Abstract
Fibrocytes hold a prominent role in inflammatory and tissue repair processes in various organ systems. In this study, we identified and quantified a reactive fibrocyte population in the vocal fold mucosa post-injury using immunohistochemistry (IHC) and stereological analysis. These cells, which expressed CD11b on their surface and prolyl-4-hydroxylase β (P4H-β) intracellularly, were largely restricted to the lamina propria, and were morphologically and immunochemically distinguishable from newly recruited epithelial cells. We validated our IHC findings using flow cytometry, and additionally characterized a reactive fibrocyte population in circulating peripheral blood using a novel detection panel (CD16−CD11b+P4H-β+). Fibrocyte recruitment peaked at three days post-injury in peripheral blood, and five days post-injury in the vocal fold mucosa. These findings suggest that circulating fibrocytes are recruited to sites of tissue injury in the vocal fold mucosa, and may play an important role in vocal fold tissue repair. The results of this study are consistent with published data from other organ systems and strongly suggest the importance of fibrocytes as therapeutic targets. Our newly reported antigen panel facilitating the direct characterization of fibrocytes via flow cytometry is a useful tool with the potential to facilitate improved study of this cell population.
Keywords: CD11b, flow cytometry, prolyl-4-hydroxylase β, scar formation, stereological analysis, tissue repair
INTRODUCTION
Fibrocytes are a recently identified leukocyte subpopulation circulating in peripheral blood.1 These cells primarily reside in the bone marrow, but are able to mobilize and enter circulation, and subsequently extravasate and migrate to sites of tissue injury in response to chemokine signals.2–7 In their new niche, fibrocytes respond to environmental cues, undergo differentiation into local cellular lineages, produce tissue-specific extracellular matrices (ECMs), and integrate into their adopted microenvironment.8 Mobilizing, migrating chemotactically, and functioning in a tissue-specific manner, fibrocytes play a prominent role in tissue repair processes in multiple organ systems.6, 9–11
The vocal fold mucosa, a membranous structure located in the larynx, plays a crucial role in phonation. Its luminal surface is covered by epithelial cells, and its subepithelial region, termed the lamina propria (LP), contains an ECM network of fibrous and interstitial proteins and glycans.12 The native LP is chiefly populated by fibroblast cells. These cells have spindle/star-shaped somata and multiple long processes and are capable of synthesizing ECM.12, 13 Pathological disruption of the vocal fold mucosa can lead to scar formation in the LP, which is characterized by altered cellularity,14 disordered ECM, suboptimal tissue viscoelasticity,15, 16 and an often intractable dysphonia. Many treatments have been developed for vocal fold scarring, including the surgical implantation of various ECM substitutes, such as autologus fat, collagen, and hyaluronic acid.17 Unfortunately, most therapeutic options have shown inconsistent and unsustained benefit.18, 19 In contrast to the use of bioimplants, direct manipulation of the cellular component of the LP is a relatively unexplored therapeutic area, in part due to limited data on different cell populations and their relative functions, particularly under various pathological conditions.
We recently demonstrated that the vocal fold mucosa undergoes distinctive and sequential changes in cellular morphology, composition, and density during the early stages of tissue repair.14 Cellular recruitment is a major event during the early post-injury phase, characterized by the arrival of quick responding neutrophil-like cells, followed by a large number of reactive cells with a fibroblast-like morphology. The morphological and temporal recruitment features of these reactive cells suggest that they (or at least a subpopulation) may belong to the fibrocyte family. In this study, we identified and quantified a reactive fibrocyte population in the vocal fold mucosa post-injury using traditional immunohistochemical (IHC) detection methods. These cells, which expressed CD11b on their surface and prolyl-4-hydroxylase β (P4H-β) intracellularly, were largely restricted to the LP region of the mucosa. We validated these IHC findings using flow cytometry analysis, and additionally characterized a reactive fibrocyte population in circulating peripheral blood using a novel CD16−CD11b+P4H-β+ detection panel.
MATERIALS AND METHODS
Animals
Thirty-three 4-month-old male Sprague-Dawley (SD) rats with a mean body weight of 290 g (±10 g) were used in this study. Four naïve rats were used for fibrocyte isolation and purification from peripheral blood. Four rats received a bilateral vocal fold mucosal stripping injury, sixteen rats received a unilateral vocal fold mucosal stripping injury, and nine age-matched naïve rats were reserved as controls. Bilateral injuries were utilized in a subset of animals to obtain a sufficient number of vocal fold mucosa cells for flow cytometry analysis; unilateral injuries were utilized in all other animals to preserve the contralateral vocal fold as a within-animal control tissue for IHC. All rats were housed in a pathogen-free facility at the University of Wisconsin School of Medicine and Public Health. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison.
Isolation and purification of fibrocytes from peripheral blood
Fibrocytes were harvested from peripheral blood and cultured as previously described.1, 20, 21 Briefly, ~15 mL peripheral blood was pooled from two naïve rats and transferred to heparinized tubes (Monoject, Kendall, Mansfield, MA). Next, peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat by gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ), according to the manufacturer’s protocol. After overnight culture (37° C, 5% CO2) on fibronectin-coated culture slides (8-well chamber, 3 × 106 PBMCs per well; Becton Dickinson, Franklin Lakes, NJ) in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies, Gaithersburg, MD) supplemented with 20% fetal calf serum (FCS, Sigma Aldrich, St. Louis, MO), non-adherent cells were removed by a single, gentle aspiration. Following 10 days of continuous culture, residual non-adherent cells were washed away and adherent cells were fixed with 4% paraformaldehyde and processed for immunocytochemical (ICC) staining.
