Summary
Differential cerebral metabolic effects of various hormone therapy formulations, and their associations with cognitive status, remain to be established. The principal aim of the current study wasto assess relationships between regional cerebral metabolism and estrogen-based hormone therapies. Postmenopausal women (n=53) at elevated risk for Alzheimer’s disease (AD) were on estrogen-containing hormone therapy for at least one year prior to enrollment in a prospective, randomized clinical trial. Subjects underwent an FDG-PET scan, along with neuropsychological, medical, and demographic assessments at time of enrollment, to be repeated one year following randomization to hormone therapy continuation versus discontinuation, and results from analyses of the baseline assessments are reported here. Across all subjects, years of endogenous estrogen exposure correlated most closely with metabolism in right superior frontal gyrus (p<0.0005). Women taking 17β-estradiol (E) performed 3 standard deviations higher in verbal memory than women taking conjugated equine estrogen (CEE), and their verbal memory performance positively correlated with metabolism in Wernicke’s (p=0.003) and auditory association (p=0.002) areas. Women taking progesterone-plus-estrogen had lower metabolism than women taking unopposed estrogen within the mesial and inferior lateral temporal regions (p<0.0005) and the inferior frontal cortex, contralateral to Broca’s area (p<0.0005). In conclusion, particular areas of relatively preserved metabolism were seen in women with more years of endogenous estrogen exposure, as well as in women taking estradiol-based formulations or estrogen therapies unopposed by progesterone, together suggesting regionally specific neuroprotective estrogenic effects.
Keywords: PET, estrogen formulations, postmenopausal, hormone therapy, AD, verbal memory
INTRODUCTION
Sex hormones exert a wide variety of effects upon brain development, aging, and function. Though estrogen influences have been the focus of particularly intensive investigation in this regard for decades, the nature of their impact upon the mature human brain remains a subject of substantial controversy. Answers to even some of the most basic questions – such as, whether the net influence of exogenous estrogen exposure on neurologic function is more harmful than beneficial – have been elusive. This in part reflects the complexity of estrogen-related effects, such that obtaining meaningful answers may require asking more specific questions: for example, whether the effects of estrogen hormone therapy (HT) exposure are influenced by the duration of endogenous estrogen exposure (i.e. length of reproductive life), whether the type of estrogen formulation (17β-estradiol (E) compared to conjugated equine estrogens (CEE)) influences brain function, and whether the use of concurrent progesterone has an effect on brain function or a moderating effect on the influence of estrogen on brain function. Experimentally addressing these more focused questions calls for prospective investigation of subpopulations characterized by greater degrees of homogeneity, and who are studied by more sophisticated neurological tools, than has typified previous clinical research in this field.
Support for neuroprotective roles of estrogen comes both from human epidemiologic studies (see below), and from experimental models of brain function in vitro and in vivo. Estrogen has long been known to influence several neurotransmitter systems, including those that are cholinergic (Luine, 1985; Dominguez et al., 2004; Kompoliti et al., 2004; Bora et al., 2005; Bartholomeusz et al., 2008; Ping et al., 2008), serotonergic (Kendall et al., 1981; Halbreich et al., 1995; Rubinow et al., 1998; Archer, 1999; Lasiuk and Hegadoren, 2007), adrenergic (Sar and Stumpf, 1981; Ungar et al., 1993; Wang et al., 2006), or dopaminergic (Roy et al., 1990). Hormones may act through inducing temporary changes in neuronal microstructure, such as dendritic spine formation (Woolley et al., 1990), affecting neurotransmitters and receptors (McEwen, 1981; Arnold and Breedlove, 1985; Meusburger and Keast, 2001), altering cell membranes (McEwen et al., 1991) and modifying cerebral glucose metabolism and blood flow (Namba and Sokoloff, 1984; Nehlig et al., 1985; Bishop and Simpkins, 1995; Eberling et al., 2000).
