Abstract
The ability to precisely pattern embryonic stem (ES) cells in vitro into predefined arrays/geometries may allow for the recreation of stem cell niche for better understanding of how cellular microenvironmental factors govern stem cell maintenance and differentiation. In this study, a new gelatin-based laser direct-write (LDW) technique was utilized to deposit mouse ES cells into defined arrays of spots, while maintaining stem cell pluripotency. Results obtained from these studies showed that ES cells were successfully printed into specific patterns and remained viable. Furthermore, ES cells retained the expression of Oct4 in nuclei after LDW, indicating that the laser energy did not affect their maintenance of an undifferentiated state. The differentiation potential of mouse ES cells after LDW was confirmed by their ability to form embryoid bodies (EBs) and to spontaneously become cell lineages representing all three germ layers, revealed by the expression of marker proteins of nestin (ectoderm), Myf-5 (mesoderm) and PDX-1 (endoderm), after 7 days of cultivation. Gelatin-based LDW provides a new avenue for stem cell patterning, with precision and control of the cellular microenvironment.
Keywords: stem cell, direct-write, micropatterning, gelatin, cell viability, pluripotency
1. Introduction
Embryonic stem (ES) cells have great potential to transform drug discovery [1–3], diagnostics [4] and therapeutics [5–8] due to their self-renewal capacity and pluripotent differentiation potential. To fully realize stem cell potential, better understanding of stem cellmicroenvironment interactions, in particular, cell-cell interactions is of paramount importance. The cellular composition of stem cell microenvironments has displayed a profound influence on both maintaining stem cell pluripotency and directing stem cell differentiation. For example, the local cell density, cellular spacing, cell-cell contact and different types of neighboring cells in cocultures have been demonstrated to influence stem cell fate decisions [9–17]. The ability to spatially control the in vitro placement of ES cells, in relation to other ES cells or other cell types in a co-culture, will allow better recreation of the in vivo stem cell microenvironment, control of cell signaling to direct a desired differentiation, and enable the investigation of cellular interactions. The in vivo spatial distribution of cells has recently been shown to influence cell differentiation and function, as well as the physiology of health and disease [18, 19]. The spatial arrangement of cells in vitro also affects stem cell differentiation [20, 21]. Therefore, the ability to precisely control the position of cells during differentiation is vital to tissue morphogenesis and regeneration.
It is impossible to control the placement and spatial arrangement of cells, with resolution or reproducibility, by manual pipetting of cell suspensions, as in conventional cell culture systems or trans-well co-culture systems. As such, cellular patterning techniques offer new opportunities to create in vivo-like cellular microenvironments for the spatial control of stem cell fate [22–26]. Current cellular patterning techniques include micropatterning [27, 28], modified ink-jet printing [29, 30], and laser cell printing [31–36]. Micropatterning is one of the most studied techniques, which utilizes microwells [37–39], elastomeric stamps [40] or stencils [41], microcontact printing [42–44], layer-by-layer deposition [45], microfluidics [46–48], micromechanical actuation [49], capillary force [50], switchable surfaces (e.g., thermoresponsive polymers [51, 52], electroactive substrate [53]), or DNA-mediated cell assembly [54] to produce various cell patterns on substrates. These micropatterning techniques usually involve the use of microfabrication tools and contain multiple steps. Laser cell printing can provide rapid, high throughput, scalable cell patterning [55]. New advances in laser cell printing, specifically laser direct-write, grant the precise control of local cell density, relative cell number in culture, specific location and proximity of cell placement, cellular heterogeneity, and the creation of combinatorial libraries of cellular microenvironments [56]. Moreover, the latest advances in laser direct-write enable the visualization of cells, both on the print ribbon prior to transfer, and on the receiving substrate following transfer [57]. This utility allows for specific cells, or groups of cells, to be targeted and transferred, and provides optical confirmation of their deposition.
We have developed a more precise patterning technique for the investigation of the spatial dependence of ES cells in culture, to ultimately direct stem cell differentiation in vitro. The first critical step toward this goal is to efficiently and accurately control the placement of ES cells, while maintaining their pluripotency. As such, this technique will allow for the investigation of stem cell differentiation directed by cellular cues in stem cell microenvironments.
In this study, we employed a new gelatin-based laser direct-write technique (matrix assisted pulsed laser evaporation direct-write, MAPLE DW) [57], herein referred to as laser direct-write (LDW), to write spatially precise patterns of mouse ES cells. Gelatin, denatured collagen, is widely used for feeder layer-free mouse ES cell culture. Here, gelatin was used as a coating for both the print ribbon and receiving substrate, and when coupled with a computeraided design/computer-aided manufacturing (CAD/CAM) laser system, enables rapid and precise patterning of cells. This gelatin-based LDW technique has been recently used to print human dermal fibroblasts [57]. In contrast to the aforementioned cells, ES cells represent a relatively sensitive cell type, in that they have the self-renewal capacity and differentiation potential to become cell types representing all three germ layers: ectoderm, mesoderm, and endoderm. As a result, their pluripotent potential poses a unique challenge for patterning ES cells: not only must a transfer yield viable cells, but the cell’s pluripotency must be preserved, or alternately driven to a specific desired fate, for the patterning technique to be deemed a success.
In this study we investigated the feasibility of using gelatin-based LDW as a method to pattern ES cells, with the maintenance of stem cell pluripotency as a benchmark for efficacy. Patterns of mouse ES cells were created using LDW and the resulting substrates were analyzed. In addition to cell viability and growth, stem cell pluripotency following LDW was further examined by the maintenance of stem cell marker (octomer binding transcription factor 4, Oct4), and the ability to form embryoid bodies (EBs) and spontaneously differentiate, in vitro, into cell lineages expressing markers of the three germ layers, nestin (ectoderm marker), Myf-5 (mesoderm marker), and PDX-1 (endoderm marker). Here we show that mouse ES cells could be LDW-patterned into pre-designed arrays, and maintain their viability and stem cell pluripotency. This establishes the feasibility of using LDW for spatially precise patterning of ES cells, and creating engineered stem cell microenvironments.