Vocal fold mucosal stripping procedure
Vocal fold mucosal injuries were created as previously reported.14, 16 Rats underwent anesthesia induction using isoflurane inhalation (2–3% delivered at 0.8–1.5 L/min), followed by maintenance with an intraperitoneal (IP) injection of ketamine hydrochloride (90 mg/kg) and xylazine hydrochloride (9 mg/kg). Atropine sulfate (0.05 mg/kg) was also injected IP to reduce the secretion of saliva and sputum in the laryngeal lumen. The rats were placed on an operation platform in a near vertical position, and a 1-mm-diameter steel wire laryngoscope was inserted to facilitate vocal fold exposure. A 1.9-mm-diameter 25° rigid endoscope (Richard Wolf, Vernon Hills, IL) connected to an external light source and video monitor was used for surgical monitoring. The vocal fold mucosa was stripped using a 25-gauge needle and the injury was confirmed by exposure of the thyroarytenoid (TA) muscle. Mucosal damage was consistent across experimental animals, and all rats recovered from surgery.
Tissue harvest and histological preparation
For IHC and stereological analyses, rats were euthanized via CO2 asphyxiation and larynges were harvested 1, 3, 5 and 7 days post-injury (four rats per time point). One or two additional age-matched naïve control rats were included at each time point. Laryngeal specimens were immediately embedded in optimum cutting temperature compound (OCT, Tissue-Tek, Sakura, Tokyo, Japan), frozen with acetone and dry ice, and stored at −80°C. Next, the larynges were sectioned at an interval of 8 μm in the coronal plane using a cryostat (CM-3050 S, Leica, Wetzlar, Germany). One-hundred serial sections, encompassing the majority of the vocal fold mucosa, were prepared from each laryngeal specimen. Every fifth slide was subjected to routine hematoxylin and eosin (H&E) staining to identify anatomical context along the anterior-posterior (A-P) axis.
Four consecutive serial sections were selected for IHC from each rat. All sections contained the mid-membranous vocal fold mucosa at the level of the laryngeal alar cartilage (LAlc). The mid-membranous mucosa was selected as it is an important tissue region for rat vocal fold oscillation22 and was in the center of the tissue injury site in all injured samples; the LAlc was selected as an anatomical landmark to ensure that all analyses were performed at a consistent A-P level in the coronal plane.
Immunohistochemistry and immunocytochemistry
Laryngeal sections and cultured cells (on all slides) were fixed in 4% paraformaldehyde, washed with phosphate-buffered saline (PBS) (sections) or 0.2% Triton X-100 (Integra, Kent, WA) in PBS (cultured cells), and incubated with Block-Ace (AbD Serotech, Raleigh, NC) and 5% goat serum (Sigma) for 30 min to block non-specific binding. Next, samples were sequentially incubated with mouse anti-rat P4H-β (clone 6-9H6, 1:100 dilution, Novus, Littleton, CO) for 90 min, Rhodamine red conjugated goat anti-mouse IgG (1:200 dilution, Jackson ImmunoResearch, West Grove, PA) for 60 min, and FITC-mouse anti-rat CD11b (clone OX-42, 1:50 dilution, Millipore, Billerica, MA) for 90 min with thorough wash steps between each incubation. Finally, slides were covered with anti-fade mounting medium (Vectashield, Vector Labs, Burlingame, CA) with or without DAPI, and cover-slipped. Control sections stained with an isotype control or without the primary or secondary antibody showed no immunoreactivity (data not shown).
Microscopy and stereological analysis
Stereological analysis of vocal fold mucosa in control and post-injury conditions was performed using Stereo Investigator (MicroBrightField, Williston, VT), as previously described.14 Live imaging was performed using a Zeiss Axioplan 2 microscope and digital microscopy camera (Carl Zeiss, Thornwood, NY), and Ludl XYZ motorized stage (Ludl, Hawthorne, NY). For each IHC stained section, a contour was drawn around the border of the mucosa, and individual cells were examined using red, green and blue fluorescent channels. CD11b+, P4H-β+, and CD11b+P4H-β+ cells were identified based on the emission wavelength of excited fluorochromes and the presence of a DAPI+ cell nucleus within the 8 μm-thick section. Each fluorescence-emitting cell type was labeled with a specific symbol, and symbols were anchored to the contour to provide coordinates. The two-dimensional (2-D) cross-sectional area of the mucosa and the spatial density of each labeled cell type within the LP were computed, and a geomorphic map comprised of the contour and cell symbols was exported for illustration and comparison within or across injury and control conditions.
Representative histological, IHC and ICC images were captured using a microscope with fluorescent and bright-field capabilities (E-600, Nikon, Melville, NY) equipped with a digital microscopy camera (DP-71, Olympus, Center Valley, PA). Images were captured at 200X (to view the entire vocal fold mucosa) and 600X (for cultured cells and detailed cell morphology on IHC stained sections) magnification. Additional IHC images were captured using a confocal laser scanning microscope (MRC 1000, Bio-Rad, Hercules, CA) to reduce non-specific fluorescent signals. Confocal microscopy images were captured at 400X or 600X magnification.
Flow cytometry analysis of peripheral blood and vocal fold mucosa cells
Peripheral blood (50 μL) was collected from the pedal vein of four experimental (bilateral injury) and two age-matched naïve control rats, pre-injury and 1, 3, 5, 7 days post-injury. Blood cells were immediately placed in 10 mM EDTA in Hank’s balanced salt solution (HBSS), washed with HBSS, and resuspended in fluorescence-activated cell sorting (FACS) buffer (2% FCS and 10 mM HEPES in HBSS). Cells were sequentially incubated with primary antibodies against CD16 (rabbit anti-CD16, 1:100 dilution, Santa Cruz Biotech, Santa Cruz, CA) and CD11b (FITC-mouse anti-rat CD11b, clone OX-42, 1:50 dilution, Millipore) for 30 min, washed with FACS buffer, and incubated with secondary antibody Alexa Fluor 594 conjugated goat anti-rabbit (1:200 dilution, Invitrogen, Carlsbad, CA) for 30 min. Next, red blood cells were lysed with FACS lysing buffer (Becton Dickinson) and remaining lymphocytes were fixed and permeabilized with Cytofix/Cytoperm (Becton Dickinson). Permeabilized cells were continuously processed for intracellular staining by incubating with mouse anti-rat P4H-β (clone 6-9H6, 1:200 dilution, Novus) freshly conjugated with Alexa 647 (Zenon mouse IgG1 labeling kit, Invitrogen; used within 30 min of conjugation) for 30 min. Finally, cells were washed and resuspended in FACS buffer, and analyzed on a BD FACSCalibur flow cytometer using CellQuest software (Becton Dickinson). Data were further analyzed using FlowJo software (Tree Star, Ashland, OR). Gates (cutoffs) were set according to unstained controls.
