Abstract
Purpose of review
The purpose of this review is to discuss the unique properties of the olfactory epithelium and the potential use of olfactory epithelial grafts to restore olfactory function.
Recent findings
Sensory neurons in the olfactory epithelium undergo continuous regeneration, grow new axons, and reestablish connections with the olfactory bulb throughout life. When transplanted into different regions of the brain, olfactory epithelial grafts cells retain their morphological and regenerative properties. Olfactory cells within the grafts grow axons that enter into the surrounding brain tissue. Recent studies have shown that the olfactory epithelium can be grafted directly to the olfactory bulb.
Summary
The olfactory epithelium has a remarkable capacity to continuously generate new sensory neurons and survives grafting into different regions of the brain. A review of the literature and the future use of olfactory grafts as a potential method to restore olfactory function is discussed.
Keywords: Anosmia, grafts, olfactory bulb, olfactory epithelium, regeneration
Introduction
The development of olfactory tissue grafts may provide physicians with new treatment options for patients with olfactory loss. About three million adults in the United States have disorders of smell or taste and about half of the population between 65 and 80 years old have impaired smell function [1]. Injury to the olfactory nerves frequently results in anosmia, the complete loss of olfactory function. Anosmia is frequently observed in patients with moderate to severe head injury [2], upper respiratory infections, and nasal and paranasal disease [3]. Spontaneous recovery of olfactory nerve function may occur within the first six months to a year after insult, however beyond one year the prognosis for recovery is very poor. The treatment options for patients with anosmia are very limited. Interestingly, cells within the olfactory epithelium (OE) have unique regenerative properties, including the ability to undergo neurogenesis and replace olfactory neurons following injury [4]. Over the past two decades the survival and regenerative capacity of olfactory tissue has been studied after transplantation to different parts of the central nervous system. More recent attention has been focused on the use of olfactory tissue grafts to replace damaged cells in the olfactory epithelium and to restore olfactory function.
Unique properties of the olfactory epithelium
The OE is a pseudo stratified epithelium consisting of basal cells, supporting cells and olfactory sensory neurons [4]. Basal cells in the OE have the remarkable capacity to undergo continuous regeneration throughout life and following injury. Since OE basal cells differentiate into sensory neurons they provide a unique population of stem cells for neuron replacement in the CNS.
Olfactory injury models have been used to investigate the restorative capacity of the OE. Graziadei [5] was the first to demonstrate regeneration of olfactory neurons in adult, non-human primates. Following transection of the olfactory nerves and the subsequent degeneration of olfactory neurons within the OE he noticed an intense mitotic activity among basal cells. By 60–90 days sufficient neurogenesis had occurred in the OE to restore the population of olfactory neurons to controls levels. Graziadei and Costanzo [6–9] used different nerve injury models to demonstrate the regenerative capacity of the OE. Their studies revealed that following nerve injury OE basal cells regenerate, differentiate into neurons, grow new axons back to the olfactory bulb, and reestablish functional connections. Although functional connections can be reestablished in the olfactory bulb, the mapping of these connections onto receptor specific targets appears to be altered [10]. The functional implications of these changes in the wiring of newly established connections n the bulb and how this effects odor identification and discrimination is not fully understood. Some evidence suggests that odor identification is altered when the bulb is rewired and that odor discrimination can be restored by odor training and relearning [6]. The formation of scar tissue following nerve injury may prevent or block axons from growing back to specific regions in the bulb. Reducing scar tissue formation could prove helpful in facilitating the reinnervation of the olfactory bulb. The administration of dexamethasone following olfactory nerve transection injury in mice proven to be successful in reducing the injury response and enhancing recovery of olfactory neurons [11].
Schwob [12] used a methyl bromide inhalation model to selectively damage sensory neurons in the nasal epithelium. This model simulates exposure of the OE to airborne toxins and chemicals that cause a direct insult to the epithelium. Following direct inhalation injury, sensory and supporting cells that are exposed at the mucosal surface undergo degeneration, while the deeper layer of basal cells often remain intact. These basal cells are capable of reconstituting the OE with new sensory and supporting cells [13]. Collectively these studies demonstrate that the OE has a remarkable capacity to recover from injury and is an ideal candidate for restorative tissue grafting.
Progress in OE transplantation and grafting
The remarkable capacity of olfactory cells to regenerate and give rise to new neurons has made them the subject of much interest for research involving transplantation, tissue grafting, and repair within the central nervous system.
