Abstract
We have developed a multi-layer approach for the synthesis of water-dispersible superparamagnetic iron oxide nanoparticles for hyperthermia, magnetic resonance imaging (MRI) and drug delivery applications. In this approach, iron oxide core nanoparticles were obtained by precipitation of iron salts in the presence of ammonia and provided β-cyclodextrin and pluronic polymer (F127) coatings. This formulation (F127250) was highly water dispersible which allowed encapsulation of the anti-cancer drug(s) in β-cyclodextrin and pluronic polymer for sustained drug release. The F127250 formulation has exhibited superior hyperthermia effects over time under alternating magnetic field compared to pure magnetic nanoparticles (MNP) and β-cyclodextrin coated nanoparticles (CD200). Additionally, the improved MRI characteristics were also observed for the F127250 formulation in agar gel and in cisplatin resistant ovarian cancer cells (A12780CP) compared to MNP and CD200 formulations. Furthermore, the drug loaded formulation of F127250 exhibited many folds of imaging contrast properties. Due to the internalization capacity of the F127250 formulation, its curcumin loaded formulation (F127250-CUR) exhibited almost equivalent inhibition effects on A2780CP (ovarian), MDA-MB-231 (breast), and PC3 (prostate) cancer cells even though curcumin release was only 40%. The improved therapeutic effects were verified by examining molecular effects using Western blotting and transmission electron microscopic (TEM) studies. F127250-CUR also exhibited haemocompatibility, suggesting a nanochemo-therapuetic agent for cancer therapy.
Keywords: Magnetic nanoparticles, multi-layer coating, MRI, drug delivery, hyperthermia
1. Introduction
Magnetic nanoparticles (MNPs) are increasingly being considered for a number of biomedical applications due to their inherent ultra fine size, biocompatibility and superparamagnetic properties [1–3]. The functional properties of the MNPs can be tailored for specific biological functions, such as drug delivery [4], hyperthermia or magnetic targeting [5–7], magnetic resonance imaging (MRI) [8, 9], cell labeling and sorting [10, 11], and immunoassays [12]. Among the MNPs, iron oxide nanoparticles (magnetite γ-Fe2O3 or magnetite Fe3O4) are the most popular formulations [4]. The applicability of iron oxide nanoparticles depends upon nanoparticles size, functionality, stability, dispensability, and interfacial surfaces [4, 13–15]. Because of high spatial resolution, polymer stabilized magnetic nanoparticles are being used for MRI, tracking cell migration and monitoring in vivo status of cell differentiation [14, 16, 17]. However, conventional iron oxide nano-formulations stabilized by natural/synthetic polymer or encapsulated in micro/nanogels, colloidosome/liposome, micelles, microcapsules, or transfecting reagents (cationic lipids, polylysine, and protamine sulfate), etc. [4, 8] have exhibited lower efficacy of drug loading or rapid release of drug molecules, loss of magnetization properties and often increase the particle size of the formulation. Some of these complexes are unstable and tend to aggregate in reaction tubes or even precipitate in the cell culture medium, resulting in cytotoxicity [18]. Such formulations eventually lead to rapid clearance from the body's circulation by the reticuloendothelial system (RES) and limit the efficacy of magnetic nanoparticle mediated drug targeting ability.
Currently a number of MNP formulations have been developed to serve specific needs; however limited efforts have been made toward developing a universal combined formulation [18] for cancer applications. Therefore, developing a multi-functional magnetic nanoparticle formulation which does not compromise basic characteristics is highly desirable. Recently, such formulations were developed to gain different biological functions [19]. These formulations can be utilized not only for drug delivery techniques but also for MRI visible targeting [20], magnetically targeted photodynamic therapy [21], targeted thermo-sensitive chemotherapy [22–24], and luminescence/near-IR/multi-model imaging [25–26] applications. In this regard, a formulation composed of iron oxide nano-core stabilized with a multi-layer coating could help to increase feasibility in drug delivery, imaging and hyperthermia properties. However, the higher hydrodynamic diameter (> 200 nm) in aqueous medium limits its use in cancer therapeutic applications [27–29]. Therefore, in our current investigation, we have applied different coating approaches to improve the efficacy of such formulations for enhanced cancer therapeutics.
Our goal is to develop MNPs with multi-functional characteristics for drug delivery, MRI, and hyperthermia applications. These applications are complimentary to each other and provide the unique ability to review the drug delivery efficiency at the tumor site [8, 19, 30]. The MNPs loaded with anti-cancer drugs with detection capabilities promotes the clinical importance of this approach. Accordingly, we have developed a formula of magnetic nanoparticles composed of iron oxide core that is subsequently coated with β-cyclodextrin (CD) and pluronic polymer (F-127). The advantages of our formulation include smaller particle size, relatively lower protein binding, higher drug loading efficacy and enhanced particles uptake in cancer cells without hampering inherent magnetization characteristics. In this investigation, we have formulated these magnetic nanoparticles which are optimized and characterized for physico-chemical properties. The magnetic and nuclear magnetic resonance (NMR) relaxometry properties were studied in detail. In addition, the nanoparticles loaded with curcumin demonstrated an enhanced uptake in cancer cells and exhibited improved therapeutic effect of curcumin in in vitro cell culture models.
2. Materials and Methods
2.1. Materials
Fe(III) chloride hexahydrate (99%), Fe(II) chloride tetrahydrate (99%), ammonium hydroxide (28% w/v in water), β-cyclodextrin (CD), pluronic polymer (F127), curcumin (≥95% purity, (E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), acetone (≥99.5, ACS reagent grade), dimethyl sulphoxide (DMSO) (anhydrous grade), ammonium acetate, hydroxylamine hydrochloride (reagent grade, 99%), 1,10-phenontroline (99%), ammonium iron (II) persulphate hexahydrate (99%), bovine serum albumin (BSA) (96%) and hydrochloric acid (HCl) (34–37%) were purchased from Sigma Chemical Co. (St Louis, MO, USA). All the chemicals and reagents were used without further purification. Bacto nutrient agar dehydrated was purchased from Difco Laboratories (Detroit, MI, USA). Millipore Milli-Q® (Burlington, MA, USA) purified water was used to make all aqueous solutions.
2.1.1. Cell culture
A2780CP ovarian cancer cells were generously provided by Dr. Stephen Howell (University of California, San Diego, USA). MDA-MB-231, MCF-7 breast cancer cells were generously provided by Dr. W. Keith Miskimins (Director, Cancer Biology Research Center, Sanford Research/USD, Sioux Falls, SD, USA), and PC3 prostate cancer cells were generously provided by Dr. Meena Jaggi, (Associate Scientist, Cancer Biology Research Center, Sanford Research/USD, Sioux Falls, SD, USA). These cells were maintained as monolayer cultures in RPMI-1640 medium (A2780CP and PC3 cells) or Dulbecco's Modified Eagle's Medium-High Glucose (DMEM-Hi) (MDA-MB-231 and MCF-7) (Hyclone Laboratories, Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA) and 1% penicillin-streptomycin (Gibco BRL, Grand Island, NY, USA) at 37 °C in a humidified atmosphere (5% CO2).
2.2. Synthesis of magnetic nanoparticles (MNPs) formulations
2.2.1. Pure magnetic nanoparticles (MNPs)
Pure magnetic nanoparticles were prepared by co-precipitating Fe2+ and Fe3+ ions in the presence of aqueous ammonia solution [31] under nitrogen atmosphere. About 45 ml of water containing 810 mg of Fe3+ and 297 mg of Fe2+ ions (molar ratio 2:1) in a 100 ml beaker was stirred at 400 rpm under nitrogen atmosphere on a stir plate for 20 min. To this solution, 3 ml of ammonium hydroxide was slowly added and the speed was increased to 900 rpm in order to uniformly precipitate magnetic nanoparticles. The resulting precipitate was stirred overnight to evaporate excess ammonia. After three washes with water, the nanoparticles were resuspended in 25 ml water and centrifuged at 1000 rpm to remove larger aggregates. The supernatant stock solution was kept refrigerated until further use.
2.2.2. β-cyclodextrin modified magnetic nanoparticles
Similar to the previous method, the surface modified magnetic nanoparticles with β-cyclodextrin (CD) were prepared using 45 ml of water containing 810 mg of Fe3+ and 297 mg of Fe2+ ions (molar ratio 2:1), and varying amounts of CD (50–300 μg), that was stirred at 400 rpm in a 100 ml beaker under nitrogen atmosphere on a stir plate for 20 min. To these solutions, 3 ml of ammonium hydroxide was slowly added and the speed was adjusted to 900 rpm. The resulting precipitate was stirred overnight to evaporate excess ammonia. After washing, the nanoparticles were resuspended in water and centrifuged to remove larger aggregates as described above. The stock solutions were designated as CD50, CD100, CD150, CD200, CD250 and CD300 formulations according to the amount of CD used.
2.2.3. β-cyclodextrin-pluronic modified magnetic nanoparticles
To prepare these formulations, 45 ml of water containing 810 mg of Fe3+ and 297 mg of Fe2+ ions (molar ratio 2:1), and 200 mg of β-cyclodextrin, was placed in a 100 ml beaker and stirred for 20 min on a stir plate at 400 rpm under nitrogen atmosphere. To this solution, 3 ml of ammonium hydroxide was slowly added and speed was adjusted to 900 rpm. After 6 hrs, 50 mg of pluronic polymer (F127) was added to the nanoparticles suspension while stirring to achieve a thin coating. The resulting precipitate was stirred overnight to evaporate excess ammonia. After triple washes with water, the nanoparticles were resuspended in 25 ml water and centrifuged at 1000 rpm to remove larger aggregates. This formulation was designated as F12750. Similarly, various F127 coated formulations (F127100, F127150, F127200, F127250 and F127300) were also prepared using 100, 150, 200, 250, and 300 mg of F127 polymer.