To further characterize fibrocyte recruitment to the vocal fold mucosa, larynges were harvested from the above experimental and naïve control rats following the seven day post-injury blood draw and vocal fold mucosae were dissected under a stereodissection microscope (Fisher Scientific, Pittsburgh, PA). Bilateral mucosae from the same animal were combined and placed in PBS containing 7.5 mg/mL collagenase I (MP Biomedical, Solon, OH) and 0.001% DNAse I (Sigma), and incubated at 37° C for 1 hour with 50 Hz shaking (Excella E24 Incubator Shaker, New Brunswick, Edison, NJ). A single cell suspension was obtained by aggressive pipetting followed by additional incubation in a 37° C water bath. Next, the suspension was passed through a 40 μm filter, centrifuged, and washed with FACS buffer. The isolated cells were stained with antibodies and subjected to flow cytometry analysis as described above for PBMCs.
Statistical analysis
Stereological and flow cytometry data from naïve control versus experimental animals were analyzed using unpaired t-tests under an assumption of unequal variance; paired stereological data representing injured versus contralateral uninjured LP within each animal were analyzed using repeated measures ANOVA, with day post-injury as a fixed effect. Analyses were performed using SAS 9.1.3 (SAS Institute, Cary, NC). An α-level of 0.05 was employed for all comparisons; all p-values were two-sided.
RESULTS
Fibrocyte culture and isolation from peripheral blood
We initially performed ICC analyses of cultured fibrocytes in order to select markers for subsequent IHC and flow cytometry experiments. Following protocols commonly used by other investigators,1, 20, 21 cells were isolated and purified from the peripheral blood of naïve rats. Approximately 1.5 × 107 PBMCs were isolated from ~15 mL peripheral blood, from which approximately 5 × 104 adherant cells remained after 10 days in culture on fibronectin-coated culture slides (similar results were obtained from two independent experiments). The fibronectin culture surface assisted initial cell attachment, although isolated PBMCs were also successfully differentiated on non-coated slides with comparable fibrocyte yield (data not shown). Immunoreactivity of these adherent cells was evaluated using antibodies anti-CD45, anti-CD11b, anti-collagen I, anti-P4H-β, and anti-α-smooth muscle actin (α-SMA). The majority of cells were CD45+, CD11b+, collagen I+, P4H-β+ and α-SMA− (data not shown). CD11b and P4H-β demonstrated particularly strong immunoreactivity in preliminary testing and were also detected in frozen sections of injured vocal folds (detailed in the following paragraphs). Therefore, the combination of active collagen production inferred by expression of P4H-β (an indispensible enzyme for this process) and expression of CD11b, a myeloid antigen, was used in this study to discriminate fibrocytes from different leukocytes and fibroblasts. Among the adherent cells in culture, ~75% were CD11b+ P4H-β+; which translates to ~0.25% of the originally plated PBMCs. These double-positive adherent cells had elongated spindle-shaped cell somata and were morphologically distinct from residual small lymphocytes (Fig. 1).
Figure 1.
Morphological and immunochemical characteristics of fibrocytes purified from the peripheral blood of naïve rats. Cells were isolated from the buffy coat, cultured on fibronectin-coated culture slides for 10 days, and stained with antibodies anti-CD11b (green) and anti-P4H-β (red), and nuclear dye DAPI (blue). Microscopy images show immunoreactivity of CD11b (in A) and P4H-β (in B), DAPI (in C), and a merged panel (in D). CD11b+P4H-β+ fibrocytes are indicated by arrows and small CD11b−P4H-β− leukocytes are indicated by arrowheads in D.
Scale Bar: 20 μm.
Vocal fold structure and cellular composition
The rat vocal fold is comprised of epithelium, LP (together considered the vocal fold mucosa) and the TA muscle (Fig. 2A). On H&E stained tissue sections, the LP was distinguished from the epithelium (Fig. 2C, D) and TA muscle (Fig. 2B) by its relatively pale appearance and cellular features. In the intact rat LP, the majority of cells had spindle/star-shaped somata and were predominantly oriented along the superior-inferior axis. The luminal surface of the LP was covered by multiple layers of epithelial cells that were closely aggregated along the mucosal surface to form a distinctive epithelial layer. The superior and medial-edge epithelium contained stratified squamous cells (SSC in Fig. 2C), which gradually transitioned into a ciliated pseudocolumnar formation (CPE in Fig. 2D) towards the inferior vocal fold boundary.
Figure 2.

Anatomical and morphological characteristics of the naïve rat vocal fold. A: Microscopy image illustrating the gross anatomy of the vocal fold in an H&E stained coronal section. A dotted contour line shows the lateral boundary of the lamina propria (LP). B: Thyroarytenoid muscle (TA). A blood vessel is indicated by an arrow; individual muscle fibers are indicated by arrowheads. C: Medial-edge segment of the vocal fold mucosa containing stratified squamous epithelial cells (SSC). D: Inferior segment of the vocal fold mucosa containing ciliated pseudocolumnar epithelial formation (CPE). In C and D, dotted contour lines show the boundary between the LP and epithelium (Epi).
Scale Bar: 250 μm in A, 25 μm in B, C, D.
The intact (uninjured control) mucosa contained almost no CD11b or P4H-β signal, aside from some CD11b+ fibers in the basement membrane and basal epithelial cell region. CD11b+, P4H-β+ and CD11b+P4H-β+ cells were identified very occasionally on isolated sections and presented with relatively low fluorescent signal intensity (Fig. 3A, C, E, G).
Figure 3.