Evidence of effects of HT on human brain aging and cognition currently is mixed. Observational studies support a decreased risk of clinically diagnosed AD for women on HT (Maki, 2006). Surgical menopause has been associated with increased risk of cognitive impairment dementia later in life (Rocca et al, 2007). Surgically menopausal HT users have also been reported to have higher verbal memory performance compared to non-users in clinical trials with randomized, placebo-controlled design (Phillips and Sherwin, 1992), as well as cross-sectional studies (Nappi et al, 1999; Verghese et al., 2000). On the other hand, the large randomized control trial (RCT), Women’s Health Initiative Memory Study (WHIMS), has reported a nearly doubled risk for all-cause dementia for women on HT compared to women not receiving HT (Shumaker et al., 2004) and estrogen plus progestin therapy in the form of CEE and medroxyprogesterone acetate (MPA) specifically diminished verbal memory performance (Resnick et al., 2006; Maki et al 2007). More recently it was found that unopposed CEE initiated in postmenopausal women aged 65 years and older did not diminish verbal memory performance, though it was associated with somewhat lower spatial rotational ability (Resnick et al., 2009a). Relative metabolic effects of CEE vs. E also remain controversial (Maki and Resnick, 2001).
Evidence from functional brain imaging studies, on the other hand, has been more consistent. Neuroimaging studies in healthy aging women have demonstrated enhanced function of medial temporal structures, including the hippocampus, amygdala, and entorhinal cortex among estrogen users vs. non-users (Maki and Resnick, 2001). Studies of specific neurochemical systems in living human brain suggest that postmenopausal hormone therapy positively modulates both cortical serotonin binding (Moses et al., 2000) and cholinergic receptor density (Smith et al., 2001). Moreover, in postmenopausal women at genetic risk for AD, HT is associated with attenuated metabolic decline in cortical regions especially affected by AD pathology, such as posterior cingulate, superior temporal, and lateral temporal cortical regions (Rasgon et al., 2001; Rasgon et al., 2005; Rasgon et al., 2008).
The present study is part of an ongoing larger prospective randomized longitudinal clinical trial, in which postmenopausal women at increased risk for eventual development of dementia and on HT at time of study enrollment have been randomly assigned to continue or discontinue HT for at least 2 years. Cognitive status and regional cerebral metabolism are assessed at baseline and 2 years after randomization. An interim analysis of the data analyzed from the first 25 subjects to complete that trial has thus far indicated that women randomized to continue HT: 1) experience less significant decline of their posterior cortical metabolism (which is most marked in right inferior parietal cortex in women who discontinue HT), and 2) preserve anterior cortical metabolism relative to overall brain metabolism in the medial prefrontal area, which is not seen in their demographically matched counterparts who discontinue HT (Rasgon et al., 2008). In the study reported here, we describe the regional cerebral metabolism for the entire cohort of subjects for whom PET was performed at time of enrollment, for a relatively homogeneous group of postmenopausal women on HT. We specifically examine the relationships between brain metabolism and three hormonal factors potentially affecting geriatric cognitive decline: length of prior endogenous estrogen exposure, whether the HT regimen includes a progesterone compound, and type of estrogen formulation being taken at time of enrollment.
METHODS
The study in its entirety was approved by the Stanford University Human Research Protection Program and the Institutional Review Board at the University of California, Los Angeles (UCLA). Cognitively normal postmenopausal women ages 50–65 at risk for AD and receiving estrogen-containing hormone therapy (HT) for at least one year were enrolled. All subjects were receiving either estrogen therapy opposed or unopposed by progesterone through any route of administration (i.e. oral, transdermal, vaginal), and no changes in their HT regimen were implemented by the investigators prior to randomization or thereafter. Assessment of endocrine reproductive markers included gathering information on age at menarche and menopause, parity, use of hormonal contraception during reproductive years, duration of perimenopausal transition in relation to time of start of HT use, and type of menopausal symptoms. All subjects had a screening and baseline visit. The screening visit included psychiatric, physical, and neurological examination, and laboratory blood measures to determine eligibility for the study. During the physical and neurological examination, subjects were screened for Parkinson disease using the motor examination (items 18–31) of the Unified Parkinson’s Disease Rating Scale (Fahn et al., 1987). After the screening visit, subjects underwent positron emission tomography (PET) scan and neuropsychological testing.
The study required the following inclusion criteria: willingness to sign human subject consent prior to enrollment into the study; willingness to be randomized to continuation/discontinuation of HT, women ages 50–65 years of age; ≥ one year post complete cessation of menses; ≥ 1 year current HT use; ≥ 8 years of education, and adequate visual and auditory acuity to allow neuropsychological testing. In addition, all subjects were required to be at elevated risk for eventual development of dementia, as defined by one or more of the following risk factors: personal history of mood disorder; personal history of hypothyroidism; family history of AD; documentation of the apolipoprotein (APOE) allele ε4, conferring increased risk for AD.