2. Materials and Methods
2.1. ES cell culture
Mouse ES cells (CCE cell line, StemCell Technologies, Vancouver, Canada) [58, 59] were cultured in gelatin-coated flasks and kept in a standard cell culture incubator. To maintain an undifferentiated state, ES cells were cultured in growth medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM with 4.5 g/l D-glucose) supplemented with 15% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1 mM MEM non-essential amino acids, 10 ng/ml murine recombinant leukemia inhibitory factor (LIF; StemCell Technologies, Vancouver, Canada), 0.1 mM monothioglycerol, 2 mM L-glutamine, and 1 mM sodium pyruvate (Sigma Aldrich, St. Louis, MO) as described previously [60]. LIF is typically used to maintain mouse ES cells in undifferentiated states as it activates latent transcription factor STAT3, by binding to LIF receptor and gp130 receptor, which allows continuous cell proliferation in vitro [61–63]. Thus, for spontaneous differentiation, growth medium without LIF was used [64].
2.2. Laser direct-write system
Mouse ES cell patterning was achieved using a new gelatin-based LDW technique, as recently described [57]. We have adapted this gelatin-based LDW technique to precisely pattern and transfer mouse ES cells, following a similar methodology.
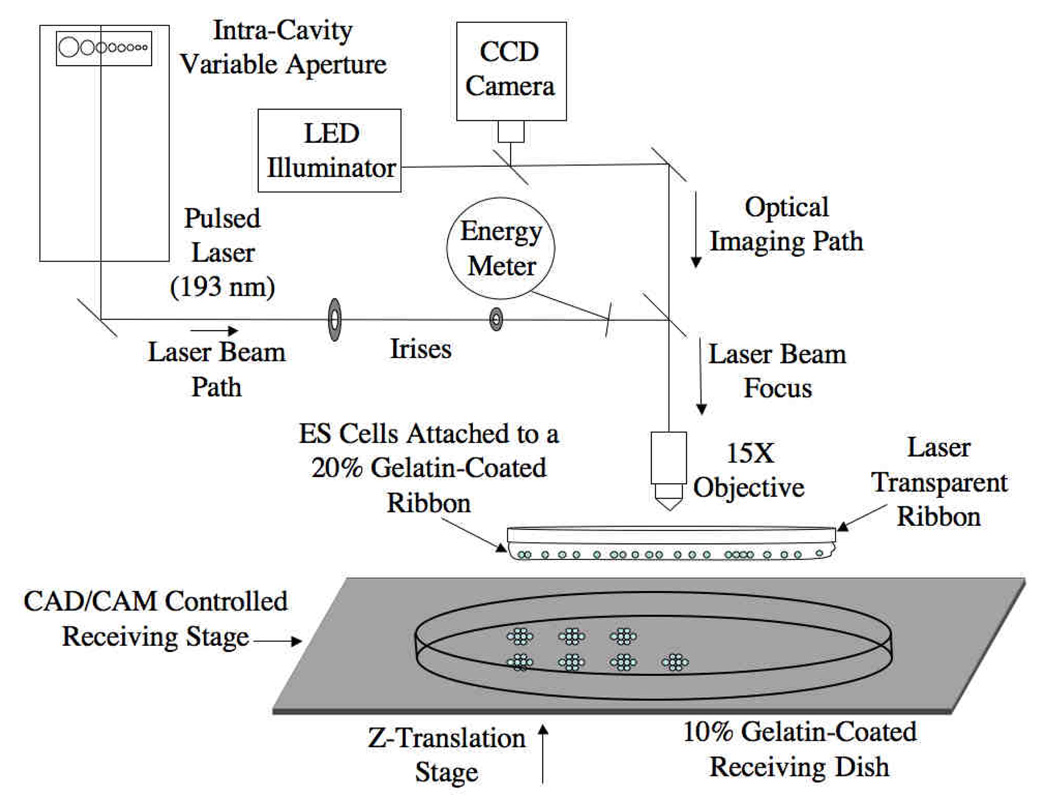
The LDW system in this study utilizes an Argon-Fluorine (ArF) pulsed excimer laser (TeoSys, Crofton, MD), operating at a wavelength of 193 nm, coupled with CAD/CAM control as well as an in situ charge-coupled device (CCD) camera (Fig 1). The laser beam, which has a near-Gaussian distribution, a pulse width of 8 ns, and a repetition rate to be varied from 1 Hz up to 300 Hz, is transmitted to the ribbon through an intra-cavity variable aperture, a series of mirrors, two irises to set the laser beam diameter, and lastly through a 15X objective to focus the beam. The beam diameter can be adjusted to prescribe the diameter (~20–500 µm) of the transferred spot of cells. User-specified pattern arrays were written in a g-code format to establish the dimensions of the arrays and the geometric spacing between transferred spots of cells, through control of the x–y motorized receiving stage and laser firing. An in situ energy meter is used to ensure that the appropriate fluence of ~1.0–1.3 J/cm2 is delivered to the ribbon. The in situ CCD camera shares the optical path with the laser as it passes through the final objective, thereby allowing visualization of cells on the ribbon, both prior to and following printing.
Figure 1.
Schematic diagram of gelatin-based laser direct-write (LDW) (adapted from [57]).
2.3. Preparation of the receiving dish and print ribbon
A 10 wt% gelatin solution was prepared using porcine skin-derived Type A gelatin (Sigma-Aldrich, St. Louis, MO) dissolved in heated Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA) to obtain a homogenous mixture. Petri dishes (100-mm diameter, FisherBrand, Pittsburgh, PA) were plasma cleaned for 1 minute, coated with 1.5 ml of poly-L-lysine (PLL) hydrobromide (0.1 mg/ml) (Sigma-Aldrich, St. Louis, MO) for 5 minutes, and allowed to air-dry in a laminar flow hood for 1 hour. Each PLL-coated receiving dish was then spin coated with 1 ml of 10 % gelatin (warmed to 60°C) at 4000 rpm, for 25 seconds. The dish was refrigerated (4 °C) for 5 minutes, then rinsed with 10 ml of DMEM (at 4 °C). The receiving dish was then incubated at 37 °C, 5% CO2, 95% RH for approximately 20 minutes. A flat, 50-mm diameter, UV-transparent quartz disk (“ribbon”) (Edmund Optics, Barrington, NJ) was cleaned with 70% ethanol, dried, and mounted on a bench-top spin coater. 1.5 ml of 20% gelatin in sterile cell culture grade water was warmed to 60 °C and pipetted onto the ribbon while spinning at 2000 rpm, for 20 seconds. The ribbon was then incubated in at 37 °C, 5% CO2, 95% RH for 3 minutes.