P4H-β+CD11b+ fibrocytes are recruited to rat vocal fold mucosa following injury. Representative frozen sections of the bilateral vocal folds three days post-injury (3d-PI) were stained with antibodies anti-CD11b (green) and anti-P4H-β (red), and nuclear dye DAPI (blue, A to H only). Microscopy images (200X) show immunoreactivity of CD11b (in A, B) and P4H-β (in C, D), DAPI (in E, F), and a merged panel (in G, H). The medial and lateral boundaries of the epithelium (Epi) are indicated by dotted contour lines. In G and H, P4H-β+CD11b+ fibrocytes are indicated by arrows, and P4H-β+CD11b− reactive fibroblasts are indicated by arrowheads. I: A merged image at higher magnification (600X) showing detailed morphology of different cell types in the lamina propria 3d-PI. A P4H-β +CD11b+ fibrocyte is indicated by a white arrow, CD11b+P4H-β− monocytes are indicated by green arrows, and CD11b−P4H-β+ reactive fibroblasts are indicated by arrowheads.
Scale bar: 25 μm in A to H, 10 μm in I.
Fibrocyte recruitment following vocal fold mucosal injury
Re-epithelialization of the damaged vocal fold mucosa was observed within three days post-injury, as previously reported.14 The majority of newly recruited epithelial cells were CD11b−P4H-β−, consistent with those in the control epithelium (Fig. 3, 4).
Figure 4.
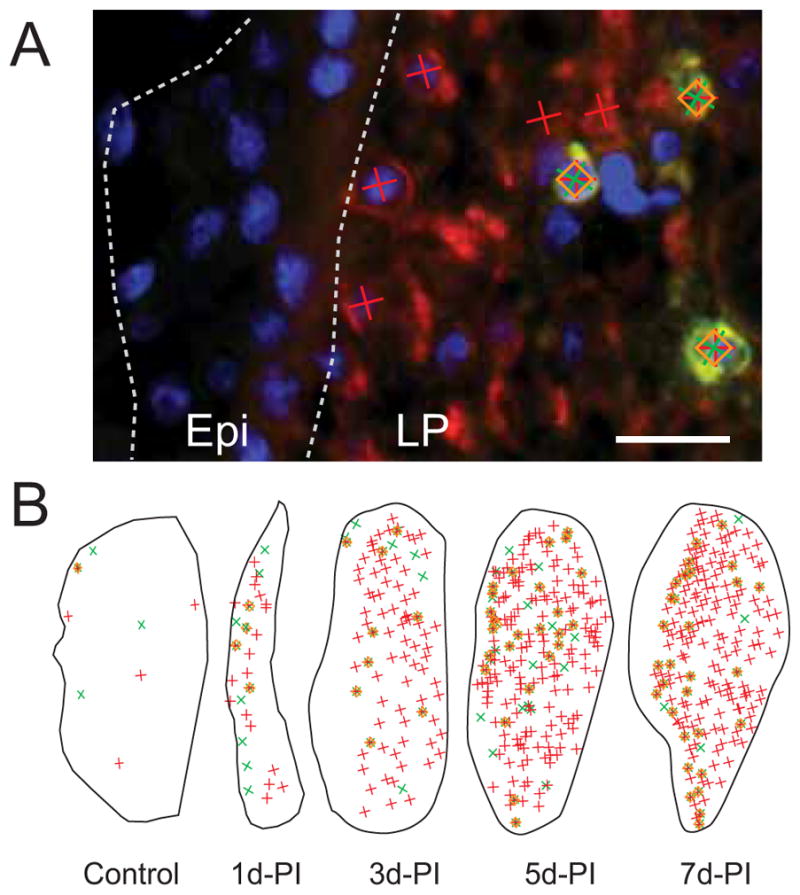
Stereological analysis of altered cellular composition in rat vocal fold mucosa following injury. Representative frozen sections were stained with antibodies anti-CD11b (green) and anti-P4H-β (red), and nuclear dye DAPI (blue). A: Representative image showing measurement probe symbols superimposed over a section of the vocal fold mucosa three days post-injury (3d-PI). The medial and lateral boundaries of the epithelium (Epi) are indicated by dotted contour lines. P4H-β+ cells are indicated by red crosses, CD11b+ cells are indicated by green crosses, CD11b+P4H-β+ fibrocytes are indicated by orange diamonds (superimposed over red and green crosses). B: Representative cellular maps of the entire vocal fold mucosa at sequential PI time points (at reduced magnification with enlarged probe symbols).
LP: lamina propria.
Scale Bar: 20 μm in A, 200 μm in B.
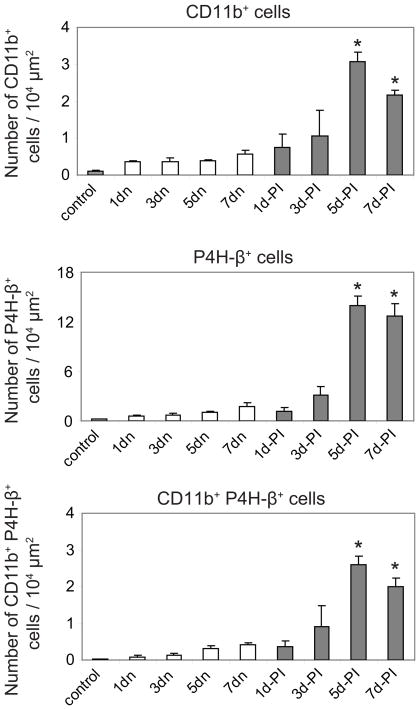
Reconstruction of the damaged LP was characterized by sequential cellular changes during the first seven days post-injury. One day post injury, LP cross-sectional area was significantly reduced compared to control (Fig. 4B). Cell density also increased relative to control, however the majority of newly recruited cells were CD11b−P4H-β− (Fig. 4B) and displayed neutrophil-like polynuclear morphology on adjacent H&E stained sections (data not shown, for examples see Ling et al.14). In addition to these neutrophil-like cells, a small number of P4H-β+, CD11b+, and CD11b+P4H-β+ cells were identified (Fig. 4B), along with a relatively high P4H-β+ extracellular background signal, suggesting cell death and replacement. By three days post injury, the injured LP had expanded to approximately its original size (Fig. 4B), and the density of CD11b+ cells, CD11b+P4H-β+ cells, and particularly P4H-β+ cells further increased (Fig. 4, 5). All three cell types continued to be recruited to the damaged LP, and peak cell density was observed five days post-injury (Fig. 4, 5). Overall, newly recruited CD11b+P4H-β+ fibrocytes were preferentially localized to the superficial LP, whereas the more abundant CD11b−P4H-β+ reactive fibroblasts were uniformly distributed throughout the LP (Fig. 4B). Quantitatively, the density of all three immunolabeled cell types was similar in the control LP (uninjured mucosa from experimentally naïve rats) and the contralateral uninjured LP from experimental rats; however, cell density for all three populations increased significantly in the injured LP over time, with the greatest magnitude change occurring in the P4H-β+ cell population (Fig. 5).