2.3. Characterization of magnetic nanoparticle formulations
2.3.1. Particles size and zeta potential
The particles size, distribution and zeta potential of magnetic nanoparticle formulations were determined using Zetasizer (Nano ZS, Malvern Instruments, Malvern, UK) based on dynamic light scattering principle technique. For these measurements, 25 μl of 1 mg/ml nanoparticles suspension was added to 3 ml of distilled water and ultra sonication was applied for 30 seconds. To determine particles size and distribution, particles suspension was measured at 3 min at 25°C. An average diameter and distribution of particles size was reported from 3 runs of each formulation. The zeta potential of nanoparticles formulations was based on the average of 3 readings (each reading = 30 runs).
2.3.2. Particles size and morphology
Nanoparticles size and morphology were evaluated using JEOL-1210 Transmission Electron Microscope (TEM) (JEOL, Tokyo, Japan) operating at 60 kV. For these measurements, 50–100 μl of nanoparticles suspension (500 μg/ml) in water was ultra sonicated for 30 seconds and carefully placed on 200 mesh formvar-coated copper TEM grid (grid size: 97 μm) (Ted Pella, Inc., Redding, CA, USA). The excess suspension on the grid was removed using a piece of fine filter paper and the samples were allowed to air dry for 10 hours prior to imaging the particles under the microscope.
2.3.3. Physical characterization
For the physical characterization of Fourier transform infrared (FTIR) spectra, X-ray diffraction (XRD) and thermo-gravimetric analyzer (TGA), the magnetic nanoparticle formulations were lyophilized to obtain dry solid particles using the Labconco Freeze Dry System (−48 °C, 133 × 10−3 mBar; Labconco, Kansas City, MO, USA). The FTIR of particles were recorded employing a Smiths Detection IlluminatIR FT-IR microscope (Danbury, CT, USA) with diamond ATR objective. FTIR spectra of samples were acquired by placing nanoparticles on the tip of the ATR objective. Data was acquired between 4000–750 cm−1 at a scanning speed of 4 cm−1 for 32 scans. The average data of 32 scans was presented as FTIR spectra. X-ray diffraction (XRD) patterns of nanoparticles were recorded employing a D/Max–B Rikagu diffractometer (Rigaku Americas Corporation, Woodlands, TX, USA) using Cu radiation at λ = 0.1546 nm and operating at 40 kV and 40 mA. The samples were mounted on double sided silicone tape and measurements were performed at 2θ from 20 to 70°. Thermo-gravimetric analysis of nanoparticles was accomplished on a TA Instruments Q50 TGA (TA Instruments, New Castle, Delaware, USA) from 25 °C to 700 °C at a heating ramp of 10 °C, under a constant flow (100 ml/min) of nitrogen gas.
2.4. Curcumin loading
Curcumin (CUR) was used as a model cancer prevention and therapeutic drug. Diluted CUR in acetone (200 μl, 10 mg/ml) was added drop-wise to an aqueous dispersion of magnetic nanoparticles (10 mg of particles in 3 ml water) while stirring at 400 rpm on a magnetic plate. The mixture was stirred overnight so that the CUR molecules would penetrate the CD or CD-F127 polymer layers surrounding the nanoparticles core. The CUR loaded nanoparticles were separated from the free drug using magnetic separation [32]. The drug-loaded nanoparticles were washed three times by re-suspending them in water and then separated with the help of magnets. Finally, the drug-loaded nanoparticles were dispersed in 2 ml sterile PBS solution in a refrigerator until further use. The curcumin loading estimation was determined using UV spectrophotometer at 450 nm, following our previously reported procedure [33].
2.5. Magnetic properties
The hysteresis for solid iron oxide formulations (2–3 mg) was measured in a small polypropylene straw with a Lakeshore vibrating sample magnetometer using maximum fields of 150 Oe. Their long axis was oriented parallel to the external field. The saturation magnetization (Ms) was determined from Ms versus plots and extrapolated to infinite fields. The heating effects of formulations (hyperthermia phenomenon) were evaluated with 1 ml of iron oxide formulations (200 μg to 5 mg of formulation/ml) at H = 150 Oe and f = 300 kHz. The temperature rise in the formulation was measured with a thermocouple immediately after the magnetic field was turned off. Similarly, the heating efficacy of these formulations was also evaluated with 1 ml of iron oxide formulations (200 μg to 5 mg of formulation/ml) containing 3% (w/v) agar solution phantom gels at H = 150 Oe and f = 300 kHz.
2.6. In vitro magnetic resonance imaging
For MRI studies, 3% (w/v) agar solution containing different amounts of magnetic nanoparticles formulations was prepared by heating agar solution at 80 °C for about 20 min and stirring thoroughly to obtain uniform solution, then allowed to cool down to room temperature. These phantom gels were employed to test the in vitro MRI properties. In vitro MRI properties were measured using a 9.4T (400MHz H1), 89 mm vertical bore MR system (Varian, Inc. Walnut Creek, CA, USA) equipped with triple axis gradients (100 G/cm) and a 4 cm Millipede transmit/receive radiofrequency coil. T1 relaxation times of the samples were measured using a spectroscopic inversion-recovery sequence. The sequence was applied using 12 inversion times and a repetition time (TR) of 10s [34]. T2 relaxation time was measured using a spin-echo NMR spectra analysis sequence with 32 echo times arrayed exponentially from 5 to 300 ms and TR of 8000 ms. The T1 and T2 relaxation times were computed using a nonlinear regression applied by the system software (VnmrJ 2.3A). For further analysis, the T1 and T2 relaxation curves were exported so that relaxivities could be extracted by graphing the relaxation rates (1/T1 and 1/T2) versus concentration using Origin 6.1 software. Additionally, images of each sample were acquired using a multiple-echo multiple-slice (MEMS) sequence with the following parameters: repetition time (TR), 1000 ms; echo time (TE), 8 ms; number of echoes (NE), 8; number of excitations (NEX), 4; matrix size of 128 × 128; and field of view 15 mm × 15 mm. The concentration of iron oxide used in each formula was assessed to be within 10–40 μg Fe/ml.
2.7. Drug delivery
2.7.1. Protein Binding
The protein binding interaction study with nanoparticles illustrates the behavior of nanoparticles in circulation. To determine an effective formulation that can be used for drug delivery application, we performed an in vitro bovine serum albumin (BSA) interaction with magnetic nanoparticle suspensions in 1× PBS solution. For this experiment, 1 ml of BSA solution (330 μg/ml) was titrated against 1 mg/ml of magnetic nanoparticle formulations. The intensity of interaction of protein molecules and nanoparticles was determined using an intrinsic fluorescence quenching in fluorescence spectrum [35]. The extent of decrease in the intensity of fluorescence due to interaction with nanoparticles was recorded between 300–500 nm at λex = 295 nm using a Shimadzu RF-5301PC Fluorimeter (Shimadzu Scientific Instruments, Columbia, MD, USA). The Chipman and Beaven methods [35,36] were employed [Equation (1)] to determine binding constant (kb) and number of binding sites or binding stoichiometry (n).
| (1) |
where F0 and Fs are relative fluorescence intensities of protein solution alone and protein solution saturated with MNPs, respectively. The relative fluorescence intensity (F) was obtained from the area under the fluorescence curve and [MNP] is the concentration of nanoparticles (mg/ml). Number of binding sites (n) was obtained from the slope of plot, log [(F0−F)/(F−Fs)] vs log [MNP]. Logarithm of dissociation constant (Kdiss) equals log [MNP] at log [(F0−F)/(F−Fs)] = 0. Binding constant (Kb) is reciprocal of Kdiss. Standard deviations were obtained from 3 replicates.
2.7.2. Nanoparticles cellular uptake
To compare the cellular uptake of magnetic nanoparticles in cancer cells (A2780CP, MDA-MB-231 and MCF-7), 5 × 105 cells were seeded in 6-well plates in 2 ml medium. After cells were attached, media was replaced with 25–100 μg of medium containing nanoparticles. After 6 hrs, cells were washed twice with 1× PBS, trypsinized, centrifuged and collected in 2 ml media. These cell suspensions (50 μl) were injected into an Acuri C6 Flow Cytometer (Accuri Cytometer, Inc., Ann Arbor, MI, USA) to determine the side scattering height fluorescence levels in FL1 channel [32]. Standard deviations were calculated from 3 replicates.
The uptake (internalization) pattern of nanoparticles was monitored by transmission electron microscopy (TEM) to further validate the cellular uptake capability of magnetic nanoparticles in cancer cells which was visually observed. For this experiment, aforementioned cells (1 × 107 cells per 150 mm plate) were incubated with 1 mg of particles in 20 ml media for 6 hrs. The treated cells were centrifuged and fixed with standard formaldehyde (4%)-glutaraldehyde (1%) fixative solution followed by OsO4 fixative solution. Next, cells were dehydrated in a graded series of acetone and embedded in Spurr resin. These cell-containing resin blocks were sectioned using an ultramicrotome and ultrathin sections (70–90 nm thickness) were transferred onto TEM grid (grid size: 97 μm) (Ted Pella Inc., Redding, CA, USA). The grids were processed with uranyl acetate and lead acetate solutions to visualize cellular ultra structures.
2.7.3. Quantitative internalization estimation of magnetic nanoparticle formulations in macrophages and A2780CP cancer cells
To determine whether our formulations are useful for drug delivery, we have evaluated their uptake in macrophage cells and A2780CP metastatic ovarian cancer cells. For this, 5 × 105 macrophage (RAW 264.7) cells or A2780CP cells were seeded in 6-well plates in 2 ml medium. After cells were attached, media was replaced with 50 or 100 μg of iron containing nanoparticles in medium. After 6 hrs, cells were washed twice with 1× PBS, trypsinized/scraped and centrifuged at 1000 rpm for 5 min. Obtained cell pellet was lysed in 500 μl HCl and analyzed for iron levels in cells using 1,10-phenonthroline colorimetric method [27]. Standard deviations were calculated from 3 replicates. To further confirm this uptake phenomenon in macrophages and A2780CP cells, a transmission electron microscopy (TEM) analysis was employed as explained in the previous section.