Because cognitive decline may be caused by a wide variety of conditions having different cerebral metabolic signatures, we excluded subjects with impairment from numerous causes (e.g., vascular disease, etc.), to enrich for those at increased risk specifically for AD. Volunteers with a history of TIAs, carotid bruits, or lacunes on MRI scan were excluded. Other exclusion criteria included evidence of current depression as determined by a score of ≥8 on the 17-item Hamilton Depression Rating Scale (Hamilton, 1960), history of drug or alcohol abuse, contraindication for MRI scan (e.g., metal in body, claustrophobia), history of mental illness (excluding mood disorders), or significant cognitive impairment, as evidenced by impairment in daily functions and/or MMSE <24 (Folstein et al., 1975), history of myocardial infarction within the previous year or unstable cardiac disease, significant cerebrovascular disease, as evidenced by neurological examination, uncontrolled hypertension (systolic BP >170 mmHg or diastolic BP >100 mmHg), history of significant liver disease, clinically significant pulmonary disease, diabetes, or cancer. Subjects were excluded if they already had possible or probable AD (McKhann et al., 1984) or any other dementia (e.g., vascular, Lewy body, frontotemporal), or evidence of neurologic or other physical illness that could be expected to imminently produce cognitive deterioration. Subjects were also excluded if they used drugs with potential to significantly affect psychometric test results, including centrally active beta-blockers, narcotics, clonidine, anti-Parkinsonian medications, antipsychotics, benzodiazepines, systemic corticosteroids, medications with significant cholinergic or anticholinergic effects, anticonvulsants, warfarin, or sporadic use of phytoestrogen-containing products, which may produce estrogen-like agonist and antagonist effects (Polkowski and Mazurek, 2000; Vincent and Fitzpatrick, 2000).
Hormone levels were assayed at baseline. Follicle-stimulating hormone (FSH) was measured by immunoassay. Estradiol was measured using the enzyme-linked immunosorbent assay (Bio-Quant Inc., San Diego, CA). Limits of detection were 0.10 mIU/mL for FSH, and the minimum detectable concentration of the estradiol assay was 2 standard deviations plus mean of a zero standard, which was estimated to be 0.4pg/ml.
PET scan acquisition and analysis
Participants were required to fast 4–6 hours for the PET imaging studies. The [F-18] fluorodeoxyglucose (FDG) PET method was used for the determination of patterns of regional cerebral metabolism. An intravenous line was placed 10 to 15 minutes prior to injection of 370 MBq FDG. Uptake of FDG proceeded while subjects were supine with low ambient lighting and sound, eyes and ears unoccluded. Scans were performed 40 minutes post FDG injection using a CTI/Siemens (Siemens Corp, Hoffman Estates, Il) HR+ tomograph (63 image planes).
We analyzed PET data by both a standardized volume of interest method as well as the statistical parametric mapping method developed by Friston, Holmes and colleagues (Friston et al., 1995; Friston et al., 1995). Briefly, images from all subjects were co-registered and reoriented into a standardized coordinate system using the SPM2 software package courteously provided by the members of the image analysis team at the Wellcome Department of Cognitive Neurology, Functional Imaging Laboratory (London, UK). Data were spatially smoothed, and normalized to mean global activity as previously described (Silverman et al., 2007), with the exception that a 12mm (full-width half-maximum) smoothing filter was applied to images prior to statistical analysis. The set of pooled data were then assessed with the t- statistic on a voxel-by-voxel basis, to identify the profile of voxels that significantly covaried with parameters characterizing each subject.
In addition, relative quantification of regional brain activity was performed using software originally developed at UCLA dedicated to the visual display and quantitative analysis of brain PET data, which has been cleared for those purposes by the U.S. Food and Drug Administration and is commercially available as the clinical package NeuroQ™ (Syntermed Inc., Atlanta). The software implements an algorithm for automatically measuring, after correction for tissue-based attenuation, the number of radioactive events emitted by a positron source (gamma-ray lines of coincidence) per second detected by the PET scanner, emanating from pixel locations assigned by a computerized reconstruction algorithm as falling within each standardized region of interest (sROI). Mean pixel activity values were calculated within each of 240 sROI’s defined throughout those transaxial planes across the field of view in which brain tissue was represented, following the transformation of each PET scan to a template space by a method previously described by Tai et al. (1997). The sROI’s were then automatically grouped into 47 clusters of regions falling within structurally defined boundaries corresponding to distinct neuroanatomical (e.g. left inferior parietal lobule) or functional (e.g., Broca’s area) standardized volumes of interest (sVOI’s), and the mean activities for each of these volumes calculated. Finally, all mean activity values were automatically normalized to the mean pixel activity measured throughout that brain scan, or to the mean activity of an individual user-specified reference sVOI within that scan.