Figure 5.
Quantitative analysis of sequential changes in cellular composition in rat vocal fold lamina propria following injury. Histograms summarize data collected using stereological analysis. Each bar represents the mean number of cells identified per 104 μm2 tissue area, derived from four rats. Error bars represent standard error values.
control: non-injured lamina propria from naïve rats; n: contralateral non-injured lamina propria from experimental rats; PI: post-injury.
*: p < 0.05 compared to both naive control and contralateral non-injured mucosa at same PI time point.
Flow cytometry analysis of fibrocytes in peripheral blood and vocal fold mucosa post-injury
To explore the origin of reactive fibrocytes identified in the injured vocal fold mucosa, we performed flow cytometry analysis of circulating fibrocytes in peripheral blood. In this experiment, 0.1~1 × 105 PBMCs were recovered from 50 μL peripheral blood. In naïve control rats, 13~18% of isolated PBMCs were CD16+; ~2% were CD16−CD11b+P4H-β+. The circulating CD16−CD11b+ population increased following the initial blood draw in all animals (experimental and control), suggesting a response to needle insertion (Fig. 6A). The circulating CD16+ cell population increased significantly within the first 24 hours post-injury (39~48% of isolated PBMCs, data not shown), suggesting an inflammatory response. The circulating CD16−CD11b+P4H-β+ fibrocyte population also increased following injury, but at a more gradual rate (Fig. 6A, B). The percentage of CD16−CD11b+P4H-β+ fibrocytes in the total PBMC population peaked at three days post-injury and gradually declined across the next four days (Fig 6A, B).
Figure 6.
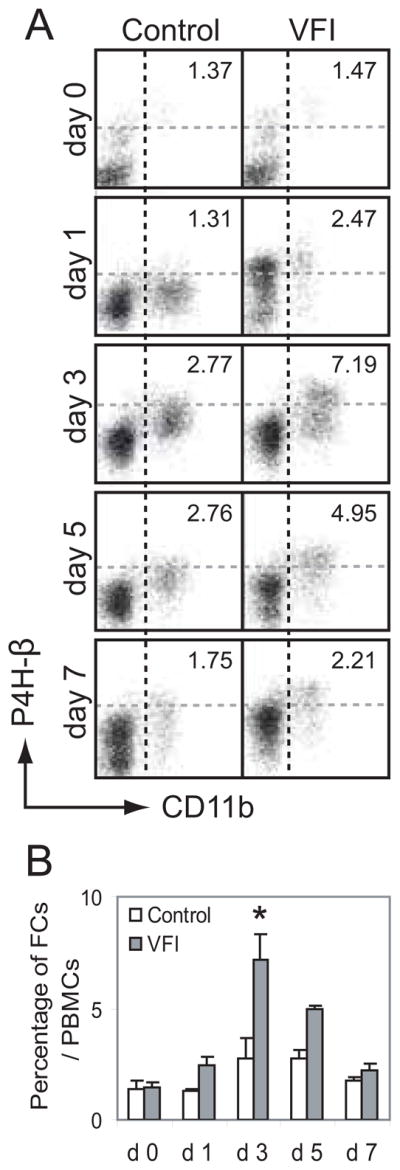
The circulating CD16−CD11b+P4H-β+ fibrocyte population is increased following rat vocal fold mucosal injury (VFI). Experimental and naïve control rats were subjected to sequential blood draws over a seven-day period. Peripheral blood mononuclear cells (PBMCs) were isolated from fresh peripheral blood, stained with antibodies anti-CD16, anti-CD11b and anti-P4H-β, and subjected to flow cytometry analysis. A: Representative dot plots gated on vital CD16− cells showing circulating CD11b+P4H-β+ fibrocytes at sequential post-injury time points. The numeric value in each plot reflects the percentage of CD16−CD11b+P4H-β+ cells in the total PBMC population. B: Histogram showing quantitative change in circulating fibrocytes identified in peripheral blood following injury. Each bar represents the mean percentage of CD16−CD11b+P4H-β+ cells in the total PBMC population, derived from two (control) or four (VFI) rats. Error bars represent standard error values.
FCs: fibrocytes.
*: p < 0.05 compared to control.
