontier E, Cario-André M, Lepreux S, Vergnes P, Bizik J, Surlève-Bazeille JE, Taïeb A. Dermal nevus cells from congenital nevi cannot penetrate the dermis in skin reconstructs. Pigment Cell Res. 2002;15:41–8. doi: 10.1034/j.1600-0749.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 50.Gontier E, Cario-André M, Vergnes P, Lepreux S, Surlève-Bazeille JE, Taïeb A. The role of E-cadherin in nevogenesis: an experimental study using epidermal reconstructs. Exp Dermatol. 2004;13:326–31. doi: 10.1111/j.0906-6705.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 51.Grinnell F. Biochemical analysis of cell adhesion to a substratum and its possible relevance to cell metastasis. Prog Clin Biol Res. 1976;9:227–36. [PubMed] [Google Scholar]
- 52.Grinnell F. Fibroblast mechanics in three-dimensional collagen matrices. J Bodyw Mov Ther. 2008;12:191–3. doi: 10.1016/j.jbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haass NK, Smalley KS, Li L, Herlyn M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–9. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 54.Hansen SL, Voigt DW, Wiebelhaus P, Paul CN. Using skin replacement products to treat burns and wounds. Adv Skin Wound Care. 2001;14:37–44. doi: 10.1097/00129334-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Herlin C, Saunière D, Huertas D. Xeroderma pigmentosum: Radical therapeutic procedure on the face using artificial dermal. Ann Chir Plast Esthet. 2009;54:594–9. doi: 10.1016/j.anplas.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann S, Saliner AG, Patlewicz G, Eskes C, Zuang V, Worth AP. A feasibility study developing an integrated testing strategy assessing skin irritation potential of chemicals. Toxicol Lett. 2008;180:9–20. doi: 10.1016/j.toxlet.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, Jain TK, Panda AK, Labhasetwar V. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm. 2008;5:849–862. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- 58.Horch RE, Kopp J, Kneser U, Beier J, Bach AD. Tissue engineering of cultured skin substitutes. J Cell Mol Med. 2005;9:592–608. doi: 10.1111/j.1582-4934.2005.tb00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoskins EE, Morris TA, Higginbotham JM, Spardy N, Cha E, Kelly P, Williams DA, Wikenheiser-Brokamp KA, Duensing S, Wells SI. Fanconi anemia deficiency stimulates HPV-associated hyperplastic growth in organotypic epithelial raft culture. Oncogene. 2009;28:674–85. doi: 10.1038/onc.2008.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu T, Kaluzhny Y, Mun GC, Barnett B, Karetsky V, Wilt N, Klausner M, Curren RD, Aardema MJ. Intralaboratory and interlaboratory evaluation of the EpiDerm 3D human reconstructed skin micronucleus (RSMN) assay. Mutat Res. 2009;673:100–108. doi: 10.1016/j.mrgentox.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, Herlyn M. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol. 2000;156(5):1515–1525. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 63.Konstantinova NV, Duong DM, Remenyik E, Hazarika P, Chuang A, Duvic M. Interleukin-8 is induced in skin equivalents and is highest in those derived from psoriatic fibroblasts. J Invest Dermatol. 1996;107:615–621. doi: 10.1111/1523-1747.ep12584215. [DOI] [PubMed] [Google Scholar]
- 64.Khorshid FA. Comparative study of keloid formation in humans and laboratory animals. Med Sci Monit. 2005;11:BR212–219. [PubMed] [Google Scholar]
- 65.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Suwa F, Wang X, Takemura A, Fang YR, Li Y, Zhao Y, Jin Y. Reconstruction of a tissue-engineered skin containing melanocytes. Cell Biol Int. 2007;31:985–990. doi: 10.1016/j.cellbi.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Marinkovich MP, Lunstrum GP, Keene DR, Burgeson RE. The dermalepidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992;119:695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;44:874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 70.Mancuso M, Gallo D, Leonardi S, et al. Modulation of basal and squamous cell carcinoma by endogenous estrogen in mouse models of skin cancer. Carcinogenesis. 2009;30:340–347. doi: 10.1093/carcin/bgn243. [DOI] [PubMed] [Google Scholar]