For the purpose of guiding interpretations of statistical analyses, a priori hypotheses were established for six specific cortical volumes bilaterally, based on their known association with physiologic memory processing and/or pathologic involvement in neurodegenerative disorders: left and right medial temporal sVOI’s, including amygdala and hippocampus/parahippocampal areas, were chosen for their established role in “new learning” (Squire et al, 1992; Stern et al., 1996; Tulving et al., 1996; Gabrieli et al., 1997). Two sVOI’s of the dorsolateral prefrontal cortex (DLPFC) were chosen for known involvement in both encoding and working memory/retrieval. The posterior cingulate cortex was chosen for its role in encoding (Shallice et al., 1994) and retrieval (Tulving et al., 1996; Fletcher et al., 1998). Finally, the parietotemporal and inferior lateral temporal cortical VOI’s were assessed due to their importance in language, semantic memory and related memory deficits in AD54,55 (Martin and Fedio, 1983; Wiggs et al., 1999). The selected volumes have also been implicated as biomarkers of impending or actual cognitive decline in functional imaging studies. Consistent patterns of hypometabolism and hypoperfusion in the parietal, temporal, and posterior cingulate cortices (Small et al., 1989; Smith et al., 1992; Small et al., 1995; Reiman et al., 1996; Ibáñez et al., 1998) have been observed in asymptomatic persons at increased genetic risk for AD, as well as in patients with AD.
Results were reported in terms of locations of the most significant effects (regionally, and/or in x,y,z Talairach-style millimeter coordinates). The probability of finding by chance an effect in any volume containing a voxel of maximal significance by statistical parametric mapping, or in any sVOI, was assessed after a Bonferroni-type adjustment for multiple comparisons, with a correspondingly less harsh correction required for the 12 volumes (6 left, 6 right) specified by a priori hypotheses, and effects at each region of the brain were considered significant if that adjusted probability was less than 0.05. For large areas, corrected values based upon the number of contiguous voxels achieving a pre-specified level of significance were additionally provided. For sVOI analyses, based on the number of regions, effects in the sVOI’s specified a priori were considered significant for pre-adjusted p≤0.004, while effects in other sVOI’s were considered significant for pre-adjusted p≤0.001; sVOI analyses serving specifically to support another pre-established result were considered corroborative for p<0.05. Analyses were also controlled for examination of multiple effects by using analysis of variance (ANOVA) methods with all three main hormonal effects entered into the statistical model (see below).
Neuropsychological assessment
An extensive neuropsychological evaluation was conducted at the baseline and final visits by the study neuropsychologist. Tests in the neuropsychological battery were chosen based on prior studies that indicated their ability to predict cognitive decline (Hänninen et al., 1995) and sensitivity to predict cognitive change (Small et al., 1995). The battery included the Auditory Consonant Trigrams (ACT), Benton Visual Retention Test (BVRT), Boston Naming Test (BNT), Color Trail Making Test (Color Trails 1 & 2), Delis Kaplan Executive Function System (DKEFS), Rey-Osterrieth Complex Figure Test (RCFT), Wechsler Adult Intelligence Scale-3rd Edition (WAIS-III) - Digit Span, Symbol Coding, and Letter Number Sequencing subtests, and the Wechsler Memory Scale-III (WMS)- Logical Memory I & II subtests. The Wechsler Abbreviated Scale of Intelligence was used to characterize intelligence (IQ). Individual test scores were z-transformed and parceled into the cognitive domains of attention, verbal memory, visual memory, word finding and category fluency, and executive functioning. Lastly, the Memory Function Questionnaire (MFQ) was administered to assess subjective memory functioning.
Reproductive endocrine variables
Information was obtained from all subjects and supported by documentation from their primary health care providers on the duration of endogenous and exogenous exposure to reproductive hormones. Duration of endogenous exposure was calculated by subtracting age at menarche from the age at menopause, duration and type of hormone therapy was recorded as well as type of menopause, parity and past use of steroidal contraception.