2.4. Laser direct-write of ES cells
To load the print ribbon, 1 ml of ES cells (2–5 × 106 cells/ml) was pipetted onto the 20% gelatin-coated ribbon. The cell-containing ribbon was placed in an incubator for 7 minutes and then put in a laminar flow hood for 4 minutes. After tilting to remove excess culture media, the ribbon was inverted and mounted approximately 0.75 mm above the 10% gelatin-coated receiving substrate/dish, so that the partially-encapsulated cells face the receiving substrate. The receiving substrate and mounted ribbon were secured on the x–y computer-controlled motorized stage, and a user-specified array program was executed, pulsing the laser once for each corresponding targeted transfer spot, depositing ES cells on the receiving dish.
2.5. Assessment of ES cell survival after laser direct-write
Cell viability after LDW was measured by live/dead stain made of calcein AM and ethidium homodimer (EthD-1) (Sigma Aldrich, St. Louis, MO) as described previously [64]. Following laser transfer, cells were incubated for 20 minutes to allow for initial attachment, at which time live/dead working solution (2 µM calcein AM and 4 µM EthD-1) was added. Cells remaining on the print ribbon following transfer were also stained with calcein AM and EthD-1. Non-laser transferred cells were used as controls. Imaging of stained cells was done on an epifluorescence microscope (Carl Zeiss, Inc., Thornwood, NY). Cell viability following laser transfer was quantified, both in the dish and on the ribbon, by calculating the percentage of cells fluorescing green (indicating viable cells), then normalizing with respect to control. This experiment was repeated twice and a standard deviation was calculated.
2.6. Observation of cell growth after laser direct-write
After mouse ES cells were transferred from the ribbon to the receiving dish, they were imaged on an inverted optical microscope (Carl Zeiss, Inc., Thornwood, NY) using phase contrast (10×). Once imaged, the receiving dishes were placed in an incubator for 30 minutes to allow for initial cell attachment, at which time 10 ml of cell culture medium warmed to 37 °C was added, and the dishes were returned to the incubator. For controls, mouse ES cells were plated using a pipette onto dishes that had been prepared as described in the receiving dish preparation, and treated in the same manner. Laser written cells and plated controls cultured in the LIF-free medium were monitored for 7 days. The cell morphology, cell growth and EB formation was observed and imaged.
2.7. Immunocytochemistry analysis of stem cell pluripotency
The maintenance of stem cell pluripotency following LDW was characterized using immunocytochemistry analysis of the stem cell marker, Oct4. Markers for the ectoderm (nestin), mesoderm (Myf-5), and endoderm (PDX-1) were characterized after 7 days, which allowed for spontaneous differentiation. Cells (LDW and controls) were treated as previously described. Standard immunocytochemistry protocol was employed to detect all the stem markers expression. For Oct4 staining mouse ES cells were incubated in maintenance medium containing LIF for three hours to allow cell adhesion following laser transfer, then fixed with 4% paraformaldehyde. Fixed cells were then rinsed with phosphate buffered saline (PBS), permeabilized in 0.1% Triton-X-100, and blocked with 5% FBS in PBS. ES cells were incubated with primary antibody for anti-Oct4 produced in rabbit (Sigma Aldrich, St. Louis, MO) and detected by the anti-rabbit secondary antibody (Sigma Aldrich, St. Louis, MO). Cells were also co-stained with 4',6-diamidino-2-phenylindole (DAPI) to reveal the nuclei in blue and to verify co-localization of Oct4 with cell nuclei.
Pluripotent stem cells have the ability to form cell types of all three germ layers (ectoderm, mesoderm, and endoderm). To evaluate the differentiation potential of cells after LDW, ES cells were fixed, permeabilized, and blocked, as described above after, and cultured for 7 days in LIF-free growth medium to allow for spontaneous differentiation. ES cells were incubated with three primary antibodies, for ectoderm (anti-nestin), mesoderm (anti-Myf-5), and for endoderm (anti-PDX-1) (Santa Cruz Biotechnology, Santa Cruz, CA), followed by the incubation with secondary antibodies (Invitrogen, Calrsbad, CA), Alexa Fluor® 594 for the detection of nestin, Alexa Fluor® 488 for Myf-5, and Alexa Fluor® 647 for PDX-1. Samples were observed using an ECLIPSE 80i epifluorescence microscope (Nikon, Melville, NY) using the filter sets for FITC, Texas Red, and Alex 647 (Micro Video Instruments, Avon, MA).
3. Results
3.1. Laser direct-write of ES cells
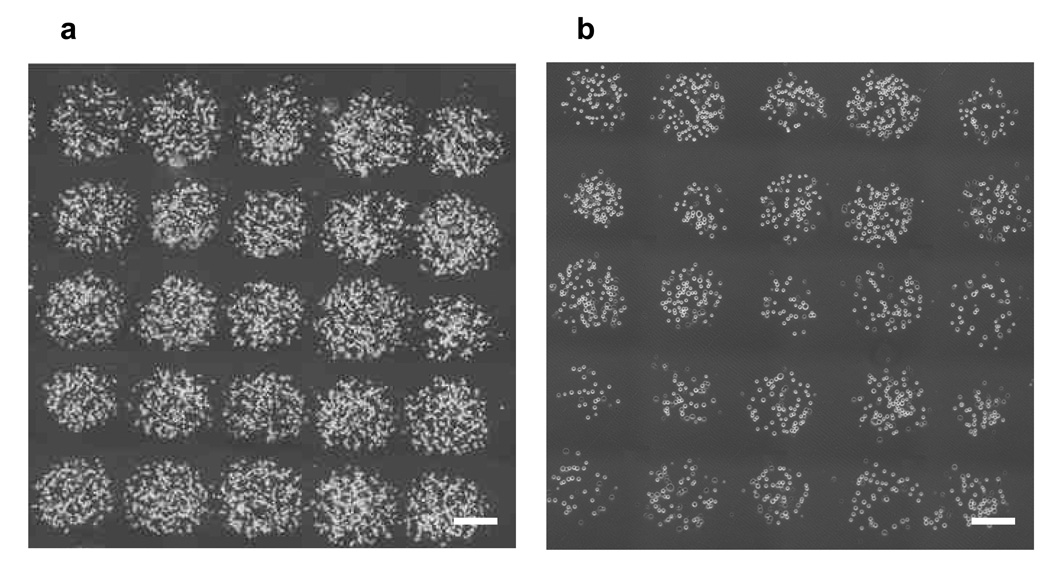
Mouse ES cells were successfully patterned using gelatin-based LDW (Figure 2). When the cell density on the ribbon was 5 × 106 cells/ml, the defined ES cell array was formed with a spot size of approximately 500 µm, spot spacing around 50 µm and ~ 130 cells/spot (Fig. 2a). When the cell density on the ribbon was reduced to 2 × 106 cells/ml, the spot size of ES cell array was approximately 500 µm, spot spacing around 200 µm, and ~ 48 cells/spot. These results demonstrated that LDW can rapidly fabricate multiple patterns of cell arrays, with different spacing and local cell density, for biological and biomedical applications.