2.7.4. In vitro cytotoxicity (MTT Assay)
In vitro cytotoxicity was assessed using a standard 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) based colorimetric assay (CellTiter 96 AQeous, Promega, Madison, WI, USA). Ovarian (A2780CP), breast (MDA-MB-231), and prostate (PC3) cancer cells were used for this experiment. Cells (5000 cells/well in 100 μl media) were cultured in RPMI-1640 or DMEM medium containing 10% FBS and 1% penicillin-streptomycin in 96-well plates and allowed to attach overnight. The media was replaced with fresh media containing different concentrations (2.5–40 μM) of CUR and CUR containing MNPs (F127250-CUR). Equivalent amounts of DMSO or F127250 MNPs without drug in PBS were used as control. These plates were incubated at 37 °C for 2 days. After day 2, the media was replaced with 100 μl fresh media and the MTT reagent (25 μl/well) was added to each well and plates were incubated for 3 hrs at 37 °C in an incubator. The color intensity was measured at 492 nm using a microplate reader (BioMate 3 UV-Vis Spectrophotometer, Thermo Electron Corporation, Hudson, NH, USA). The anti-proliferation potential of CUR and F127250-CUR treatments was calculated as a percentage of cell growth with respect to the DMSO and F127250 formulation in PBS controls. Standard deviations were obtained from 6 replicates.
2.7.5. Colony Formation
Colony formation assay was performed to determine long-term anti-cancer potential of our formulations. For this assay, cancer cells (A2780CP, MDA-MB-231, and PC3) were seeded in 2 ml media in 6-well plates (1000 per well) and allowed 2 days to initiate the colonies. Cells were then treated with different concentrations (2–10 μM) of CUR or F127250-CUR over a period of 10 days. The plates were washed three times with 1× PBS, fixed in chilled methanol, stained with hematoxylin (Fisher Scientific, Fair Lawn, NJ, USA), washed with water and air dried. The number of colonies was counted using MultimageTM Cabinet (Alpha Innotech Corporation, San Leandro, CA, USA) and AlphaEase Fc software. The percent colonies were calculated using the number of colonies formed in treatment divided by the number of colonies formed in DMSO or F127250 without drug in PBS. Standard deviations were obtained from 3 replicates.
2.7.6. Western blot analysis
The immunoblot analyses were performed to determine anti-cancer effects of our formulation at the molecular level. For these experiments, cancer cells were collected after 2 days of treatment with 10 μM and 20 μM CUR and 10 μM and 20 μM of F127250-CUR and processed for protein extraction and Western blotting using standard procedures [33, 37]. The cell lysates were separated by gel electrophoresis on polyacrylamide gels containing sodium dodecyl sulfate and then transferred to PVDF membranes. The membranes were blocked with Tris-buffered saline (TBS) containing 5% (w/v) skimmed milk. After washing thrice with Tween 20, the membranes were incubated overnight with primary antibody specific to Bcl-xL or PARP at 4 °C. After washing, the membranes were incubated for 1 hr with secondary antibody (Promega, Madison WI, USA). Protein bands were visualized using the Lumi-Light Detection Kit (Roche, Nutley, NJ, USA) and detected with a BioRad Gel Doc (BioRad, Hercules, CA, USA).
2.8. Haemocompatibility
For this study, 8 ml healthy male human blood (Donor # 53554, Registration # 2577632, Biological Specialty Corp, Colmar, PA, USA) was centrifuged at 2000 rpm for 10 min and supernatant was discarded and red blood cells (RBC) were collected. RBCs were resuspended in 8 ml RPMI1640 growth media. A total 100 μl cell suspension containing RBCs was treated with CUR containing MNP, CD200 or F127250 formulations (10–100 μg). The cells were incubated for 2 hrs at 37 °C, centrifuged and supernatant was collected to determine the degree of haemolysis at λmax 570 nm using absorbance spectrophotomer. The treated RBC pellets were redispersed in PBS and a drop of these solutions was placed and spread on a glass slide and images were taken under an Olympus BX 41 phase contrast microscope (Olympus, Center Valley, PA, USA)
2.9. Statistical analysis
Values were processed using Microsoft Excel 2007 software and presented as mean ± standard error of the mean (S.E.M.). Statistical analyses were performed using an unpaired, two tailed student t-test. The level of significance was set at *p < 0.05. All the graphs were plotted using Origin 6.1 software.
3. Results and discussion
Recent literature reveals that there are many methods to produce stable water dispersible superparamagnetic iron oxide nanoparticles [4, 8]. Although most of the conventional methodologies provide a uniform magnetic nanoparticle formulation they often fail in achieving additional features such as (i) smaller particles size, (ii) good aqueous stability over a period of time, (iii) higher magnetization, (iv) surface functionality and antibody conjugation capability, (v) higher drug/bio-macromolecular encapsulation, and (vi) bioavailability. We have employed a simple precipitation approach [28, 31] to develop a magnetic nanoparticle formulation with multi-functional properties, in which iron (II) and iron (III) salts are reduced by ammonia in the presence of β-cyclodextrin (CD) and pluronic polymer [(F-127, poly(ethylene-co-propylene glycol)]. Using nitrogen atmosphere throughout the preparation to prevent oxidation resulted in formulations in the form of magnetite. The developed formulations are schematically illustrated in Scheme 1. The selection of CD for this formulation is based upon its combination of hydrophilic units (−OH) which can bind to iron oxide nanoparticle surface and the presence of a hydrophobic cavity to load anti-cancer drug(s) [28, 31, 38]. F127 consists of a hydrophobic (polypropylene, PPO) chain which can bind to the hydrophobic cavities of CD and the hydrophilic (polyethylene glycol, PEO) chain provides additional hydrophilicity and stability to overall formulation [39–42]. Therefore, this formulation is comprised of an iron oxide core with the presence of hydrophobic and hydrophilic layers.
Scheme 1.
Synthesis route of multi-layer coated magnetic nanoparticle (MNP) formulation and curcumin drug loading process. (A) Iron salt precipitation into iron oxide (magnetic) nanoparticles and β-cyclodextrin and F127 polymer coatings leads to F127-CD-MNP (F127250) nanoformulation. (B) Curcumin (200 μl of curcumin in acetone, 10 mg/ml) loading into F127250 magnetic nanoparticles (10 mg of particles in 3 ml 1XPBS buffer) is carried out via diffusion process. Loading is estimated using UV-vis spectrophotometer.
3.1. Characterization of magnetic nanoparticles
3.1.1. Particles size and morphology
The initial focus of our investigation was to elucidate which formulation has smaller particles size and distribution in aqueous medium after surface engineering with CD or CD and F127 polymer. The particles size and distribution measurements were obtained using a dynamic light scattering (DLS) instrument (Table 1). Pure magnetic nanoparticles (MNPs) have shown a larger particles size of 201.4 nm with a polydispersity index (PI) of 0.22%. Coating of CD (50–150 mg) onto MNPs resulted in a slight increase in particles size (208.43 nm to 256.17 nm) due to random coating and/or the formed coating layers of CD molecules on the surface were not stable. But further increases in CD (200–300 mg) for coating onto MNPs produced more uniform formulations with average particles size ~175 nm. Therefore, we have selected 200 mg of CD that is optimized to provide a good dispersion formulation. Further, it is shown that F127 polymer layer coating (50–250 mg) improves its overall stability in aqueous dispersion by reducing the size of CD200 particles from ~175 nm to ~90 nm. Overall, the DLS data suggest that CD200 and F127 coatings prevent aggregation of iron oxide formulations, unlike bare magnetic nanoparticles, conventional and multi-layer MNP formulations [4, 28, 29, 43–45] (Fig. 1A). The average cluster formation of MNPs consistently decreased with the CD and F-127 coatings. This behavior can be seen visually in TEM studies. Pure magnetic nanoparticles aggregate to have an average cluster size of > 300 nm (Fig. 1B (a)). When the particles were coated with 200 mg of CD, the cluster size is decreased to 170 nm (Fig. 1B (b)). F127 polymer coating further reduced particle size and provided uniformly suspended particles with an average size of 90 nm (Fig. 1B (c)). These data suggest that CD and F127 layers on iron oxide nanoparticles not only coat particles but also attenuate their cluster behavior in aqueous media. Further, F127250 formulation particles size (90.72 ± 0.23 nm) is considerably smaller compared to many double layered iron oxide formulations prepared by co-precipitation approach [28, 29]. However, an individual nanoparticle grain size of this formulation is slightly increased after coating with CD and F127 polymers (Table 1). But, all the individual particle grain sizes ranged between 7–10 nm which is commonly observed with many precipitation procedures [4, 14, 28, 39–42] (Fig. 1C).
Table 1.