- 71.Mavilio F, Pellegrini G, Ferrari S, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- 72.Mazzoleni G, Di Lorenzo D, Steimberg N. Modelling tissues in 3D: the next future of pharmaco-toxicology and food research? Genes Nutr. 2009;4:13–22. doi: 10.1007/s12263-008-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meier F, Nesbit M, Hsu MY, et al. Human melanoma progression in skin reconstructs: biological significance of bFGF. Am J Pathol. 2000;156:193–200. doi: 10.1016/S0002-9440(10)64719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menon GK. New insights into skin structure: scratching the surface. Adv Drug Deliv Rev. 2002;54(Suppl 1):S3–17. doi: 10.1016/s0169-409x(02)00121-7. [DOI] [PubMed] [Google Scholar]
- 75.Moll R, Moll I. Epidermal adhesion molecules and basement membrane components as target structures of autoimmunity. Virchows Arch. 1998;432:487–504. doi: 10.1007/s004280050197. [DOI] [PubMed] [Google Scholar]
- 76.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 77.Nakazawa K, Kalassy M, Sahuc F, Collombel C, Damour O. Pigmented human skin equivalent--as a model of the mechanisms of control of cell-cell and cell-matrix interactions. Med Biol Eng Comput. 1998;36:813–820. doi: 10.1007/BF02518888. [DOI] [PubMed] [Google Scholar]
- 78.Nemecek GM, Dayan AD. Safety evaluation of human living skin equivalents. Toxicol Pathol. 1999;27:101–103. doi: 10.1177/019262339902700118. [DOI] [PubMed] [Google Scholar]
- 79.Nolte SV, Xu W, Rennekampff HO, Rodemann HP. Diversity of fibroblasts -- a review on implications for skin tissue engineering. Cells Tissues Organs. 2008;187:165–176. doi: 10.1159/000111805. [DOI] [PubMed] [Google Scholar]
- 80.Okazaki M, Suzuki Y, Yoshimura K, Harii K. Construction of pigmented skin equivalent and its application to the study of congenital disorders of pigmentation. Scand J Plast Reconstr Surg Hand Surg. 2005;39:339–43. doi: 10.1080/02844310500300362. [DOI] [PubMed] [Google Scholar]
- 81.Pageon H, Asselineau D. An in vitro approach to the chronological aging of skin by glycation of the collagen: the biological effect of glycation on the reconstructed skin model. Ann N Y Acad Sci. 2005;1043:529–532. doi: 10.1196/annals.1333.060. [DOI] [PubMed] [Google Scholar]
- 82.Pasonen-Seppänen S, Suhonen TM, Kirjavainen M, Miettinen M, Urtti A, Tammi M, Tammi R. Formation of permeability barrier in epidermal organotypic culture for studies on drug transport. J Invest Dermatol. 2001;117:1322–1324. doi: 10.1046/j.0022-202x.2001.01529.x. [DOI] [PubMed] [Google Scholar]
- 83.Paulitschke V, Schicher N, Szekeres T, et al. 3,3′,4,4′,5,5′-hexahydroxystilbene impairs melanoma progression in a metastatic mouse model. J Invest Dermatol. 2010;130:1668–1679. doi: 10.1038/jid.2009.376. [DOI] [PubMed] [Google Scholar]
- 84.Phillips TJ. New skin for old: developments in biological skin substitutes. Arch Dermatol. 1998;134:344–349. doi: 10.1001/archderm.134.3.344. [DOI] [PubMed] [Google Scholar]
- 85.Ponec M, Gibbs S, Weerheim A, Kempenaar J, Mulder A, Mommaas AM. Epidermal growth factor and temperature regulate keratinocyte differentiation. Arch Dermatol Res. 1997;289:317–326. doi: 10.1007/s004030050198. [DOI] [PubMed] [Google Scholar]
- 86.Ponec M. Skin constructs for replacement of skin tissues for in vitro testing. Adv Drug Deliv Rev. 2002;54(Suppl 1):S19–30. doi: 10.1016/s0169-409x(02)00112-6. [DOI] [PubMed] [Google Scholar]
- 87.Portes P, Grandidier MH, Cohen C, Roguet R. Refinement of the Episkin protocol for the assessment of acute skin irritation of chemicals: follow-up to the ECVAM prevalidation study. Toxicol In Vitro. 2002;16:765–770. doi: 10.1016/s0887-2333(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 88.Pruniéras M, Régnier M, Woodley D. Methods for cultivation of keratinocytes with an air-liquid interface. J Invest Dermatol. 1983;81:28s–33s. doi: 10.1111/1523-1747.ep12540324. [DOI] [PubMed] [Google Scholar]
- 89.Rees JL. Genetics of hair and skin color. Annu Rev Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- 90.Régnier M, Caron D, Reichert U, Schaefer H. Barrier function of human skin and human reconstructed epidermis. J Pharm Sci. 1993;82:404–407. doi: 10.1002/jps.2600820414. [DOI] [PubMed] [Google Scholar]