Figure 2.
Laser direct-write of mouse ES cells in spotted arrays with cell density on the ribbon of (a) 5 × 106 cells/ml, and (b) 2 × 106 cells/ml. Scale bar = 200 µm.
3.2. Cell viability and growth potential after laser direct-write
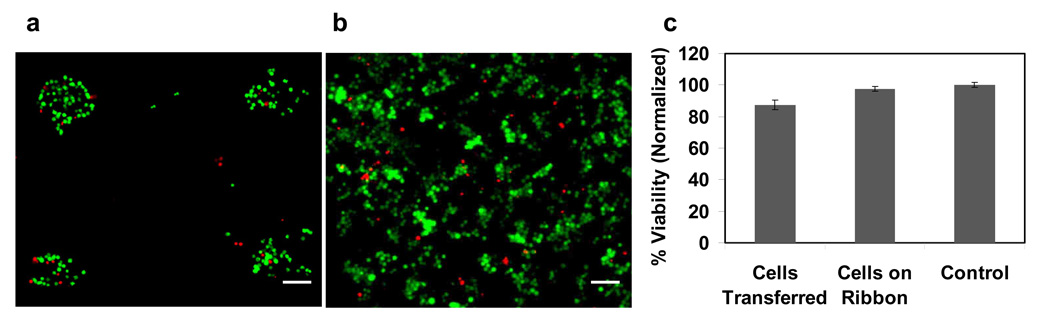
After LDW, the viability of mouse ES cells in the receiving dish and of cells on the ribbon was examined using live/dead stain with calcein AM and EthD-1. A relatively high percentage (~87%) of viable cells was observed following LDW in comparison to cells on the ribbon (Fig 3). Laser-transferred mouse ES cells in the receiving dish consist of approximately 87% viable cells (Fig. 3a), which represents only a 10% loss compared to viability on the ribbon (Fig. 3b). Quantitative analysis of these studies is shown in Figure 3c. These results support the fact that the laser energy used in transferring cells has little effect on ES cell viability as majority of ES cells maintained viable.
Figure 3.
Viability of mouse ES cells after laser direct-write was detected by live/dead stain (green: calcein AM stained live cells; red: EthD-1 stained dead cells). Fluorescence images of ES cells (a) in receiving dish after transfer, and (b) on the ribbon. (c) Quantitative analysis of percentage of live cells after laser direct-write. Scale bar = 100 µm.
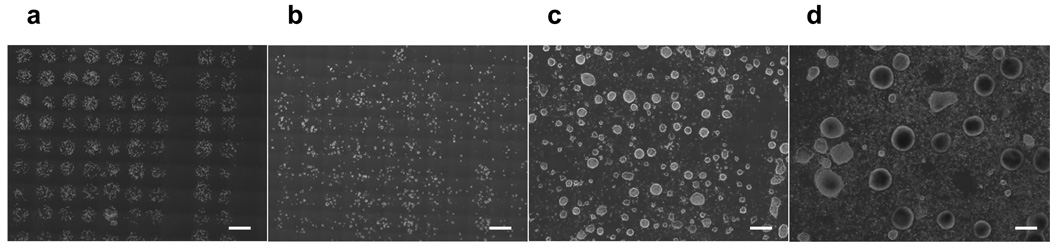
Long-term cell growth was further confirmed by culturing mouse ES cells in medium after LDW. A majority of laser transferred mouse ES cells adhered to the receiving dish and remained in their location after the addition of growth medium (Fig. 4a). On Day 1, mouse ES cells showed maintained registry within the pattern array while keeping their original phenotype (Fig. 4b). Mouse ES cells demonstrated adherent and proliferative morphology and formed EBs at Day 4 (Fig. 4c), and Day 7 (Fig. 4d).
Figure 4.
Growth profiles of mouse ES cells after laser direct-write on (a) day 0, (b) day 1, (c) day 4, and (d) day 7. Embryoid bodies formed after culturing for 7 days. Scale bar = 500 µm.
3.3. Maintenance of stem cell pluripotency after laser direct-write
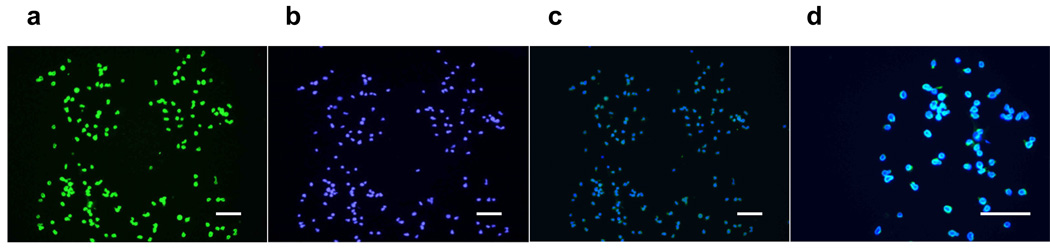
In addition to the demonstration of the ES cells’ ability to form EBs following LDW, the maintenance of pluripotency was further examined by the expression of the canonical stem cell pluripotency marker, Oct4, as well as in vitro differentiation after long-term culture. First, immunocytochemistry analysis of Oct4 expression in mouse ES cells was performed after three hours post LDW (Fig. 5a). Cells were co-stained with DAPI to reveal the nuclei in blue (Fig. 5b). The merged images demonstrated that after LDW, over 99% of mouse ES cells exhibit Oct4 immunoreactivity (green) in their nuclei (blue) (Fig. 5c and d). This illustrated that all mouse ES cells that survived the LDW process remained undifferentiated.