A number of studies have used OE tissue grafts as a source of stem cells to induce neural regeneration in the CNS. One study demonstrated that transplantation of the olfactory placode from Xenopus embryos to different locations could give rise to olfactory cells capable of integrating into the optic nerve stalk as well as the diencephalon [14]. In another study, OE grafts from neonatal rats survived transplantation to the adult parietal cortex and the 4th ventricle [15]. In one study transplantation of olfactory mucosa grafts helped to minimize axonal branching and promoted the recovery of vibrissae motor performance after facial nerve repair in rats.[16]. OE grafts not only survive placement in different regions of the brain but they also retain cell characteristics typically found in the normal olfactory epithelium [17–19]. In a study where strips of OE from young mice (5 to 20 days old) were grafted into the parietal cortex of adult mice OE grafts examined at 120 days survival contained fully developed olfactory sensory neurons [18]. This was confirmed by the presence of the characteristic olfactory marker protein (OMP) in graft cells. These mature olfactory neurons integrated into the surrounding cortex and grew axons processes. Axons from sensory cells in OE grafts are capable of growing axons deep into the surrounding cortex. Although functional connections to cells in the OB have not been confirmed many of the OE graft studies suggest that synapse formation is likely.
Cells harvested from the OE can be used for grafting back into the damaged OE or into other regions of the CNS. Several investigators have attempted to use olfactory ensheathing cells to promote regeneration in spinal cord injury [20;21]. Goldstein [13] harvested precursor cells from adult OE in rats and transplanted them to the olfactory mucosa of rats having received a methyl gas lesion to destroy the olfactory sensory neurons. These precursor cells participated in the reconstitution of the olfactory epithelium suggesting that they have the capacity to serve a therapeutic role in regeneration and repair. Chen and colleagues [17] selectively isolated different cell types from the OE of GFP expressing donor mice and engrafted them into the OE of normal mice. They found that specific cells types, such as the globose basal cells, were capable of giving rise to either neurons or sustentacular supporting cells within the host OE.
Some investigators have developed cell culture systems to maintain and expand olfactory progenitor cells. Three dimensional spheres of cultured cells derived from the OE of transgenic mice expressing a green fluorescent protein were dissociated after a few days in vitro and then directly transplanted into the epithelium of wild-type mice. Methyl bromide-lesioned mice receiving such a transplant via nasal infusion revealed integration of sphere-cultured cells into the lesioned epithelium but not cells cultured in two dimensional layers [22]. The ability to culture and perhaps modify olfactory cells in culture could provide the basis for future cell replacement therapies.
Recent attention has been focused on grafting OE tissue directly to the olfactory bulb. OE grafts transplanted to the olfactory bulb have good survival rates and express the olfactory marker protein present in mature olfactory neurons [19]. One advantage of grafting OE tissue directly to the OB is that regenerating axons would have a short distance to gain direct access to their target cells in the bulb. In contrast, grafting OE cells within the OE of the nasal cavity would require axons to grow longer distances to reestablish connections with second order neurons in the bulb. In addition they would have to locate and grow through the ethmoid bone at the base of the skull.