Particles size, zeta potential and drug encapsulation data of iron oxide nanoformulations
| Iron oxide formulation code | Particles size (nm)a,b | Polydispersity (%)a,b | Individual particle size (nm)c | Zeta potential (mV)a,d | Curcumin loadinge |
|---|---|---|---|---|---|
| Iron oxide nanoparticles (MNP) | 201.4 ± 2.45 | 0.42 ± 0.02 | 7.92 ± 3.49 | 6.17 | 62 ± 4.6 |
| Cyclodextrin coated Iron oxide nanoformulationsa | |||||
| CD50 | 208.43 ± 1.75 | 0.21 ± 0.015 | 8.54 ± 2.46 | −32.31 | 74 ± .9 |
| CD100 | 236.12 ± 8.25 | 0.29 ± 0.03 | 8.62 ± 3.62 | −20.1 | 82 ± 3.8 |
| CD150 | 256.17 ± 3.95 | 0.29 ± 0.005 | 8.80 ± 3.42 | −26.09 | 89 ± 3.6 |
| CD200 | 174.47 ± 2.41 | 0.38 ± 0.005 | 8.79 ± 2.98 | −18.85 | 92 ± 3.3 |
| CD250 | 177.26 ± 1.62 | 0.19 ± 0.003 | 8.92 ± 2.61 | 0.59 | 93 ± 4.4 |
| Cyclodextrin (CD200)-F127 coated iron oxide nanoformulationsb | |||||
| F12750 | 108.33 ± 0.23 | 0.35 ± 0.003 | 9.56 ± 3.18 | −9.42 | 87 ± 4.7 |
| F127100 | 107.96 ± 4.97 | 0.36 ± 0.047 | 9.29 ± 3.22 | −8.55 | 89 ± 5.2 |
| F127150 | 92.46 ± 0.93 | 0.44 ± 0.002 | 9.46 ± 2.93 | −2.31 | 94 ± 3.9 |
| F127200 | 91.21 ± 0.31 | 0.34 ± 0.008 | 9.73 ± 3.16 | −8.02 | 93 ± 3.7 |
| F127250 | 90.72 ± 0.23 | 0.29 ± 0.005 | 9.72 ± 2.65 | −5.47 | 96 ± 4.2 |
| F127300 | 181.76 ± 0.23 | 0.21 ± 0.015 | 9.79 ± 2.27 | −10.79 | 96 ± 4.6 |
Particles size and zeta potential of iron oxide formulations were measured by diluting 10 μl of 1 mg/ml solution in 2.75 ml water at 25 °C.
Particles size (nm) and polydispersity (%) values were presented an average value of 3 readings ± S.E.M.
Individual particle size (nm) of formulations was presented an average value of 100 particles measured using high resolution TEM images ± S.E.M.
Zeta potential of formulations was presented average reading obtained for 6 min.
Curcumin loading (%) = weight of curcumin (micro grams) present in 1 mg of nanoparticles.
Fig. 1.
Particles size characterization of magnetic nanoparticle formulations: (A) Dynamic light scattering particles size data of (a) pure magnetic nanoparticles (MNP), (b) magnetic nanoparticles coated with 200 mg of CD (CD200) and (c) magnetic nanoparticles coated with 200 mg of CD and 250 mg of F127 polymer (F127250). (B) Transmission electron microscopic images of (a) pure magnetic nanoparticles, (b) magnetic nanoparticles coated with 200 mg of CD and (c) magnetic nanoparticles coated with 200 mg of CD and 250 mg of F127 polymer. (C) Transmission electron microscopic image of (a) pure magnetic nanoparticles, (b) magnetic nanoparticles coated with 200 mg of CD and (c) magnetic nanoparticles coated with 200 mg of CD and 250 mg of F127 polymer. Data showing individual particle grain size of 7–10 nm.
Good stability in aqueous medium of CD or CD and F127 polymer coated magnetic nanoparticle formulations is due to their negative zeta potential values (Table 1). The CD coated formulations (CD50 to CD250) have − 32 to + 0.59 mV while CD200 with F127 coated formulations (F12750 to F127250) exhibited − 9.42 to − 10.79 mV. Such negative zeta potential formulations help repel each particle in the suspension, ensuring long-term stability and avoiding particles aggregation [46–48], whereas MNP formulations exhibited positive zeta potential, i.e., 6.17 mV indicates some degree of aggregation phenomenon.
3.1.2. Physical characterization
X-ray diffraction (XRD) patterns of different formulations were analyzed to determine the crystal phase of the iron oxide nanoparticles and surface engineered iron oxide nanoparticles (Fig. 2A). All the formulations (MNPs, CD200, and F127250) have shown diffraction peaks at 2θ = 30.1°, 36.2°, 42.4°, 52.5°, 57.5° and 62.2° due to face centered cubic lattice structures 220, 311, 400, 422, 440 and 511 which are characteristic peaks of Fe3O4 crystal structure [31]. All of the diffraction peaks in Fig. 2A can be indexed and assigned to the cubic structure of Fe3O4 which is consistent with the theoretical values (JCPDS card no.: 01-088-0315). Additionally, there are no peaks at 31° corresponding to γ-Fe2O3 and α-Fe2O3 for 210 and 213 in XRD patterns, supporting the purity of synthesized iron oxide nanoparticles. This clearly suggests that iron oxide formulations are composed of magnetite (Fe3O4), not maghemite. Further, formulations were stored under nitrogen atmosphere to prevent possible oxidation which is responsible for producing maghemite from magnetite.
Fig. 2.
Physical characterization of magnetic nanoparticle formulations: (A) X-ray diffraction patterns, (B) Fourier transform infrared spectra, and (C) thermograms of MNP, CD200, and F127250 nanoparticle formulations. (D) Curcumin release profiles from curcumin loaded MNP, CD200, and F127250 formulations. Cumulative release was estimated using UV-vis spectrophotometric method. Data presented is a mean of three replicates. Note: (B) also presents curcumin and curcumin containing F127250 nanoparticle formulations (F127250-CUR).
To confirm the presence of β-cyclodextrin or F127 polymer layer(s) on magnetic nanoparticles, FTIR analysis was taken into consideration (Fig. 2B). Pure magnetic nanoparticles exhibited a broad peak between 3500–3000 cm−1 due to the presence of hydroxyl/amino groups on the surface and a strong peak in the 550 cm−1 region due to –O–Fe of iron oxide skeleton [32] (Fig. 2B, black line). In addition, β-cyclodextrin coated MNPs (CD200) showed an intense band at 1010 cm−1 due to glycosidic (C-O-C) vibration and the coupled (C-C/C-O) stretch vibrations. (Fig. 2B, red line) [31]. The F127 polymer coated on the CD-MNP formulation (F127250) demonstrated the same peaks that appeared in CD200. The peak at 1010 cm−1 belongs to the CH2 rocking and C-O-C stretch vibrations of F127 polymer (green line) [28].
We performed thermogravitic analyses to further confirm the presence of CD and F127 layer(s) on magnetic nanoparticle formulations (Fig. 2C). Pure magnetic nanoparticles have a weight loss ~6.78 wt.% (iron oxide core content 93.22 wt.%), whereas CD coated formulations lost ~11.38 wt.% (6.78 wt.% degradation of NPs + 4.6 wt.% is due to CD coating) indicating 88.62 wt.% of iron oxide core in the formulation. In the case of F127250 formulations it was noticed 87.76 wt.% iron oxide core (6.78 wt.% degradation of NPs + 5.46 wt.% due to CD and F127 polymer layer coatings). Thus, we can confirm that additional weight loss in the case of CD200 (4.6 wt.%) and F127250 (5.46 wt.%) formulations is due to coating of CD and F127 polymer layer(s).
3.1.3. Curcumin loading and release
The curcumin loading was estimated using a UV-vis spectrophotometer as described in our previous report [49]. It was confirmed that the loading capacity continuously increased as the amount of CD used for nanoparticles coating increased. This indicates that curcumin molecules were entering into the CD layer on nanoparticles via hydrophobic-hydrophobic interactions. Our recent report supports this behavior of curcumin encapsulation into the hydrophobic bucket structure of the CD molecule [38]. In addition, F127 polymer promotes its loading to a greater extent due to PPO hydrophobic chains [28]. It was also confirmed that in pure magnetic nanoparticles, curcumin is primarily on the surface of the nanoparticles. Because curcumin molecules are loosely bound to surface of nanoparticles, curcumin release is much faster (Fig. 2D), whereas CD200 and F127250 magnetic nanoparticle formulations have shown a bi-phasic release characteristic. The initial burst of release was due to immediate dissociation of surface bound curcumin molecules that exist on the CD or F127 polymer matrix. The remaining sustained drug release was due to the slow release of the drug entrapped inside CD and/or F127 polymer layers. The curcumin existence in nanoparticle layers was confirmed by FTIR analysis. Curcumin exhibited sharp absorption bands at 1605 cm−1 (stretching vibrations of benzene ring), 1502 cm−1 (C=O and C=C vibrations of benzene), 1435 cm−1 (olefinic C-H bending vibration), 1285 cm−1 (aromatic C-O stretching vibrations), and 1025 cm−1 (C-O-C stretching vibrations of CUR) (Fig. 2B, inset, black line). The curcumin encapsulated formulation F127250-CUR also exhibited all these peaks in addition to the parent F127250 formulation (Fig. 2B, blue line), indicating the presence of curcumin in the formulation.
3.2. Hyperthermia Application
Magnetic nanoparticles are especially susceptible to induce localized hyperthermia in an alternating magnetic field which may potentially shirk or destroy the tumors and sensitize to radiation or chemo-therapies [50–54]. Iron oxide based magnetic nanoparticle formulations are often employed for this purpose because of fewer side effects [55, 56]. For a given magnetic targeted application, an iron oxide formulation needs to meet superparamagnetic properties (high saturation magnetization, Ms) with greater heat effect. Therefore, our formulations were evaluated for magnetic properties, i.e., magnetic saturation (Ms), using a vibrating sample magnetometer at 300 K and at alternating ± 12000 Oe magnetic field (Fig. 3A). The MNP, CD200 and F127250 formulations have saturation magnetization values of 54.47, 47.51 and 52.58 emu/g, respectively. These saturation magnetization values correlate with the reported values for many polymer stabilized iron oxide nanoparticle formulations [32, 34, 57]. This slight variation between the formulations is due to coating with F127 polymer and/or β-cyclodextrin, and a small variation in overall particles size. Further, no coercivity and remanence were observed for any of the formulations at lower magnetic field curves, confirming superparamagnetic characteristics (Fig. 3A, inset).
Fig. 3.