Figure 5.
Fluorescence images of mouse ES cells expressing (a) pluripotency marker, Oct4, and (b) DAPI stain to reveal the cell nuclei. (c) and (d) Merged image to demonstrate the expression of Oct4 in nuclei. Scale bar = 100 µm.
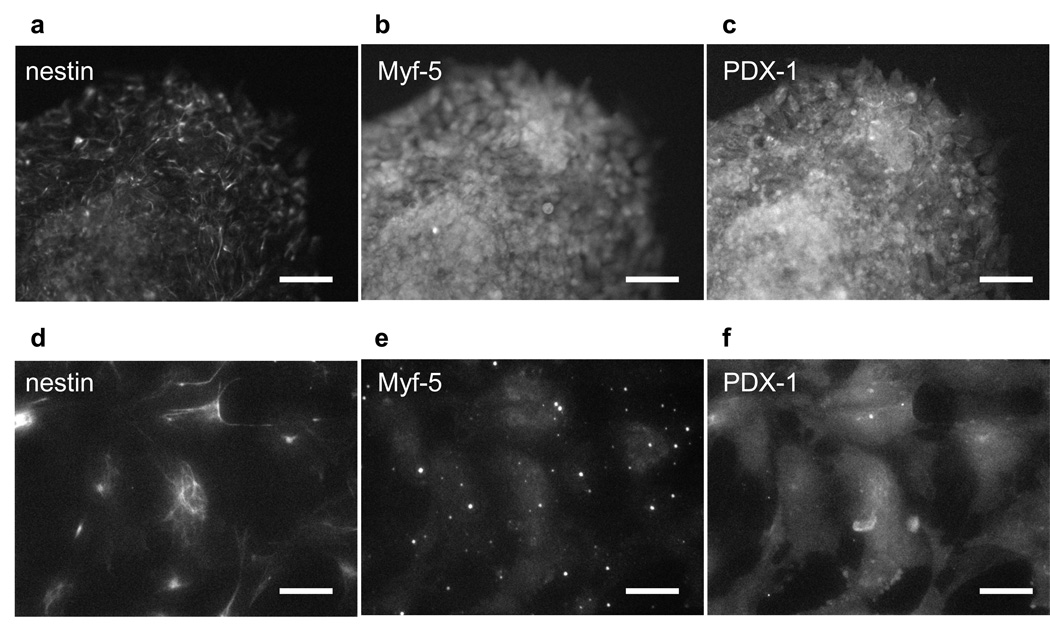
Furthermore, the differentiation potential of mouse ES cells following LDW was demonstrated by their ability to spontaneously differentiate into cell types expressing markers for ectoderm (nestin), mesoderm (Myf-5), and endoderm (PDX-1). After LDW, mouse ES cells were cultured in medium without LIF, which allows spontaneous differentiation. After 7 days in culture following LDW, mouse ES cells were tri-stained with antibodies recognizing nestin, Myf-5 and PDX-1. The outgrowths of cells from EBs differentiated spontaneously into multiple lineages. The expression of markers of neural cells (nestin), skeletal muscle cells (Myf-5), and pancreatic cells (PDX-1), demonstrated that mouse ES cells maintained their differentiation potential for ectoderm, mesoderm, and endoderm after LDW (Fig. 6).
Figure 6.
The differentiation potential of mouse ES cells after laser direct-write was revealed by the expression of markers of (a and d) ectoderm—nestin, (b and e) mesoderm—Myf-5, and (c and f) endoderm—PDX-1, after ES cells were cultured in medium without LIF for 7 days. (a–c) Scale bar = 100 µm. (d–f) Scale bar = 50 µm.
4. Discussion
Traditional LDW is a powerful tool for effectively printing a variety of cell types, including human dermal fibroblasts, HT-29 colon cancer cells, MG63 osteosarcoma cells, A431 epithelial carcinoma cells, bovine pulmonary artery endothelial cells, rat bone marrow stem cells, neural stem cells and B35 neuroblastoma cells, mouse C2C12 myoblasts and P19 embryonal carcinoma cells, and others [32, 56, 65–70]. All of the cell types involved in these studies maintained viability and retained the ability to proliferate after direct-write. In addition, laserprinted embryonal carcinoma cells exhibited minimal destruction to DNA and displayed differentiation potential to both neural and muscular lineages [32]. A recent LDW study utilizing a human colon cancer cell line, demonstrated that parameters in laser cell printing, such as fluence and cell density, can be modified and optimized in order to achieve high cell viability post-transfer [70].
In a recent study, a similar laser cell printing technique, termed laser induced forward transfer (LIFT), was able to print viable mouse ES cells onto a Matrigel™-coated growth surface. However, no attempts were made to quantify the potential effects on ES cell maintenance and differentiation [71]. In their study of ES cells, as well as other laser-based direct write techniques, the cellular patterns were constructed utilizing Matrigel™ basement membrane in the process, either to coat the print ribbon, to coat the receiving surfaces, or both [36]. In the directwrite process, Matrigel™ provides a laser interaction zone and a moist environment to facilitate successful transfer, as well as a scaffold rich with ECM proteins to encourage cell attachment and growth on the receiving substrate. However, Matrigel™ contains a number of intrinsic growth factors (e.g., bFGF, TGF-β, IGF-1, PDGF and EGF) [72], and the amount of these growth factors, as well as other constituent materials, varies significantly from lot-to-lot. Such variability in constituents, particularly in growth factors, can have significant implications on the cellular response. This is particularly important when using sensitive cells such as ES cells. The growth factors and constituent variability may cause unwanted and uncontrolled maintenance and differentiation of ES cells [73]. Despite the many advantages offered by Matrigel™, these shortcomings preclude, or greatly limit, its use in the direct-write of stem cells.