Significant progress has been made in developing a mouse model for studying OE grafts in the OB [23]. The 30 day survival rates of OE graft in the OB (5 out of 6) are similar to those for OE grants transplanted into cerebral cortex (10 out of 12). The morphology of cells observed in the OE grafts demonstrates the presence of olfactory sensory cells within an epithelium lined with cilia at the surface (See Figure 1). These OE grafts reorganized to form vesicle like structures within the OB that look like miniature nasal cavities. At high magnification olfactory sensory neurons were observed with well defined dendrites and cilia lining the epithelial surface.
Figure 1.
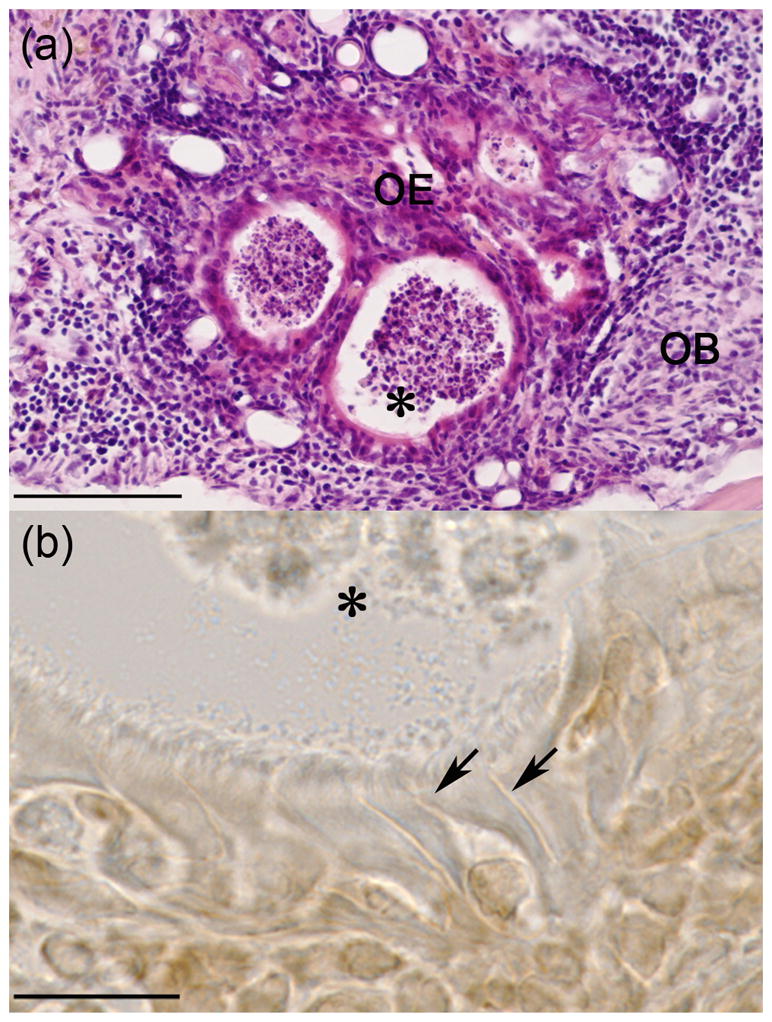
Histological sections of OE graft in OB of a mouse at 30 day survival time point. A. H&E stained section of OE graft tissue showing formation of circular vesicles lined with epithelial cells. B. High magnification of epithelial cells stained for the Olfactory Marker Protein (OMP) confirming the presence of olfactory sensory neurons with characteristic vessel shape, apical dendrites (arrows) and olfactory cilia. Astric (*) marks a common reference point in images A and B. Scale bars: A, 100um; B, 15um.
Challenges
Several technical issues and the further refinement of OE grafts must be addressed before application and use in humans can be considered. First, the functional properties of OE grafts must be established in animal models. Can the grafts survive when positioned along the external surface of the bulb? Will the axons from graft neurons grow into the bulb and establish synaptic connections with higher order olfactory neurons? Animal models for testing the functional capacity of OE grafts are currently in development and may provide answers to these questions. Additional challenges related to the translation of OE grafts models to humans center around surgical access and the graft sites located at the base of the skull. A small opening in the cribriform plate would be necessary to access the olfactory bulb and position the OE grafts over the ventral surface of the bulb. The mucosal surface of the graft must be exposed to the nasal cavity and a tight seal established at the cribriform plate to prevent CSF leaks.
Although it is likely that many of the technical issues can be addressed through advances in ESS surgery and material sciences, the question of neural integration and functional connectivity remains. Will the graft axons connect to cells in the OB and will the mapping of odor information onto the bulb be sufficient to restore odor detection and odor discrimination?
Conclusions
Patients with a complete loss of smell function (anosmia) often present a therapeutic challenge to the treating otolaryngologist. When there is injury or tearing of the olfactory nerves, such as in traumatic head injury, there is little that the surgeon can do to restore function. Recent studies reporting the survival of OE tissue grafts in the olfactory bulb now offer new hope. Future research and refinement of OE graft methods will ultimately determine the functional and sensory capacity of OE grafts.
Keypoints.
Bullet point 1: Injury to the olfactory nerves often results in a complete loss of smell function (anosmia)
Bullet point 2: The olfactory epithelium has a remarkable capacity to continuously generate new sensory neurons.
Bullet point 3: Grafts of olfactory neurons survive transplantation into different regions of the brain and retain olfactory characteristics.
Bullet point 3: Olfactory grafts may provide new treatment options for restoring olfactory function in patients with anosmia.
Acknowledgments
This work was supported in part by the National Institute on Deafness and Other Communication Disorders (NIDCD) and the Richmond Eye and Ear Health Alliance Foundation.