(A) Hysteresis loops of MNP, CD200 and F127250 nanoparticle formulations at room temperature. (B) Time course of the raised temperature of MNP, CD200 and F127250 nanoparticle formulations under an alternating magnetic field operating at 300 kHz. (C) Temperature of various concentrations of F127250 nanoparticles in solution and agarose gels after altering magnetic field applied for 15 min.
The deficiency of hyperthermic cancer treatment is due to the difficulty of raising the tissue temperature properly [58]. Hyperthermia using magnetic nanoparticles can raise the temperature in the tumor locally up to 41–45 °C and is capable of damaging the tumor cells without damaging the healthy cells [59]. Our formulations were tested for these heating effects and results are presented in Fig. 3B. The temperature of formulations is plotted as a function of time at the field of 150 Oe and a constant frequency of 300 kHz. The heating effects (hyperthermia) of formulations resulted from absorbing energy from the alternating magnetic field which was transformed into heat by means of hysteresis loss during reversal of magnetization. The order of heating effects of formulations was found to be F127250 > CD200 > MNP. This can be explained since iron oxide core is available for excellent heat effects in the modified formulation of F127250 due to its freely dispersed stage, in contrast to other formulations. On the other hand, smaller clustered particles have a higher specific surface area which generates higher heat [59]. Therefore, we speculate the F127250 nanoparticles formulation would be highly useful as thermoseeds for localized hyperthermia treatment of cancers [60–62]. Additionally, our formulations exhibited a continuous increase in temperature with respect to an increased concentration of F127250 formulations employed (Fig. 3C). This behavior is similar whether it is in solution form or in the gel form. This is further support that the heat buildup is uniform throughout the sample.
3.3. Magnetic Resonance Imaging
Nanomedicine platforms combine therapeutic function with imaging abilities which have proven to be the next generation of medicine [41]. Unlike traditional contrast agents or drugs, image visible nanomedicine has the ability for simultaneous diagnosis and therapy in one formulation [20, 21, 23]. We have evaluated our iron oxide formulations for in vitro MRI agent characteristics. These tailor-made cocktails can address the challenges of tumor heterogeneity and adaptive resistance which can ultimately help achieve the goal of personalized medicine for cancer therapy [63].
Based upon the collected images (Fig. 4A), increased concentration of MNPs resulted in a greater reduction in signal. Relative to the control gel, the increase in iron oxide concentration also resulted in shorter transverse relaxation times (Fig. 4B). For example, as the concentration of iron oxide in the MNP increased from 10 μg Fe/ml to 40 μg Fe/ml, T2 relaxation times diminished from 20.8ms to 6.3ms. Similar to T2 relaxation times, longitudinal relaxation T1 noted a reduction in relaxation time with iron concentration. By plotting the transverse relaxation rate, R2, (1/T2) as a function of the concentration of Fe in each sample, R2 increased linearly with the concentration of Fe (Fig. 4C) in all the formulations according to the equation: R2 = 1/T2 = 1/T°2 + r2*[Fe], where 1/T2 is the relaxation rate in the presence of iron oxide, 1/T°2 is the relaxation rate of pure water, r2 is the transverse relaxivity, and [Fe] is the concentration of iron in each sample. The T2 relaxivity (r2) was found to decrease in the following order for the tested compounds: F127250-CUR > F127250-CUR in cells > F127250 > F127250 in cells > MNP > CD200. In comparison to T2 relaxation, application of F127250 to curcumin with and without cells resulted in an increase in relaxivity. For example, the r1 for F127250-CUR was found to be 28.6 × 10−3 s−1 μg−1 ml; whereas, F127250 had a relaxivity of 12.5 s−1 μg−1 ml. The observed difference suggests that curcumin increases local inhomogeneity in the magnetic field. For T2-weighted imaging procedures, the F127250 MNP can potentially improve observation through contrast enhancement. It has been proven in previous reports that these multi-layer coated magnetic nanoparticles have superior imaging characteristics over Feridex IV® formulations [28].
Fig. 4.
Magnetic resonance image (MRI) characteristics of magnetic nanoparticle formulations: (A) Signal intensity T2 weighted MR images of magnetic nanoparticle formulations in phantom agar gel at 10–40 μg/ml concentration at 25 °C. (a) MNP, (b) CD200, (c) F127250, (d) F127250 in A2780CP cells, (e) F127250-CUR and (f) F127250-CUR in A2780CP cells. (B) T2 relaxation curves of various magnetic nanoparticle formulations in phantom agar gel. (C) T2 relaxation rates (1/T2) plotted as a function of the Fe concentration for various magnetic nanoparticle formulations.
3.4. Drug Delivery
3.4.1. F127250 formulation lowers protein binding characteristics
One major shortcoming of the magnetic nanoparticles is their destabilization following adsorption of plasma proteins which leads to nonspecific uptake by the reticulum-endothelial system (RES). To evade clearance by RES and avoid agglomeration, and to improve the circulation time of particles, iron oxide nanoparticles (MNP) were coated with CD (CD100) or CD and F127 polymers (F127250). This coating process led to composite heterogeneous particles composed of an iron oxide inner core with a modifying CD or CD-F127 outer coating. Agglomeration is prevented by the firm coating, emulsifying and adhesive properties of F127 efficiently. To prove this concept, our formulations were tested for in vitro protein adsorption (BSA) in 1× PBS solutions. The BSA adsorption was measured by the dimension of fluorescence of tryptophan residue of BSA. It was noticed that fluorescence intensity reduced with increase of particle addition to BSA solution (Fig. 5A). This reduction is probably dependent upon the formulation. The order of reduction was found to be MNP > CD200 > F127250 formulation. From this data, the number of binding sites (n) and binding constant (k) were calculated for each formulation presented in Fig. 5B. It was found that F127250 has a low number of binding sites and binding constant (n = 1.57 and k = 0.025 μg/μg) while CD200 and MNP formulations have n = 1.74 and 2.08, k = 0.037 μg/μg and 0.038 μg/μg, respectively. These analyses suggest that the F127250 formulation will have greater circulation time than remaining formulations due to its number of binding sites and lower binding constant [28]. Therefore, in the next section we have evaluated the internalization efficacy of F127250 formulation to determine its utility as a drug delivery carrier.
Fig. 5.
(A) BSA protein interaction with magnetic nanoparticle (MNP) formulations. Fluorescence emission spectra of BSA solution with various concentrations (0–70 μg) of nanoparticles (a) MNP, (b) CD200, and (c) F127250 at room temperature. (B) Binding constant (k) and number of binding sites calculation graph from fluorescence spectral data. Fo, F and Fs are the relative fluorescence emission intensity of BSA alone, in the presence of nanoparticles, and infinity saturated nanoparticles, respectively. Data is a mean of three replicates.
3.4.2. F127250 formulation intracellular uptake
Intracellular uptake of nanoparticles improves therapeutic outcome because the internalized nanoparticles containing drug are retained in cancer cells. Higher internalization is an index for more accumulation of drug molecules, which release slowly and have sustained effects on cancer cells. We have evaluated our formulations for cellular uptake in three different cancer cells (A2780CP, MDA-MB-231, and MCF-7) by using Flow Cytometeric (Fluorescence activated cell shorter, FACS) analysis [32, 38, 49]. Particles uptake by cancer cells leads to shifting in the side scattered (SSC) height in the Flow Cytometeric analysis. An increased uptake was noticed with increased amount of nanoparticles (0–100 μg) incubated for internalization (Fig. 6A, black to green). The qualitative estimation of particles uptake by various cancer cells demonstrates higher uptake by cisplatin resistant ovarian cancer cells (A2780CP) compared to MCF-7 and MDA-MB-231 breast cancer cells at all the concentrations (Fig. 6B). The order of particles uptake by cancer cells is A2780CP > MDA-MB-231 > MCF-7. This phenomenon is clearly observed in the transmission electron microscopy particle uptake experiments (Fig. 6C). A large portion of magnetic nanoparticles (F127250) are accumulated in the epithelial membrane, endoplasmic reticulum, golgi and cytosolic of A2780CP cancer cells. The particles aggregation is also observed in MDA-MB-231 at a few spots. Compared to A2780 and MDA-MB-231 cells, less uptake/accumulation of particles was observed in MCF-7 (non-metastatic) cells. This data demonstrate that our nanoparticles are capable of internalizing within 6 hrs, even in resistant and metastatic cancer cells. In addition, we have also observed that the magnetic nanoparticles uptake in A2780CP cancer cells is relatively higher compared to our recently fabricated PLGA nanoparticles [49].
Fig. 6.
Cellular uptake of magnetic nanoparticle formulations in cancer cells. (A) Side scattered measurements of nanoparticles uptake by cancer cells using FACS. (B) The quantitative internalization of magnetic nanoparticle formulations in A2780CP (cisplatin resistant ovarian cancer cells), MDA-MB-231 (metastatic breast cancer cells) and MCF-7 (non-metastatic breast cancer cells) are based on the side scattered fluorescence height values. Data represents mean of 3 repeats for each treatment. (C) Transmission electron micrographs of F127250 nanoparticle uptake in (a) A2780CP, (b) MDA-MB-231 and (c) MCF-7 cancer cells. Arrow points indicate F127250 nanoparticles internalization with a distinct contrast.
3.4.3. Internalization of formulations in macrophage and A2780CP ovarian cancer cells
A drug delivery carrier must be present in the blood stream for an appropriately long time to reach and accumulate at its therapeutic site. The opsonization/phagocytosis or removal of drug delivery vehicles from the body by a mononuclear phagocytic system which is also known as the reticuloendothelial system (RES) is a major obstacle to achieving efficient drug delivery. Knowledge on how to design particles to escape phagocytosis could help to overcome this limitation. The macrophages of the mononuclear phagocytic system have the capability to drain nanoparticles from the circulation within seconds. In other words, lower uptake of particles by macrophages determines their efficient use for the drug delivery applications. Therefore, we compared the uptake (indication of phagocytosis) efficiency of MNP, CD200 and F127250 formulations in RAW 264.7 (Mouse leukaemic monocyte macrophage cell line) as determined by Yallapu et al. [27]. Our MNP and CD200 formulations exhibited a greater level of phagocytosis (uptake) compared to the F127250 formulation (Fig. 7A) at two concentrations (50 and 100 μg/ml). The reason for lower phagocytosis of the F127250 formulation is probably due to the protective coating of F127 polymer (pluronic) which helps to form stable nanoparticles in aqueous media [28, 29]. Altogether, these observations reveal clear differences in the phagocytosis pattern of different particles. The lower macrophage uptake of F127250 formulation suggests that this would be a better choice for drug delivery application.