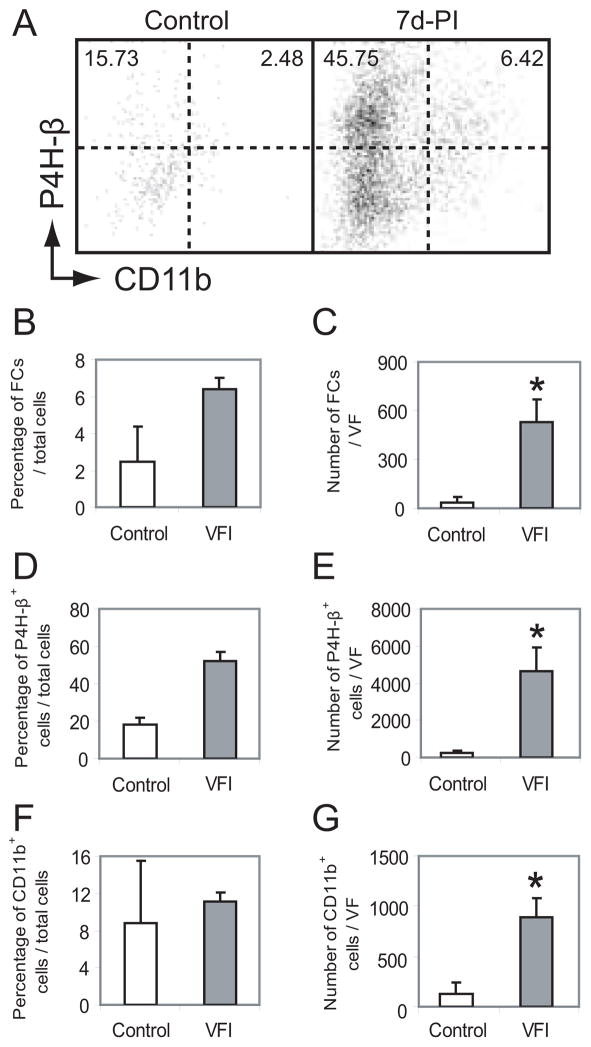
The vocal fold mucosal cell population was also processed for flow cytometry analysis seven days post-injury. A limited number of cells were isolated from naive control mucosae (0.5–2 × 103 cells per vocal fold), due to the low cell density of this tissue and dense crosslinking of its ECM. Consequently, bilaterally injured mucosae were pooled within each experimental and control animal. The majority of cells were CD16−; 2–4% were CD16−CD11b+P4H-β+ fibrocytes (Fig. 7A). Mucosal injury triggered significant cellular recruitment and resulted in a significant increase in the total number of isolated cells seven days post-injury. Compared to control, the mean number of cells isolated per vocal fold increased more than 15-fold for the CD16−CD11b+P4H-β+ population (Fig. 7C), 20-fold for the P4H-β+ population (Fig. 7E) and 7-fold for the CD11b+ population (Fig. 7G). The percentage of each cell type in the total population of isolated cells also increased following injury, however these changes were non-significant due to the simultaneous change in cell density (Fig. 7A, B, D, F).
Figure 7.
The rat vocal fold mucosa CD16−CD11b+P4H-β+ fibrocyte population is increased seven days post-injury (7d-PI). Cells were isolated from vocal fold mucosa, stained with antibodies anti-CD16, anti-CD11b and anti-P4H-β, and subjected to flow cytometry analysis. A: Representative dot plots gated on vital CD16− cells showing an increased CD11b+P4H-β+ fibrocyte population in the vocal fold mucosa at 7d-PI compared to control. In each plot, the left numeric value reflects the mean percentage of CD16−CD11b−P4H-β+ reactive fibroblasts in the total population of isolated cells, and the right numeric value reflects the percentage of CD16−CD11b+P4H-β+ fibrocytes; derived from two (control) or four (7d-PI) rats. B: Histograms showing quantitative change in percentage and number of CD16−CD11b+P4H-β+ fibrocytes, CD16−P4H-β+ cells and CD16−CD11b+ cells in the vocal fold mucosa at 7d-PI compared to control. Each bar represents the mean percentage or number of labeled cells in the total population of isolated cells, derived from two (control) or four (7d-PI) rats. Error bars represent standard error values.
VF: vocal fold; FCs: fibrocytes.
*: p < 0.05 compared to control.
DISCUSSION
We previously reported massive cellular recruitment to the rat vocal fold mucosa post-injury and noted that the majority of newly recruited LP cells presented with morphological features consistent with the fibroblast super-family.14 In this study, IHC and stereological analyses showed that a subpopulation of these newly recruited LP cells express the fibrocyte markers CD11b on their surface and P4H-β intracellularly. Fibrocyte identification was further validated by flow cytometry analysis: The vocal fold mucosa contained an increased fibrocyte popluation post-injury, and the degree of observed recruitment corresponded to our IHC findings. Further, vocal fold mucosal injury was associated with increased fibrocyte recruitment in circulating peripheral blood in advance of peak recruitment at the injury site, suggesting involvement of this reactive cell population in vocal fold injury response and tissue repair.
In humans, fibrocytes express the leukocyte pan marker CD45; monocyte markers CD11a, CD11b, CD32, and CD64; progenitor/stem cell markers CD34 and CD105; and mesenchymal cell markers vimentin, collagen I, P4H-β, and α-SMA; in addition to many integrin molecules (such as CD18, CD29 and CD49b) and chemokine receptors (such as CXCR1, CXCR3 and CXCR4).3, 21, 23, 24 Each of these markers is also expressed by other cell types, thus any effort to unambiguously identify fibrocytes is hampered by the lack of a single distinguishing marker. In response to this problem, the combination of collagen production and expression of one of the hematopoietic (such as CD45, CD34) or myeloid (CD11b, CD13) antigens is often used to discriminate fibrocytes from leukocytes, dendritic cells, epithelial cells, and tissue-resident fibroblasts in vitro and in vivo.1, 4, 21, 25 In a recent study, over 100 commercial antibodies were screened in an attempt to streamline this process and identify cell surface and intracellular markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages and fibroblasts.26 This work principally employed single marker immunolabeling of cultured cells; however, among the numerous proteins expressed by fibrocytes, many change with cell maturation/differentiation status and in response to environmental alterations. For example, CD34 expression has been shown to decrease over time in inflammatory scar,20 while P4H-β expression appears to increase under certain pathological conditions.10, 27 Such changes may indicate a transition in fibrocyte function from an inflammatory to a remodeling phase.