To address these shortcomings, we recently developed a gelatin-based LDW method for spatially-precise cellular patterning in which gelatin, rather than Matrigel™, is utilized on both the print ribbon and receiving substrate [57]. On the receiving substrate, gelatin is present temporarily to dissipate kinetic energy and maintain a moist environment to facilitate transfer. The gelatin layer is removed following LDW with incubation and culture medium exchange to provide an unobstructed growth surface for the patterned cells. This technique, as demonstrated using human dermal fibroblasts, patterned cells with high post-transfer viability (>90%), a high degree of registry to the desired initial pattern (within 5.6 ± 2.5 µm), and no evidence of doublestrand DNA damage, nor any effect on cellular behaviors in extended culture (e.g., morphology and fibronectin production) [57]. The optical set-up in the LDW process is truly unique, and provides significant advantages over many other cell patterning systems; it allows specific cell targeting and transfer by the user, as well as visual confirmation of cell deposition, post-transfer, in real time.
These ES cells represent a relatively sensitive cell type and have the potential to be differentiated into specialized cell types, which are composed of all three germ layers. Therefore, it was important to investigate whether the prior success of gelatin-based LDW could be applied to mES cells. Mouse ES cells used in this study have been adapted to grow in gelatin-coated tissue culture wares. This has been a conventional feeder-free culture method of mouse ES cells, as the gelatin coating facilitates mouse ES cell maintenance in the presence of LIF [74, 75]. In our experiments, the receiving substrate and long-term growth surface was spin coated with 1 ml of 10% gelatin prior to laser transfer, which proved to be an effective surface treatment for cell viability and proliferation. The 20% gelatin-coated quartz print ribbon ensured that the laser energy was non-damaging to the cells, yet sufficient for cell transfer. Live/dead staining showed high ES cell viability despite the use of laser energy to propel the cells from one surface to another.
After LDW, mouse ES cells maintained ES cell-specific characteristics. Mouse ES cells remained undifferentiated immediately after LDW, as indicated by expressing stem cell marker Oct4 in their nuclei, which is the canonical pluripotency marker of mouse ES cells. The Oct4 gene maintains mouse ES cells in an undifferentiated state [76]. In the absence of LIF, after LDW, mouse ES cells formed EBs, which is an essential step for in vivo-like stem cell differentiation. The maintenance of pluripotency was further confirmed by the ability to spontaneously differentiate into cell lineages expressing marker proteins of ectoderm (nestin for neuronal cells), mesoderm (Myf-5 for skeletal muscle cells), and endoderm (PDX-1 for pancreatic cells) after 7 days of culture. These results demonstrate that LDW can be utilized to pattern mouse ES cells such that the process itself does not cause premature and unwanted stem cell differentiation, and longer-term ES cell growth exhibits expected differentiation potential. This shows the efficacy of LDW for the spatial patterning of mES cells. LDW provides a promising platform for the investigation of how various geometric arrangements, spacing between spots of cells, patterns, and co-cultures with both normal and cancerous cells effect embryonic stem cell differentiation.
The spatial arrangements and interconnectivities of cells are critical concerns for the engineering of functional tissue complexes [54]. The heterotypic cell-cell interaction, and the ratio of cell populations in co-culture, greatly affect bioengineered tissue function [27]. The local cell density, cellular spacing or proximity, cell-cell contact and different types of neighboring cells are critical cellular microenvironmental elements to direct stem cell fates [9–17]. For example, Ruiz and Chen demonstrated that the geometry affects the differentiation of hMSCs into different lineages [20]. hMSCs that were spatially placed in different geometries (circle, square, rectangle, ellipses), using microcontact printing, showed differentiation towards adipogenesis in the center of the shapes and the concave region of ellipses, while osteogenesis was induced in the outer layer and convex edges. Phillippi et al. showed that muscle-derived stem cells patterned on a bone morphogenetic factor (BMP-2)-containing fibrin matrix via inkjet bioprinting underwent osteogenesis, while those on the surrounding area differentiated into myotubes [26]. Tang et al. performed spatial patterning of bone marrow stromal cells by micropatterning, which showed that stem cell differentiation was controlled by cell-cell contact, where the differentiation towards adipogenesis and osteogenesis was increased linearly with the number of cells in contact with each other [12]. All of these studies displayed how cellular patterning is essential and vital in order to investigate stem cell differentiation. However, it is often difficult to precisely place cells to recreate the complex in vivo cellular microenvironment. The ability to use LDW to deposit ES cells into defined arrays of various patterns makes this technique attractive for engineering cellular complexity in vitro. Cell density and proximity can be controlled using this printing technique to permit better study of interactions between cells and signaling molecules. Precise placement of various cell types to investigate interactions between cells of different type or lineage could also be achieved by this technique. Furthermore, the controlled and reproducible re-construction of two- and three-dimensional organization of cellular microenvironment can also be realized using this technique. LDW grants the precision and control of these cellular elements to recreate stem cell microenvironments for the investigation of stem cell fate decisions, which will favorably advanced stem cell research, and lead to functional tissue engineering.
Moreover, Williams has recently defined the ‘material’ as a single, well defined and characterized entity, and included engineered systems as ‘biomaterials’; which can range from engineered drugs and gene vectors, micropatterned cells, functionalized tissues, to printed organs [77]. LDW provides a valuable tool to precisely place these diverse ‘biomaterials’ into a welldefined, in vivo-like microenvironment. These engineered microenvironments can serve as an important platform for understanding how ‘biomaterials’ interactions affect functional tissue regeneration, leading to effective diagnosis or therapy for the treatment of human diseases.
5. Conclusions
In this study, we have successfully shown precise spatial patterning of mouse ES cells using gelatin-based LDW. Defined arrays of cells and precise control over cell position have been achieved. Mouse ES cells demonstrated high viability following LDW and continued to grow and form EBs. Laser direct-written ES cells maintained their pluripotency, as indicated by their nuclear expression of the canonical stem cell marker Oct-4 and their ability to in vitro differentiate into cell types of all three germ layers (ectoderm, mesoderm, and endoderm). Gelatin-based LDW provides a new and valuable cell-patterning technique that can be utilized to create precise and controlled stem cell microenvironments.