Fig. 7.
Quantitative estimation of magnetic nanoparticle formulations in (A) macrophage cells (RAW 264.7 Mouse leukaemic monocyte macrophage cell line) and (B) A2780CP cancer cells. Data indicates mean of 3 repeats for each treatment. (C) Transmission electron micrographs of F127250 nanoparticle uptake in (a) macrophage cells and (b) A2780CP cancer cells.
Next, we monitored the internalization of MNP, CD200, and F127250 formulations with respect to 50 and 100 mg dosages (Fig. 7B) in A2789CP metastatic ovarian cancer cells. The entry of MNP, CD200, and F127250 formulations into the cancer cells was determined [27] and it is varied in different formulations. Evidently, with increase of dose, their uptake is increased. The cellular uptake of the nanocarriers is mainly dependent upon the route of entry, i.e., endocytosis or phagocytosis. Phagocytosis is considered when the particles size was above 300 nm [8, 14, 19]. Whereas endocytic pathways for nanocarriers are subdivided into four categories: namely, clathrin-mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis, and clathrinand caveolae-independent endocytosis [8, 14, 19]. Taken together, our studies suggest that the internalization of F127250 formulation is 5% in macrophages and is > 75% in cancer cells, indicating uptake is based on endocytosis but not phagocytosis (Fig. 7A–B). This phenomenon is also evident in the TEM analysis of particles uptake in macrophage and A2780CP cancer cells (Fig. 7C). A large number of F127250 nanoparticles can be seen inside the A2780CP cancer cells but not in macrophages (RAW 264.7).
3.4.4. CUR encapsulated F127250 formulation anticancer efficacy
The therapeutic efficacy of curcumin encapsulated F127250 was evaluated in three different cancer cell lines (A2780CP: ovarian, MDA-MB-231: breast, and PC3: prostate) by MTS cell viability assay [38]. All the studied cell lines have shown typical dose dependant anti-proliferative effects (5–40 μM) by both native curcumin and curcumin encapsulated F127250 formulation (Fig. 8). The control (DMSO or F127250) treatments did not show any effects on cell growth. The in vitro 50% cell growth inhibitory concentration (IC50) is the quantitative measure for the cell toxicity induced by chemotherapeutic drug. The IC50 values were 12.6, 18.8 and 10.6 μM with curcumin and 12.1, 11.9, and 12.8 μM with curcumin encapsulated F127250 formulation, for A2780CP, MDA-MB-231 and PC3 cancer cells, respectively. This data demonstrates that F127250 containing curcumin is equally efficient in suppressing cell growth even though the release is only 40 percent of nanoformulations within the 48 hour treatment time. Therefore, we can consider that inhibition of cell proliferation is more effective and sustained for long period of times.
Fig. 8.
Anti-proliferative effect of CUR and F127250-CUR treatment in ovarian (A2780CP), breast (MDA-MB-231), and prostate (PC3) cancer cells. Cells were treated with CUR or F127250-CUR in solution, medium was changed on day 2 and cell viability was measured using MTT assay using UV-vis spectrophotometer at 492 nm. Data is mean ± SEM (n = 6). DMSO and F127250 (magnetic nanoparticles contain β-cyclodextrin and pluronic polymer F127 coatings) control did not show any effect at these concentrations.
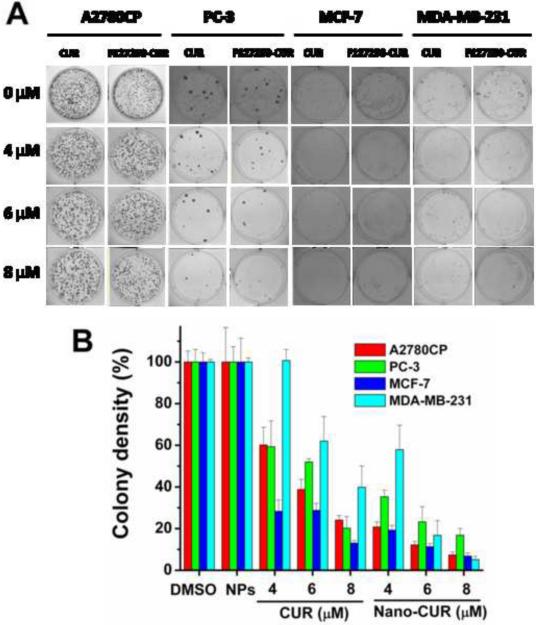
Previous literature suggests that colony formation assay provides the ability to evaluate long-term anti-cancer efficacy of the developed drug(s) or drug(s) formulations. To prove our MNP curcumin formulation has greater effects in long term anti-cancer efficacy assays, we have studied free CUR and F127250 curcumin formulation in A2780CP, MDA-MB-231 and PC3 cancer cell lines at equivalent doses 4, 6, and 8 μM. Equivalent quantities of DMSO or F127250 formulations were used as controls for CUR and F127250 CUR formulation, respectively. A significant (p<0.05) decrease in the density of colonies was observed with F12725 curcumin formulation compared to free curcumin (Fig. 9). For example, at 4 μM, F127250 CUR formulation showed 38, 52, 21 and 56% colony densities, whereas free curcumin showed 59, 58, 30, and 100% colony densities after 10 day treatment in A2780CP, MDA-MB-231, and PC3 cancer cells, respectively. The lower colonies indicate an improved therapeutic efficacy of F127250 CUR formulation, resulting from an intracellular uptake effect and sustained release properties.
Fig. 9.
(A) Representative photographs of colony formation assays of prostate cancer cells (PC3) treated with CUR or F127250-CUR (nano-CUR). (B) Quantitation of colony densities in CUR and F127250-CUR treatment groups in three prostate cancer cell lines. Data represent mean of 3 repeats for each treatment, mean ± SEM (n =3). DMSO and NPs (F127250, magnetic nanoparticles contain β-cyclodextrin and pluronic polymer F127 coatings) control did not show any effect at these concentrations.
3.4.5. Molecular pathway
Bcl-xl is a transmembrane molecule in the mitochondria. It is one of several anti-apoptotic proteins which are members of the Bcl-2 family of proteins. It has been implicated in the survival of cancer cells [33, 64, 65]. The expression level analysis suggests strong suppression of Bcl-xL expression after treatment with 10 or 20 μM F127250-CUR compared to equivalent amounts of CUR, DMSO and F127250. This data demonstrate less cancer cell survival with the F127250-CUR formulation compared to CUR treatment (Fig. 10A). No change in Bcl-xL expression was observed with control (DMSO and F127250 formulation) treatments. From this study, it was clear that F127250-CUR at 10 and 20 μM exhibited less cancer cell survival and implies induction of apoptosis or cancer cell death. To study its apoptosis, we have investigated the pattern of Poly(ADP-ribose) polymerase (PARP) cleavage which is a protein involved in a number of cellular processes including DNA repair and cell death [66, 67]. Cleavage of PARP is an indicator of DNA damage and apoptosis in response to a diverse range of cytotoxic agents. Because of good PARP expression and its cleavage in response to stress signal, we have chosen PC3 cells for PARP apoptosis assay. PC3 cells treated with 20 μM CUR or equivalent amounts of F127250-CUR exhibited considerable cleavage of full length PARP (116 kDa) into cleaved PARP (86 kDa), which indicates the cancer cells are undergoing cell death via apoptosis pathway (Fig. 10B). The PARP cleavage caused by F127250-CUR formulation is much stronger compared to pure CUR treatment, which suggests an improved efficacy of F127250-CUR formulation for cancer therapy.
Fig. 10.
(A–B) Immunoblot analysis for Bcl-xL expression and PARP cleavage in CUR or F127250-CUR (nano-CUR) cancer cells. Note: F127250-CUR (nano-CUR) has shown a decrease in Bcl-xL expression compared to free curcumin (CUR) which indicates reduced cell survival. F127250-CUR has also shown enhanced PARP cleavage compared to free CUR. DMSO and NPs (F127250, magnetic nanoparticles contain β-cyclodextrin and pluronic polymer F127 coatings) control did not show any effects. (C) Ultrastructural cellular changes induced by CUR or F127250-CUR treatments.
The variation of ultrastructural changes in the cancer cells upon exposure to control (DMSO and F127250 formulation), 20 μM CUR or 20 μM F127250-CUR were observed by transmission electron microscopy (TEM) analysis. The control treatments did not cause any ultrastructural changes in PC3 cancer cells (Fig. 10C (a–b)), but both F127250-CUR and 20 μM CUR treated cells demonstrated the formation of endosomal-lysosomal-vacules, which is an indication of cell death (Fig. 10C (c–d). However, this effect was more pronounced in F127250-CUR treated cells, suggesting its improved therapeutic efficacy (Fig. 10C (d)). The vacuole formation is usually caused by the destabilization of subcellular organelle, mitochondrial swelling, opening of the permeability, dissipation of the mitochondrial potential which is a hallmark of typical apoptosis. The internalization of the F127250-CUR and sustained release of active curcumin in the cells probably enhanced the apoptosis in cancer cells, which as a result, further improved therapeutic efficacy of our formulation.