In this study, the combination of active collagen production (P4H-β) and expression of myeloid (CD11b) and hematopoietic (CD16) antigens was used to identify fibrocytes in the vocal fold mucosa and peripheral blood. Double- (ICC/IHC) and triple-labeling (flow cytometry) strategies were employed to distinguish fibrocytes from monocytes, macrophages and fibroblasts. P4H-β, an enzyme responsible for catalyzing proline hydroxylation, is essential to the synthesis and maturation of stable and functional collagen molecules.28 P4H is comprised of α- and β-subunits: Three isoforms of the α-subunit interact with the β-subunit to form active P4H-α2β2 tetramers.29 As the three α-isoforms exhibit differential abundance and tissue expression patterns,30 we used an antibody against the P4H-β subunit to evaluate the intracellular expression of P4H. P4H-β is predominantly expressed by cells actively producing and modifying collagens, but to a lesser extent is also involved in the hydroxylation of other proteins containing collagen-like sequences, such as complement protein C1q30 and the argonaute proteins, essential components of RNA-induced silencing complexes.31 Because of this, we cannot exclude the possibility that a small number of P4H-β positive cells in our dataset were non-collagen containing cells. Collagen producing fibrocytes have been identified by both P4H-β and collagen I immunoreactivity, although the collagen I marker has been more commonly employed in previous studies.1, 4, 21 In our preliminary flow cytometry analyses, the circulating collagen I+ and CD11b+ collagen I+ populations were slightly higher but comparable to the P4H-β+ and CD11b+P4H-β+ populations in naïve control rats. Further, over 95% of cultured P4H-β+ fibrocytes co-expressed collagen I (data not shown). Despite these similarities on flow cytometry and in culture, P4H-β demonstrated significantly improved specificity for cells actively producing collagens on IHC, whereas the collagen I signal was difficult to attribute to the cell soma due to its high abundance in the ECM, especially post-injury (data not shown). To maintain consistency and facilitate comparison between ICC/IHC and flow cytometry assays, we therefore focused on CD11b and P4H-β.
The vocal fold mucosa contains both fibrocytes and fibroblasts. Both are collagen-producing cells and share many morphological and functional features.1, 32 Fibroblast proliferation and collagen synthesis are tightly regulated under normal conditions, however this regulation can be eluded under pathological conditions,32, 33 during which collagen-producing reactive fibroblasts show the P4H-β+ phenotype.32 P4H-β+ immunoreactivity was barely detected in the uninjured vocal fold mucosa in this study, due to the limited number of fibrocytes and predominantly inactive status of resident fibroblasts. In contrast, a large number of P4H-β+ cells were recruited and/or activated in the LP following mucosal injury. The preferential localization of CD11b+P4H-β+ reactive fibrocytes to the superficial LP post-injury suggests that these cells follow a region-specific tissue repair program. In addition to CD11b+P4H-β+ reactive fibrocytes, an even larger population of CD11b−P4H-β+ cells was identified in both IHC and flow cytometry analyses. Most likely, these cells are reactive fibroblasts. Although fibrocytes can be distinguished from fibroblasts by their expression of CD11b,26 the functional differences between these two reactive cell populations are unknown and require further study.
CD11b is one of two protein subunits that form the heterodimeric integrin αMβ2 molecule, also known as macrophage-1 antigen (Mac-1) or complement receptor 3 (CR3).34 In addition to fibrocytes, it is expressed on the surface of many leukocytes involved in the innate immune system, including monocytes, granulocytes, macrophages, and natural killer cells.34 In our initial flow cytometry analyses of circulating cells, CD11b+P4H-β+ cells comprised over 10% of the PBMC population in naïve rats. This population more than doubled in the first 24 hours post-injury, but rapidly declined to pre-injury levels by three days post-injury, suggesting that it might contain a subpopulation of leukocytes involved in the innate immune response (data not shown). To distinguish and to exclude leukocytes from our fibrocyte population of interest, we constructed a novel detection panel employing an antibody against CD16 as a negative selection marker. CD16 is found on the surface of natural killer cells, neutrophils, polymorphonuclear leukocytes, monocytes and macrophages,35 but not on fibrocytes and their precursors.36 Since peripheral blood lymphocytes, monocytes, dendritic cells and granulocytes are also P4H-β−,32 CD11b+CD16− leukocytes can be further distinguished from fibrocytes by the P4H-β marker. Although it has been shown that certain macrophages are capable of producing collagens,37 in this study the majority of circulating CD11b+ cells identified in the peripheral blood were P4H-β−. Therefore, P4H-β appears to be a suitable marker for differentiating CD11b+P4H-β−macrophages (both CD16− and CD16+ subpopulations) from CD16−CD11b+P4H-β+ reactive fibrocytes in this injury system.
Traditionally, circulating fibrocytes have been largely derived from the buffy coat of peripheral blood drawn from humans and various animal species. PBMCs are cultured for one-to-two weeks, non-adhesive leukocytes are removed by washing, and residual adhesive fibrocytes are then isolated, typically representing 0.1~0.5% of the originally plated PBMCs.1, 3, 21, 38 Although positively charged or hydrophilic glass and plastic surfaces provide adequate support for fibrocyte differentiation,39 certain molecules, such as fibronectin, may assist cellular attachment at the onset of culture and detachment at the conclusion of culture.40 This indirect, culture-deletion isolation method can produce a relatively pure fibrocyte population, but may not provide accurate quantitative information, due to the extended culture period and multiple-steps involved in the isolation process, during which some cell loss, selective cell proliferation and other changes in cell biochemistry may occur. An alternative approach to fibrocyte isolation and characterization in peripheral blood is direct flow cytometry analysis.2, 41 In this study, we analyzed circulating fibrocytes in naïve rats using both an indirect culture-deletion method and direct flow cytometry. Consistent with previous studies,1, 3, 21, 38 the indirect culture-deletion method yielded a relatively small fibrocyte population (~2.5 × 103 fibrocytes per mL peripheral blood) that comprised approximately 0.25% of the originally plated PBMC population. Comparatively, our direct flow cytometry method yielded 0.2~2 × 106 PBMCs per mL peripheral blood, of which 1~2% were CD16−CD11b+P4H-β+ fibrocytes. These comparative results suggest that direct flow cytometry analysis may facilitate greater cell yield and therefore more accurate quantitative analysis. Direct flow cytometry analysis has been previously utilized to detect CD45+ Collagen I+ fibrocytes in peripheral blood;41, 23 however, limited fibrocyte separation was observed in these studies due to widespread expression of the leukocyte pan marker CD45 on circulating mononuclear cells, in addition to other technical issues. To overcome these problems, we employed a novel CD16−CD11b+P4H-β+ antigen panel to negatively select contaminating leukocytes and more accurately distinguish our cell populations of interest.