Acknowledgements
This work was partially supported by the NIH sponsored 1R56DK088217-01 (Corr/Xie), NSF sponsored CBET 0846270 (Xie), and UAlbany FRAP-A award (Xie).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pouton CW, Haynes JM. Embryonic stem cells as a source of models for drug discovery. Nat Rev Drug Discov. 2007;6:605–616. doi: 10.1038/nrd2194. [DOI] [PubMed] [Google Scholar]
- 2.Rubin LL. Stem cells and drug discovery: the beginning of a new era? Cell. 2008;132:549–552. doi: 10.1016/j.cell.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Janne J, Johan H, Petter B. Human embryonic stem cell technologies and drug discovery. J Cell Physiol. 2009;219:513–519. doi: 10.1002/jcp.21732. [DOI] [PubMed] [Google Scholar]
- 4.Keith WN. Cancer stem cells: opportunities for novel diagnostics and drug discovery. Eur J Cancer. 2006;42:1195–1196. doi: 10.1016/j.ejca.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Adams GB, Scadden DT. A niche opportunity for stem cell therapeutics. Gene Ther. 2007;15:96–99. doi: 10.1038/sj.gt.3303063. [DOI] [PubMed] [Google Scholar]
- 6.Daley GQ, Scadden DT. Prospects for stem cell-based therapy. 2008;132:544–548. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Singec I, Jandial R, Crain A, Nikkhah G, Snyder EY. The leading edge of stem cell therapeutics. Ann Rev Med. 2007;58:313–328. doi: 10.1146/annurev.med.58.070605.115252. [DOI] [PubMed] [Google Scholar]
- 8.Lysaght MJ, Jaklenec A, Deweerd E. Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng Part A. 2008;14:305–315. doi: 10.1089/tea.2007.0267. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- 10.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christian MM, Jeffrey CM, Christopher JD, Juan JdP, Bernard JVW, Sean PP. Engineering the stem cell microenvironment. Biotechnol Prog. 2007;23:18–23. doi: 10.1021/bp060350a. [DOI] [PubMed] [Google Scholar]
- 12.Tang J, Peng R, Ding J. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials. 2010;31:2470–2476. doi: 10.1016/j.biomaterials.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 13.El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442:403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 14.Peerani R, Onishi K, Mahdavi A, Kumacheva E, Zandstra PW. Manipulation of signaling thresholds in "engineered stem cell niches" identifies design criteria for pluripotent stem cell screens. PLoS One. 2009;4:e6438. doi: 10.1371/journal.pone.0006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem Cells. Mol Ther. 2007;15:467–480. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Advanced Drug Delivery Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurtley S. Location, location, location. Science. 2009;326:1205. doi: 10.1126/science.326.5957.1205. [DOI] [PubMed] [Google Scholar]
- 19.Chang HY. Anatomic demarcation of cells: genes to patterns. Science. 2009;326:1206–1207. doi: 10.1126/science.1175686. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vunjak-Novakovic G. Patterning stem cell differentiation. Cell Stem Cell. 2008;3:362–363. doi: 10.1016/j.stem.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SY, Park SY, Namgung S, Kim B, Im J, Kim JY, et al. Carbon nanotube monolayer patterns for directed growth of mesenchymal stem cells. Adv Mater. 2007;19:2530–2534. [Google Scholar]
- 23.Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol. 2008;19:534–540. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eshghi S, Schaffer DV. Engineering microenvironments to control stem cell fate and function. In: StemBook, editor. The Stem Cell Research Community, StemBook. Available from URL: doi/10.3824/stembook.1.5.1 http://www.stembook.org. [PubMed] [Google Scholar]
- 25.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Nat Acad Sci. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells towards muscle- and bone-like subpopulations. Stem Cells. 2008;26:127–134. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 28.Goubko CA, Cao X. Patterning multiple cell types in co-cultures: A review. Mater Sci Eng C. 2009;29:1855–1868. [Google Scholar]
- 29.W. Cris Wilson J, Thomas B. Cell and organ printing 1: Protein and cell printers. The Anat Rec A. 2003;272A:491–496. doi: 10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- 30.Boland T, Tao X, Damon BJ, Manley B, Kesari P, Jalota S, et al. Drop-on-demand printing of cells and materials for designer tissue constructs. Mater Sci and Eng C. 2007;27:372–376. [Google Scholar]
- 31.Chrisey DB. Materials processing: the power of direct writing. Science. 2000;289:879–881. doi: 10.1126/science.289.5481.879. [DOI] [PubMed] [Google Scholar]
- 32.Ringeisen BR, Kim H, Barron JA, Krizman DB, Chrisey DB, Jackman S, et al. Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 2004;10:483–491. doi: 10.1089/107632704323061843. [DOI] [PubMed] [Google Scholar]
- 33.Odde DJ, Renn MJ. Laser-guided direct writing of living cells. Biotechnol Bioeng. 2000;67:312–318. doi: 10.1002/(sici)1097-0290(20000205)67:3<312::aid-bit7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Liberski A, Zhang R, Bradley M. Laser printing mediated cell patterning. Chem Comm. 2009;48:7509–7511. doi: 10.1039/b914279g. [DOI] [PubMed] [Google Scholar]
- 35.Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R, et al. Laser printing of skin cells and human stem cells. Tissue Eng C: Methods. 2010;16(5):847–854. doi: 10.1089/ten.TEC.2009.0397. [DOI] [PubMed] [Google Scholar]
- 36.Schiele NR, Corr DT, Huang Y, Abdul Raof N, Xie Y, Chrisey DB. Laser-based direct-write techniques for cell printing. Biofabrication. 2010;2(3) doi: 10.1088/1758-5082/2/3/032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenthal A, Macdonald A, Voldman J. Cell patterning chip for controlling the stem cell microenvironment. Biomaterials. 2007;28:3208–3216. doi: 10.1016/j.biomaterials.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Moeller H-C, Mian MK, Shrivastava S, Chung BG, Khademhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008;29:752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tien J, Nelson CM, Chen CS. Fabrication of aligned microstructures with a single elastomeric stamp. Proc Nat Acad Sci. 2002;99:1758–1762. doi: 10.1073/pnas.042493399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostuni E, Kane R, Chen CS, Ingber DE, Whitesides GM. Patterning mammalian cells using elastomeric membranes. Langmuir. 2000;16:7811–7819. [Google Scholar]