3.5. Toxicity evaluation
In general, nanoparticles have extremely fast systemic translocation rates following in vivo administration. Blood is one of the common translocation routes to organs for any nanoparticle-mediated therapy. Thus, we want to evaluate our CUR encapsulated F127250 formulation for haemocompatibility. For this, different concentrations (10–100 μg) of CUR containing MNP, CD200, and F127250 formulations were incubated in 100 μl of human blood for 2 hrs. OD measurements recorded on a UV-vis spectrophotometer at λmax 570 nm indicate both CUR containing CD200 and F127250 formulations are haemocompatible but CUR containing MNP formulation is toxic after a concentration of 30 μg (Fig. 11A). A similar observation can be found with the red blood cells morphology studies (Fig 11B). Data in Fig. 11B(a–b) shows a clear morphology of red blood cells without any particles. After 2 hrs of MNP-CUR particles exposure to red blood cells, a rigorous change in the morphology and a higher amount of deposition of particles were noticed (Fig. 11B(c)). Other CUR containing formulations (CD200 and F127250) showed slight deposition of particle clumps; however, no change in the morphology of red blood cells was noted (Fig. 11B(d–e)). In conclusion, F127250-CUR formulation is haemocompatible. The possible reason for diminished haemolytic activity or haemocompatibility is low toxicity coatings of cyclodextrin and/or pluronic polymer F127. These results are consistent with reported nanoparticles [68] coated with pluronic polymers which showed almost no toxicity.
Fig. 11.
Haemocompatibility of CUR containing magnetic nanoparticle (nano-CUR) formulations. (A) nano-CUR formulations were incubated with red blood cells for 2 hrs, centrifuged and supernatant absorbance at 570 nm in UV-vis spectrophotometer was recorded. Controls: magnetic nanoparticles (without curcumin) showed results similar to F127250-CUR formulation (data not shown). Note: MNP formulation shows toxicity on red blood cells because of deposition towards greater aggregation. (B) Red blood cells morphology after incubation with different formulation.
4. Conclusion
It is essential to develop a tailor made magnetic nanoparticle formulation for multi-functional biomedical applications. A number of magnetic nanoparticle formulations have been generated in the recent past. However, their effective utility has been limited due to higher particle size, loss of magnetization, other inherent properties and lower internalization capacity into the cancer cells which ultimately results in poor therapeutic efficacy in cancer treatment. Our present study illustrates that multi-layer β-cyclodextrin and F127 polymer coated magnetic nanoparticles offer good stability, enhanced cellular uptake, sustained release characteristic of encapsulated anti-cancer drug (curcumin) with improved anti-cancer therapeutic efficacy. The F127250-CUR formulation has shown almost similar growth inhibitory effects as pure curcumin in the cancer cells. The F127250-CUR formulation has also exhibited haemocompatibility, representing an excellent drug delivery approach. On the other hand, recent reports on these types of nanoformulations have shown similar anti-proliferative effects [28, 29, 38, 49] but they do not provide MRI and hyperthermia properties. Additionally, our formulation has shown enhanced molecular effects in cancer cells toward apoptosis.
Acknowledgments
We thank Cathy Christopherson (Sanford Research/USD, Sioux Falls) for editorial assistance, Robert Japs (Sanford Health), Sara Basiaga (Department of Chemistry, UN-Lincoln), Shah R Valloppilly (Nebraska Center for Materials and Nanoscience, Department of Physics and Astronomy, UN-Lincoln), and Crittenden J. Ohlemacher (Applied Polymer Research Center, University of Akron) for their help in characterization of our samples. We also thank Dr. Omathanu P. Perumal (Department of Pharmaceutical Sciences, South Dakota State University, Brookings, SD) for providing access to DLS instrument for particles size and zeta potential measurements. The authors also thank Dr. Diane Maher, Dr. Vasudha Sundram and Mara Ebeling (Sanford Research/USD) for their suggestions throughout this study. This work was supported by grants from Sanford Research/USD, Department of Defense (DOD) (PC073887), Governor's Cancer 2010, and NIH RO1 (CA142736) awarded to SCC and Department of Defense (DOD) (PC073643) and Governor's Cancer 2010 grants awarded to MJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 2007;46:1222–44. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- [2].Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 2005;100:1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- [3].Saiyed Z, Telang S, Ramchand C. Application of magnetic techniques in the field of drug discovery and biomedicine. Biomagn Res Technol. 2003;1:2. doi: 10.1186/1477-044X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Namdeo M, Saxena S, Tankhiwale R, Bajpai M, Mohan YM, Bajpai SK. Magnetic nanoparticles for drug delivery applications. J Nanosci Nanotechnol. 2008;8:3247–71. doi: 10.1166/jnn.2008.399. [DOI] [PubMed] [Google Scholar]
- [5].Zhang L, Yu F, Cole AJ, Chertok B, David AE, Wang J, et al. Gum arabic-coated magnetic nanoparticles for potential application in simultaneous magnetic targeting and tumor imaging. Aaps J. 2009;11:693–9. doi: 10.1208/s12248-009-9151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, et al. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperthermia. 2005;21:637–47. doi: 10.1080/02656730500158360. [DOI] [PubMed] [Google Scholar]
- [7].Wilhelm C, Fortin JP, Gazeau F. Tumour cell toxicity of intracellular hyperthermia mediated by magnetic nanoparticles. J Nanosci Nanotechnol. 2007;7:2933–7. doi: 10.1166/jnn.2007.668. [DOI] [PubMed] [Google Scholar]
- [8].Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60:1252–65. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang M. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small. 2006;2:785–92. doi: 10.1002/smll.200600009. [DOI] [PubMed] [Google Scholar]
- [10].Bruce IJ, Sen T. Surface modification of magnetic nanoparticles with alkoxysilanes and their application in magnetic bioseparations. Langmuir. 2005;21:7029–35. doi: 10.1021/la050553t. [DOI] [PubMed] [Google Scholar]
- [11].Wilhelm C, Gazeau F. Universal cell labelling with anionic magnetic nanoparticles. Biomaterials. 2008;29:3161–74. doi: 10.1016/j.biomaterials.2008.04.016. [DOI] [PubMed] [Google Scholar]
- [12].Osaka T, Matsunaga T, Nakanishi T, Arakaki A, Niwa D, Iida H. Synthesis of magnetic nanoparticles and their application to bioassays. Anal Bioanal Chem. 2006;384:593–600. doi: 10.1007/s00216-005-0255-7. [DOI] [PubMed] [Google Scholar]
- [13].Dey T. Polymer-coated magnetic nanoparticles: surface modification and end-functionalization. J Nanosci Nanotechnol. 2006;6:2479–83. doi: 10.1166/jnn.2006.534. [DOI] [PubMed] [Google Scholar]
- [14].Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine. 2007;2:23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- [15].Lee SY, Harris MT. Surface modification of magnetic nanoparticles capped by oleic acids: characterization and colloidal stability in polar solvents. J Colloid Interface Sci. 2006;293:401–8. doi: 10.1016/j.jcis.2005.06.062. [DOI] [PubMed] [Google Scholar]
- [16].Flexman JA, Minoshima S, Kim Y, Cross DJ. Magneto-optical labeling of fetal neural stem cells for in vivo MRI tracking. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5631–4. doi: 10.1109/IEMBS.2006.259982. [DOI] [PubMed] [Google Scholar]
- [17].Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99:16267–72. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Montet-Abou K, Montet X, Weissleder R, Josephson L. Cell internalization of magnetic nanoparticles using transfection agents. Mol Imaging. 2007;6:1–9. [PubMed] [Google Scholar]
- [19].McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev. 2008;60:1241–51. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guthi JS, Yang SG, Huang G, Li S, Khemtong C, Kessinger CW, et al. MRI-visible micellar nanomedicine for targeted drug delivery to lung cancer cells. Mol Pharm. 2010;7:32–40. doi: 10.1021/mp9001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cinteza LO, Ohulchanskyy TY, Sahoo Y, Bergey EJ, Pandey RK, Prasad PN. Diacyllipid micelle-based nanocarrier for magnetically guided delivery of drugs in photodynamic therapy. Mol Pharm. 2006;3:415–23. doi: 10.1021/mp060015p. [DOI] [PubMed] [Google Scholar]
- [22].Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doblinger M, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 142:108–21. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [23].Shubayev VI, Pisanic TR, 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–77. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rubio-Retama J, Zafeiropoulos NE, Serafinelli C, Rojas-Reyna R, Voit B, Cabarcos EL, et al. Synthesis and characterization of thermosensitive PNIPAM microgels covered with superparamagnetic gamma-Fe2O3 nanoparticles. Langmuir. 2007;23:10280–5. doi: 10.1021/la7009594. [DOI] [PubMed] [Google Scholar]
- [25].Wang L, Yang Z, Zhang Y, Wang L. Biofunctional nanoparticles with magnetization and luminescence. J Phys Chem C. 2009;113:3955–9. [Google Scholar]
- [26].Guo R, Zhang L, Qian H, Li R, Jiang X, Liu B. Multifunctional nanocarriers for cell imaging, drug delivery, and near-IR photothermal therapy. Langmuir. 2010;26:5428–34. doi: 10.1021/la903893n. [DOI] [PubMed] [Google Scholar]