In summary, we observed a rapid and significant increase in the CD16−CD11b+P4H-β+ circulating fibrocyte population following vocal fold mucosal injury, suggesting that fibrocyte precursors respond to inflammatory signals by proliferating, differentiating into fibrocytes, and entering circulation. We further observed sequential recruitment of this cell population to the vocal fold mucosa post-injury, consistent with fibrocyte extravasation and migration from peripheral blood. This study represents the first report of fibrocyte isolation from vocal fold mucosa, although CD34+ cells displaying typical fibrocyte morphology have been reported in the tumor-free region of surgically resected tissue obtained from human patients with laryngeal cancer.42 To date, reactive fibrocytes have been identified in peripheral blood and a number of non-hematopoietic organs, including injured skin;1, 3, 5 suggesting that this cell population plays a critical role in the injury response of multiple organ systems. Consequently, understanding and regulating these cells and their response mechanism may prove pivotal in improving tissue repair outcomes.
Acknowledgments
This work was funded by grant R01 DC004428 from the National Institute on Deafness and Other Communication Disorders, and philanthropic support from David and Katherine Bradley. Statistical consultation was provided by Glen Leverson, Ph.D.
References
- 1.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 4.Varcoe RL, Mikhail M, Guiffre AK, Pennings G, Vicaretti M, Hawthorne WJ, Fletcher JP, Medbury HJ. The role of the fibrocyte in intimal hyperplasia. J Thromb Haemost. 2006;4:1125–33. doi: 10.1111/j.1538-7836.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 5.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–56. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–31. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- 9.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–44. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, stergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci USA. 2008;105:10179–84. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- 13.Hirano M, Sato K, Nakashima T. Fibroblasts in human vocal fold mucosa. Acta Otolaryngol. 1999;119:271–6. doi: 10.1080/00016489950181800. [DOI] [PubMed] [Google Scholar]
- 14.Ling C, Yamashita M, Waselchuk EA, Raasch JL, Bless DM, Welham NV. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 2010;18:89–97. doi: 10.1111/j.1524-475X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rousseau B, Hirano S, Scheidt TD, Welham NV, Thibeault SL, Chan RW, Bless DM. Characterization of vocal fold scarring in a canine model. Laryngoscope. 2003;113:620–7. doi: 10.1097/00005537-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Tateya T, Tateya I, Sohn JH, Bless DM. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005;114:183–91. doi: 10.1177/000348940511400303. [DOI] [PubMed] [Google Scholar]
- 17.Dailey SH, Ford CN. Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngol Clin N Am. 2006;39:23–42. doi: 10.1016/j.otc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Neuenschwander MC, Sataloff RT, Abaza MM, Hawkshaw MJ, Reiter D, Spiegel JR. Management of vocal fold scar with autologous fat implantation: perceptual results. J Voice. 2001;15:295–304. doi: 10.1016/S0892-1997(01)00031-5. [DOI] [PubMed] [Google Scholar]
- 19.Bjorck G, D’Agata L, Hertegard S. Vibratory capacity and voice outcome in patients with scarred vocal folds treated with collagen injections--case studies. Logoped Phoniatr Vocol. 2002;27:4–11. doi: 10.1080/140154302760146925. [DOI] [PubMed] [Google Scholar]
- 20.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009;52:1008–1020. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 26.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson-Sjoland A, Erjefalt JS, Bjermer L, Eriksson L, Westergren-Thorsson G. Fibrocytes are associated with vascular and parenchymal remodelling in patients with obliterative bronchiolitis. Respir Res. 2009;10:103. doi: 10.1186/1465-9921-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22:15–24. doi: 10.1016/s0945-053x(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 29.Kukkola L, Koivunen P, Pakkanen O, Page AP, Myllyharju J. Collagen prolyl 4-hydroxylase tetramers and dimers show identical decreases in Km values for peptide substrates with increasing chain length: mutation of one of the two catalytic sites in the tetramer inactivates the enzyme by more than half. J Biol Chem. 2004;279:18656–61. doi: 10.1074/jbc.M401514200. [DOI] [PubMed] [Google Scholar]
- 30.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Qi HH, Ongusaha PP, Myllyharju J, Cheng D, Pakkanen O, Shi Y, Lee SW, Peng J, Shi Y, Lee SW, Peng J, Shi Y. Prolyl 4-hydroxylation regulates Argonaute 2 stability. Nature. 2008;455:421–4. doi: 10.1038/nature07186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konttinen YT, Nykanen P, Nordstrom D, Saari H, Sandelin J, Santavirta S, Kouri T. DNA synthesis in prolyl 4-hydroxylase positive fibroblasts in situ in synovial tissue. An autoradiography-immunoperoxidase double labeling study. J Rheumatol. 1989;16:339–45. [PubMed] [Google Scholar]
- 33.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–72. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solovjov DA, Pluskota E, Plow EF. Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. J Biol Chem. 2005;280:1336–45. doi: 10.1074/jbc.M406968200. [DOI] [PubMed] [Google Scholar]
- 35.Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology. 6. New York: Garland Publishing; 2005. [Google Scholar]
- 36.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, Rauterberg J, Lorkowski S. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol. 2008;180:5707–19. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 39.Pilling D, Vakil V, Gomer RH. Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods. 2009;351:62–70. doi: 10.1016/j.jim.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan TE, Bucala R. Culture and analysis of circulating fibrocytes. Methods Mol Med. 2007;135:423–434. doi: 10.1007/978-1-59745-401-8_28. [DOI] [PubMed] [Google Scholar]
- 41.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, O’Byrne PM, Strieter RM, Kolb M. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 42.Barth PJ, Schenck zu Schweinsberg T, Ramaswamy A, Moll R. CD34+ fibrocytes, alpha-smooth muscle antigen-positive myofibroblasts, and CD117 expression in the stroma of invasive squamous cell carcinomas of the oral cavity, pharynx, and larynx. Virchows Arch. 2004;444:231–4. doi: 10.1007/s00428-003-0965-1. [DOI] [PubMed] [Google Scholar]