- 42.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, et al. Engineering cell shape and function. Science. 1994;264(5159):696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 43.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 44.Elloumi Hannachi I, Itoga K, Kumashiro Y, Kobayashi J, Yamato M, Okano T. Fabrication of transferable micropatterned-co-cultured cell sheets with microcontact printing. Biomaterials. 2009;30:5427–5432. doi: 10.1016/j.biomaterials.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 45.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, et al. Layer-by-layer deposition of hyaluronic acid and poly-l-lysine for patterned cell co-cultures. Biomaterials. 2004;25:3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 46.Huang CP, Lu J, Seon H, Lee AP, Flanagan LA, Kim H-Y, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab on a Chip. 2009;9:1740–1748. doi: 10.1039/b818401a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan W, Desai TA. Microscale multilayer cocultures for biomimetic blood vessels. J Biomed Mater Res. 2005;72A:146–160. doi: 10.1002/jbm.a.30182. [DOI] [PubMed] [Google Scholar]
- 48.Chiu DT, Jeon NL, Huang S, Kane RS, Wargo CJ, Choi IS, et al. Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc Nat Acad Sci. 2000;97:2408–2413. doi: 10.1073/pnas.040562297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc Nat Acad Sci. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SH, Heinz AJ, Shin S, Jung Y-G, Choi S-E, Park W, et al. Capillary based patterning of cellular communities in laterally open channels. Anal Chem. 2010;82:2900–2906. doi: 10.1021/ac902903q. [DOI] [PubMed] [Google Scholar]
- 51.Yamato M, Konno C, Utsumi M, Kikuchi A, Okano T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2002;23:561–567. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda N, Shimizu T, Yamato M, Okano T. Tissue engineering based on cell sheet technology. Adv Mater. 2007;19:3089–3099. [Google Scholar]
- 53.Yousaf MN, Houseman BT, Mrksich M. Using electroactive substrates to pattern the attachment of two different cell populations. Proc Nat Acad Sci. 2001;98:5992–5996. doi: 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gartner ZJ, Bertozzi CR. Programmed assembly of 3-dimensional microtissues with defined cellular connectivity. Proc Nat Acad Sci. 2009;106:4606–4610. doi: 10.1073/pnas.0900717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. doi: 10.1016/j.actbio.2009.09.029. In Press. [DOI] [PubMed] [Google Scholar]
- 56.Schiele NR, Koppes RA, Corr DT, Ellison KS, Thompson DM, Ligon LA, et al. Laser direct writing of combinatorial libraries of idealized cellular constructs: Biomedical applications. Appl Surf Sci. 2009;255:5444–5447. [Google Scholar]
- 57.Schiele NR, Chrisey DB, Corr DT. Gelatin-based laser direct-write technique for the precise spatial patterning of cells. Tissue Engi C. doi: 10.1089/ten.tec.2010.0442. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robertson E, Bradley A, Kuehn M, Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986;323:445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- 59.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang X, Xie Y, Powell HM, James Lee L, Belury MA, Lannutti JJ, et al. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials. 2007;28:450–458. doi: 10.1016/j.biomaterials.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 61.Burdon T, Chambers I, Stracey C, Niwa H, Smith A. Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs. 1999;165:131–143. doi: 10.1159/000016693. [DOI] [PubMed] [Google Scholar]
- 62.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes & Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Xie Y, Kang X, Lee LJ, Kniss DA. Assembly of three-dimensional polymeric constructs containing cells/biomolecules using carbon dioxide. J Am Chem Soc. 2006;128:14040–14041. doi: 10.1021/ja066157u. [DOI] [PubMed] [Google Scholar]
- 65.Doraiswamy A, Narayan RJ, Harris ML, Qadri SB, Modi R, Chrisey DB. Laser microfabrication of hydroxyapatite-osteoblast-like cell composites. J Biomed Mat Res. 2007;80A:635–643. doi: 10.1002/jbm.a.30969. [DOI] [PubMed] [Google Scholar]
- 66.Doraiswamy A, Narayan RJ, Lippert T, Urech L, Wokaun A, Nagel M, et al. Excimer laser forward transfer of mammalian cells using a novel triazene absorbing layer. Appl Surf Sci. 2006;252:4743–4747. [Google Scholar]
- 67.Hopp Bl, Smausz T, Kresz N, Barna N, Bor Z, Kolozsvà ri L, et al. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2006;11:1817–1823. doi: 10.1089/ten.2005.11.1817. [DOI] [PubMed] [Google Scholar]
- 68.Patz TM, Doraiswamy A, Narayan RJ, He W, Zhong Y, Bellamkonda R, et al. Three-dimensional direct writing of B35 neuronal cells. J Biomed Mat Res. 2006;78B:124–130. doi: 10.1002/jbm.b.30473. [DOI] [PubMed] [Google Scholar]
- 69.Patz TM, Doraiswamy A, Narayan RJ, Modi R, Chrisey DB. Two-dimensional differential adherence and alignment of C2C12 myoblasts. Mater Sci Eng B. 2005;123:242–247. [Google Scholar]
- 70.Lin Y, Huang G, Huang Y, Tzeng T-RJ, Chrisey DB. Process-induced cell injury in laser direct writing of human colon cancer cells. Tissue Eng C. doi: 10.1089/ten.TEC.2009.0606. in press. [DOI] [PubMed] [Google Scholar]
- 71.Kattamis NT, Purnick PE, Weiss R, Arnold CB. Thick film laser induced forward transfer for deposition of thermally and mechanically sensitive materials. Appl Physics Lett. 2007;91:171120–171123. [Google Scholar]
- 72.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 73.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Nat Acad Sci. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davey RE, Onishi K, Mahdavi A, Zandstra PW. LIF-mediated control of embryonic stem cell self-renewal emerges due to an autoregulatory loop. FASEB J. 2007;21:2020–2032. doi: 10.1096/fj.06-7852com. [DOI] [PubMed] [Google Scholar]
- 75.Smith AG. Embryo-derived stem cells: of mice and men. Ann Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 76.Maurizio P, Hans RS. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 77.Williams DF. On the nature of biomaterials. Biomaterials. 2009;30:5897–5909. doi: 10.1016/j.biomaterials.2009.07.027. [DOI] [PubMed] [Google Scholar]