- 91.Régnier M, Staquet MJ, Schmitt D, Schmidt R. Integration of Langerhans cells into a pigmented reconstructed human epidermis. J Invest Dermatol. 1997;109:510–512. doi: 10.1111/1523-1747.ep12336627. [DOI] [PubMed] [Google Scholar]
- 92.Régnier M, Patwardhan A, Scheynius A, Schmidt R. Reconstructed human epidermis composed of keratinocytes, melanocytes and Langerhans cells. Med Biol Eng Comput. 1998;36:821–824. doi: 10.1007/BF02518889. [DOI] [PubMed] [Google Scholar]
- 93.Régnier M, Duval C, Schmidt R. Potential cosmetic applications of reconstructed epidermis. Int J Cosmet Sci. 1999;21:51–58. doi: 10.1046/j.1467-2494.1999.183571.x. [DOI] [PubMed] [Google Scholar]
- 94.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 95.Schoop VM, Mirancea N, Fusenig NE. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J Invest Dermatol. 1999;112:343–353. doi: 10.1046/j.1523-1747.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- 96.Schulz JT, 3rd, Tompkins RG, Burke JF. Artificial skin. Annu Rev Med. 2000;51:231–244. doi: 10.1146/annurev.med.51.1.231. [DOI] [PubMed] [Google Scholar]
- 97.Smalley KS, Lioni M, Herlyn M. Life isn’t flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim. 2006;42:242–247. doi: 10.1290/0604027.1. [DOI] [PubMed] [Google Scholar]
- 98.Sok J, Pineau N, Dalko-Csiba M, Breton L, Bernerd F. Improvement of the dermal epidermal junction in human reconstructed skin by a new c-xylopyranoside derivative. Eur J Dermatol. 2008;18:297–302. doi: 10.1684/ejd.2008.0392. [DOI] [PubMed] [Google Scholar]
- 99.Souto LR, Vassallo J, Rehder J, Pinto GA, Puzzi MB. Immunoarchitectural characterization of a human skin model reconstructed in vitro. Sao Paulo Med J. 2009;127(1):28–33. doi: 10.1590/S1516-31802009000100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sobral CS, Gragnani A, Cao X, Morgan JR, Ferreira LM. Human keratinocytes cultured on collagen matrix used as an experimental burn model. J Burns Wounds. 2007;7:e6. [PMC free article] [PubMed] [Google Scholar]
- 101.Stark HJ, Szabowski A, Fusenig NE, Maas-Szabowski N. Organotypic cocultures as skin equivalents: A complex and sophisticated in vitro system. Biol Proced Online. 2004a;6:55–60. doi: 10.1251/bpo72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stark HJ, Willhauck MJ, Mirancea N, Boehnke K, Nord I, Breitkreutz D, Pavesio A, Boukamp P, Fusenig NE. Authentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co-culture. Eur J Cell Biol. 2004b;83:631–45. doi: 10.1078/0171-9335-00435. [DOI] [PubMed] [Google Scholar]
- 103.Stark HJ, Boehnke K, Mirancea N, Willhauck MJ, Pavesio A, Fusenig NE, Boukamp P. Epidermal homeostasis in long-term scaffold-enforced skin equivalents. J Investig Dermatol Symp Proc. 2006;11:93–105. doi: 10.1038/sj.jidsymp.5650015. [DOI] [PubMed] [Google Scholar]
- 104.Steingrímsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- 105.Tiznado-Orozco T, Orea-Solano M. Dendritic cells and their role in atopic dermatitis. Rev Alerg Mex. 2004;51:119–123. [PubMed] [Google Scholar]
- 106.Tjabringa G, Bergers M, van Rens D, de Boer R, Lamme E, Schalkwijk J. Development and validation of human psoriatic skin equivalents. Am J Pathol. 2008;173:815–823. doi: 10.2353/ajpath.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Welss T, Basketter DA, Schröder KR. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicol In Vitro. 2004;18:231–243. doi: 10.1016/j.tiv.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 108.Wong T, McGrath JA, Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol. 2007;156:1149–1155. doi: 10.1111/j.1365-2133.2007.07914.x. [DOI] [PubMed] [Google Scholar]
- 109.Yang J, Balakrishnan A, Hamamoto S, Beattie CW, Das Gupta TK, Wellings SR, Nandi S. Different mitogenic and phenotypic responses of human breast epithelial cells grown in two versus three dimensions. Exp Cell Res. 1987;167:563–569. doi: 10.1016/0014-4827(86)90196-5. [DOI] [PubMed] [Google Scholar]
- 110.Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y, Sotiropoulou PA, Blanpain C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 111.Yu H, McDaid R, Lee J, et al. The role of BRAF mutation and p53 inactivation during transformation of a subpopulation of primary human melanocytes. Am J Pathol. 2009;174:2367–2377. doi: 10.2353/ajpath.2009.081057. [DOI] [PMC free article] [PubMed] [Google Scholar]