Abbreviations
- OE
olfactory epithelium
- OB
olfactory bulb
- OMP
olfactory marker protein
Contributor Information
Richard M. Costanzo, Email: rcostanz@vcu.edu, Departments of Physiology & Biophysics, and Otolaryngology-Head & Neck Surgery, VCU School of Medicine, 1101 East Marshall Street, Richmond, Virginia 23298-0551, Phone: 804 828-4774, Fax: 804 828-7382
Sayaka Yagi, Email: yagisa@med.kanazawa-u.ac.jp, Department of Otorhinolaryngology-Head & Neck Surgery, Kanazawa University, Graduate School of Medical Science, 13-1, Takara-machi, Kanazawa, Ishikawa, JAPAN 920-8640, Phone: +81-76-265-2413, Fax:+81-76-234-4265
Reference List
* of special interest
** of outstanding interest
- 1.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 2.Costanzo RM, Miwa T. Posttraumatic olfactory loss. Adv Otorhinolaryngol. 2006;63:99–107. doi: 10.1159/000093753. [DOI] [PubMed] [Google Scholar]
- 3**.Doty RL. Clinical studies of olfaction. Chem Senses. 2005;30(Suppl 1):i207–i209. doi: 10.1093/chemse/bjh187. This article sumarizes data from clinical studies of olfaction. [DOI] [PubMed] [Google Scholar]
- 4*.Schwob JE, Costanzo RM. Regeneration of the Olfactory Epithelium. In: Smith DV, Firestein S, Beauchamp GK, editors. Olfaction and Taste. San Diego: Academic Press; 2008. pp. 591–612. This review article describes the structure of the olfactory mucosal layers and the regenerative capacity of stem cells witin the olfactory epithelium. [Google Scholar]
- 5.Graziadei PPC, Karlan MS, Monti Graziadei GA, Bernstein JJ. Neurogenesis of sensory neurons in the primate olfactory system after section of the fila olfactoria. Brain Res. 1980;186:289–300. doi: 10.1016/0006-8993(80)90976-2. [DOI] [PubMed] [Google Scholar]
- 6.Yee KK, Costanzo RM. Changes in odor quality discrimination following recovery from olfactory nerve transection. Chem Senses. 1998;23:513–519. doi: 10.1093/chemse/23.5.513. [DOI] [PubMed] [Google Scholar]
- 7.Costanzo RM, Graziadei PP. A quantitative analysis of changes in the olfactory epithelium following bulbectomy in hamster. J Comp Neurol. 1983;215:370–381. doi: 10.1002/cne.902150403. [DOI] [PubMed] [Google Scholar]
- 8.Costanzo RM. Rewiring the olfactory bulb: changes in odor maps following recovery from nerve transection. Chem Senses. 2000;25:199–205. doi: 10.1093/chemse/25.2.199. [DOI] [PubMed] [Google Scholar]
- 9.Costanzo RM. Neural regeneration and functional reconnection following olfactory nerve transection in hamster. Brain Res. 1985;361:258–266. doi: 10.1016/0006-8993(85)91297-1. [DOI] [PubMed] [Google Scholar]
- 10.Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Costanzo RM. Olfactory nerve recovery following mild and severe injury and the efficacy of dexamethasone treatment. Chem Senses. 2009;34:573–580. doi: 10.1093/chemse/bjp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwob JE, Youngentob SL, Ring G, Iwema CL, Mezza RC. Reinnervation of the rat olfactory bulb after methyl bromide-induced lesion: timing and extent of reinnervation. J Comp Neurol. 1999;412:439–457. doi: 10.1002/(sici)1096-9861(19990927)412:3<439::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein BJ, Fang H, Youngentob SL, Schwob JE. Transplantation of multipotent progenitors from the adult olfactory epithelium. NeuroReport. 1998;9:1611–1617. doi: 10.1097/00001756-199805110-00065. [DOI] [PubMed] [Google Scholar]
- 14.Magrassi L, Graziadei PP. Interaction of the transplanted olfactory placode with the optic stalk and the diencephalon in Xenopus laevis embryos. Neuroscience. 1985;15:903–921. doi: 10.1016/0306-4522(85)90088-0. [DOI] [PubMed] [Google Scholar]
- 15.Morrison EE, Graziadei PPC. Transplants of olfactory mucosa in the rat brain I. A light microscopic study of transplant organization. Brain Res. 1983;279:241–245. doi: 10.1016/0006-8993(83)90184-1. [DOI] [PubMed] [Google Scholar]
- 16.Guntinas-Lichius O, Wewetzer K, Tomov TL, Azzolin N, Kazemi S, Streppel M, Neiss WF, Angelov DN. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci. 2002;22:7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- 18.Holbrook EH, DiNardo LJ, Costanzo RM. Olfactory epithelium grafts in the cerebral cortex: an immunohistochemical analysis. Laryngoscope. 2001;111:1964–1969. doi: 10.1097/00005537-200111000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monti Graziadei AG, Graziadei PPC. Experimental studies on the olfactory marker protein. V. Olfactory marker protein in the olfactory neurons transplanted within the olfactory bulb. Brain Res. 1989;484:157–167. doi: 10.1016/0006-8993(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 20.Raisman G. Olfactory ensheathing cells - another miracle cure for spinal cord injury? Nat Rev Neurosci. 2001;2:369–375. doi: 10.1038/35072576. [DOI] [PubMed] [Google Scholar]
- 21.Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. Journal of Neuroscience. 1998;18:6176–6185. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang W, Lambropoulos J, Woo JK, Peluso CE, Schwob JE. Maintaining epitheliopoietic potency when culturing olfactory progenitors. Exp Neurol. 2008;214:25–36. doi: 10.1016/j.expneurol.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Yagi S, Costanzo RM. Grafting the olfactory epithelium to the olfactory bulb. Am J Rhinol Allergy. 2009;23:239–243. doi: 10.2500/ajra.2009.23.3307. This study describes characteristics and survival rates for OE graphs transplanted to the olfactory bulb and cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]