- [27].Yallapu MM, Foy SP, Jain TK, Labhasetwar V. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pham Res. 2010 doi: 10.1007/s11095-010-0260-1. DOI: 10.1007/s11095-010-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- [29].Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5:316–27. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- [30].Gao J, Gu H, Xu B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc Chem Res. 2009;42:1097–107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- [31].Banerjee SS, Chen D-H. Magnetic Nanoparticles Grafted with Cyclodextrin for Hydrophobic Drug Delivery. Chem Mater. 2007;19:6345–9. [Google Scholar]
- [32].Bhattarai SR, Kc RB, Kim SY, Sharma M, Khil MS, Hwang PH, et al. N-hexanoyl chitosan stabilized magnetic nanoparticles: Implication for cellular labeling and magnetic resonance imaging. J Nanobiotechnology. 2008;6:1. doi: 10.1186/1477-3155-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yallapu MM, Maher DM, Sundram V, Bell MC, Jaggi M, Chauhan SC. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J Ovarian Res. 2010;3:11. doi: 10.1186/1757-2215-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Luo B, Song XJ, Zhang F, Xia A, Yang WL, Hu JH, et al. Multi-functional thermosensitive composite microspheres with high magnetic susceptibility based on magnetite colloidal nanoparticle clusters. Langmuir. 2010;26:1674–9. doi: 10.1021/la902635k. [DOI] [PubMed] [Google Scholar]
- [35].Beaven GH, Chen S-H, D'albis A, Gratzer WB. A spectroscopic study of the haemin-human-serum-albumin system. Eur J Biochem. 1974;41:539–46. doi: 10.1111/j.1432-1033.1974.tb03295.x. [DOI] [PubMed] [Google Scholar]
- [36].Chipman DM, Grisaro V, NSharon N. The binding of oligosaccharides containing N-acetylglucosamin and N-acetylmuramic acid to lysozyme. J Biol Chem. 1967;242:4388–94. [PubMed] [Google Scholar]
- [37].Chauhan SC, Vannatta K, Ebeling MC, Vinayek N, Watanabe A, Pandey KK, et al. Expression and functions of transmembrane mucin MUC13 in ovarian cancer. Cancer Res. 2009;69:765–74. doi: 10.1158/0008-5472.CAN-08-0587. [DOI] [PubMed] [Google Scholar]
- [38].Yallapu MM, Jaggi M, Chauhan SC. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces. 2010;79:113–25. doi: 10.1016/j.colsurfb.2010.03.039. [DOI] [PubMed] [Google Scholar]
- [39].Bae KH, Ha YJ, Kim C, Lee KR, Park TG. Pluronic/chitosan shell cross-linked nanocapsules encapsulating magnetic nanoparticles. J Biomater Sci Polym Ed. 2008;19:1571–83. doi: 10.1163/156856208786440451. [DOI] [PubMed] [Google Scholar]
- [40].Lim JK, Majetich SA, Tilton RD. Stabilization of superparamagnetic iron oxide core-gold shell nanoparticles in high ionic strength media. Langmuir. 2009;25:13384–93. doi: 10.1021/la9019734. [DOI] [PubMed] [Google Scholar]
- [41].Lin JJ, Chen JS, Huang SJ, Ko JH, Wang YM, Chen TL, et al. Folic acid-Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials. 2009;30:5114–24. doi: 10.1016/j.biomaterials.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [42].Xiong XY, Tam KC, Gan LH. Release kinetics of hydrophobic and hydrophilic model drugs from pluronic F127/poly(lactic acid) nanoparticles. J Control Release. 2005;103:73–82. doi: 10.1016/j.jconrel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- [43].Dorris A, Rucareanu S, Reven L, Barrett CJ, Lennox RB. Preparation and characterization of polyelectrolyte-coated gold nanoparticles. Langmuir. 2008;24:2532–8. doi: 10.1021/la703003m. [DOI] [PubMed] [Google Scholar]
- [44].Latham AH, Williams ME. Controlling transport and chemical functionality of magnetic nanoparticles. Acc Chem Res. 2008;41:411–20. doi: 10.1021/ar700183b. [DOI] [PubMed] [Google Scholar]
- [45].Peracchia MT, Vauthier C, Puisieux F, Couvreur P. Development of sterically stabilized poly(isobutyl 2-cyanoacrylate) nanoparticles by chemical coupling of poly(ethylene glycol) J Biomed Mater Res. 1997;34:317–26. doi: 10.1002/(sici)1097-4636(19970305)34:3<317::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [46].Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [47].Billotey C, Wilhelm C, Devaud M, Bacri JC, Bittoun J, Gazeau F. Cell internalization of anionic maghemite nanoparticles: quantitative effect on magnetic resonance imaging. Magn Reson Med. 2003;49:646–54. doi: 10.1002/mrm.10418. [DOI] [PubMed] [Google Scholar]
- [48].Smirnov P. Cellular magnetic resonance imaging using superparamagnetic anionic iron oxide nanoparticles: applications to in vivo trafficking of lymphocytes and cell-based anticancer therapy. Methods Mol Biol. 2009;512:333–53. doi: 10.1007/978-1-60327-530-9_19. [DOI] [PubMed] [Google Scholar]
- [49].Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010 doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- [50].Salomir R, Vimeux FC, de Zwart JA, Grenier N, Moonen CT. Hyperthermia by MR-guided focused ultrasound: accurate temperature control based on fast MRI and a physical model of local energy deposition and heat conduction. Magn Reson Med. 2000;43:342–7. doi: 10.1002/(sici)1522-2594(200003)43:3<342::aid-mrm4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [51].Salomir R, Palussiere J, Vimeux FC, de Zwart JA, Quesson B, Gauchet M, et al. Local hyperthermia with MR-guided focused ultrasound: spiral trajectory of the focal point optimized for temperature uniformity in the target region. J Magn Reson Imaging. 2000;12:571–83. doi: 10.1002/1522-2586(200010)12:4<571::aid-jmri9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [52].Le Renard PE, Jordan O, Faes A, Petri-Fink A, Hofmann H, Rufenacht D, et al. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials. 2010;31:691–705. doi: 10.1016/j.biomaterials.2009.09.091. [DOI] [PubMed] [Google Scholar]
- [53].Le Renard PE, Buchegger F, Petri-Fink A, Bosman F, Rufenacht D, Hofmann H, et al. Local moderate magnetically induced hyperthermia using an implant formed in situ in a mouse tumor model. Int J Hyperthermia. 2009;25:229–39. doi: 10.1080/02656730802713557. [DOI] [PubMed] [Google Scholar]
- [54].Gentilini C, Evangelista F, Rudolf P, Franchi P, Lucarini M, Pasquato L. Water-soluble gold nanoparticles protected by fluorinated amphiphilic thiolates. J Am Chem Soc. 2008;130:15678–82. doi: 10.1021/ja8058364. [DOI] [PubMed] [Google Scholar]
- [55].Latorre M, Rinaldi C. Applications of magnetic nanoparticles in medicine: magnetic fluid hyperthermia. P R Health Sci J. 2009;28:227–38. [PubMed] [Google Scholar]
- [56].Duguet E, Vasseur S, Mornet S, Devoisselle JM. Magnetic nanoparticles and their applications in medicine. Nanomedicine. 2006;1:157–68. doi: 10.2217/17435889.1.2.157. [DOI] [PubMed] [Google Scholar]
- [57].Xia H-B, Yi J, Foo P-S, Liu B. Facile fabrication of water-soluble magnetic nanoparticles and their spherical aggregates. Chem Mater. 2007;19:4087–91. [Google Scholar]
- [58].Kato H, Ishida T. Present and future status of noninvasive selective deep heating using RF in hyperthermia. Med Biol Eng Comput. 1993;31(Suppl):S2–11. doi: 10.1007/BF02446643. [DOI] [PubMed] [Google Scholar]
- [59].Motoyama J, Hakata T, Kato R, Yamashita N, Morino T, Kobayashi T, et al. Size dependent heat generation of magnetite nanoparticles under AC magnetic field for cancer therapy. Biomagn Res Technol. 2008;6:4. doi: 10.1186/1477-044X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Deger S, Taymoorian K, Boehmer D, Schink T, Roigas J, Wille AH, et al. Thermoradiotherapy using interstitial self-regulating thermoseeds: an intermediate analysis of a phase II trial. Eur Urol. 2004;45:574–9. doi: 10.1016/j.eururo.2003.11.012. discussion 80. [DOI] [PubMed] [Google Scholar]
- [61].Kawashita M, Tanaka M, Kokubo T, Inoue Y, Yao T, Hamada S, et al. Preparation of ferrimagnetic magnetite microspheres for in situ hyperthermic treatment of cancer. Biomaterials. 2005;26:2231–8. doi: 10.1016/j.biomaterials.2004.07.014. [DOI] [PubMed] [Google Scholar]
- [62].Serrano MC, Portoles MT, Pagani R, de Guinoa JS, Ruiz-Hernandez E, Arcos D, et al. In vitro positive biocompatibility evaluation of glass-glass ceramic thermoseeds for hyperthermic treatment of bone tumors. Tissue Eng Part A. 2008;14:617–27. doi: 10.1089/tea.2007.0205. [DOI] [PubMed] [Google Scholar]
- [63].Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Orrenius S, Zhivotovsky B. The future of toxicology-Does it matter how cells die? Chem Res Toxicol. 2006;19:729–33. doi: 10.1021/tx0600651. [DOI] [PubMed] [Google Scholar]
- [65].Malugin A, Kopeckova P, Kopecek J. HPMA copolymer-bound doxorubicin induces apoptosis in ovarian carcinoma cells by the disruption of mitochondrial function. Mol Pharm. 2006;3:351–61. doi: 10.1021/mp050065e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McGowan AJ, Ruiz-Ruiz MC, Gorman AM, Lopez-Rivas A, Cotter TG. Reactive oxygen intermediate(s) (ROI): common mediator(s) of poly(ADP-ribose)polymerase (PARP) cleavage and apoptosis. FEBS Lett. 1996;392:299–303. doi: 10.1016/0014-5793(96)00838-1. [DOI] [PubMed] [Google Scholar]
- [67].de Murcia G, Menissier de Murcia J. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994;19:172–6. doi: 10.1016/0968-0004(94)90280-1. [DOI] [PubMed] [Google Scholar]
- [68].Liu F, Park JY, Zhang Y, Conwell C, Liu Y, Bathula SR, Huang L. Targeted cancer therapy with novel high drug-loading nanocrystals. J Pharm Sci. 2010;99:3542–3551. doi: 10.1002/jps.22112. [DOI] [PubMed] [Google Scholar]