RESULTS
Eighty-one subjects were initially recruited; baseline data from 53 subjects were included in the final analysis; the other 28 subjects had incomplete data sets caused by schedule conflicts, technically limited MRI or PET imaging, or due to a variety of reasons not noted during initial screening (hysterectomy at a young age, discontinued HT prior to randomization, TIA, claustrophobia emerging during imaging, severe mood episode with menopause transition, congenital ovarian agenesis, progesterone-only therapy). Demographic and personal characteristics of these 53 subjects are presented in Table 1. All subjects had MMSE and cognitive performance scores within the normal range for persons of their same age and educational level. There was a history of depression in 41 subjects and 26 were currently taking antidepressants.
Table 1. Demographics and Hormone Therapy Details.
The number of subjects (N), mean, standard deviation (SD), and range or category are given above. HT=hormone therapy, E=estradiol, CEE=conjugated equine estrogen, P=progesterone, MPA=medroxyprogesterone acetate, AD= Alzheimer’s Disease
| Study Sample Demographics and Clinical Characteristics (N=53) | |||
|---|---|---|---|
| Mean | SD | Range | |
| Age | 57.8 | 4.8 | 49, 69 |
| Years of Education | 16.3 | 2.0 | 12, 20 |
| Length of HT | 9.7 | 5.7 | 1, 22.5 |
| Age at Menarche | 13.0 | 1.6 | 9, 16 |
| Age at Menopause | 47.2 | 5.7 | 29, 58 |
| Endogenous Estrogen Exposure | 34.2 | 5.4 | 17, 44 |
| BMI | 25.5 | 3.6 | 19, 37 |
| Estradiol (pg/mL) | 44.6 | 32.9 | 9, 185 |
| FSH (mIU/mL) | 60.7 | 23.7 | 22.7, 112.2 |
| N | |||
| Natural or Surgical Menopause | Natural = 33, Surgical = 20 | ||
| APOE Type | E2/E3=3, E2/E4=2, E3/3=30, E3/4=16, E4/4=2 | ||
| Family History of AD (FamHxAD) | FamHxAD+ = 25, FamHxAD− = 28 | ||
| Type of HT | E+P = 19, E+MPA = 5, CEE+MPA = 9, CEE+P = 2, Unopposed E = 11, Unopposed CEE = 7 | ||
| Vasomotor Symptoms | Present = 38, Not Present =15, | ||
All times are expressed in years. HT=hormone therapy, E=estradiol, CEE=conjugated equine estrogen, P=progesterone, MPA=medroxyprogesterone acetate, AD=Alzheimer’s Disease
Endogenous Estrogen Exposure
As a potential factor related to cognitive status in postmenopausal women, duration of pre-menopausal endogenous estrogen exposure was explored by statistical parametric mapping for associations with regional cerebral metabolism. The total duration of endogenous estrogen exposure (i.e. age at menopause minus age at menarche) positively correlated most closely with metabolism in the posterior part of the right superior frontal gyrus (t=5.64, p<0.0005 at voxel of peak significance: 22,32,58), remaining significant after full correction for multiple comparisons (p=0.005), as well as after adjustment for variance in age and education (t=5.19, p<0.0005; p=0.024 after multiple comparison correction). The sVOI analyses corroborated SPM analyses in the right superior frontal gyrus (r=0.27, p=0.047). Again adjusting for age and education, years of endogenous estrogen exposure also positively correlated with activity in the right peri-insular area at the axial level of the anterior commissure (48,16,0; t=5.29, p<0.0005, p=0.017 after multiple-comparison correction); an area of similar size and significance was seen in the contralateral hemisphere but centered slightly more superiorly and anteriorly, in the left inferior frontal gyrus overlapping with Broca’s area (−48, 20, 14; t=5.28, p<0.0005, p=0.017 after multiple-comparison correction). Endogenous estrogen exposure also tended to positively correlate with metabolism in the left parahippocampal gyrus (−28,−30,−20; t=4.05, p<0.0005), area in the vicinity of the subgenual anterior cingulate (−4,44,−14; t=4.25, p<0.0005), and an area of the left superior lateral temporal cortex (−48,−24,10; t=4.58, p<0.0005) (Figure 1). The sVOI analyses also corroborated the correlation with metabolism in the left superior lateral temporal cortex (r=0.28, p=0.037). Not surprisingly, age of menopause, which was highly correlated with the duration of endogenous estrogen exposure (r=0.92, p<0.00001), also positively correlated with metabolism in these regions.
Figure 1. Metabolic Correlation with Years of Endogenous Estrogen Exposure.
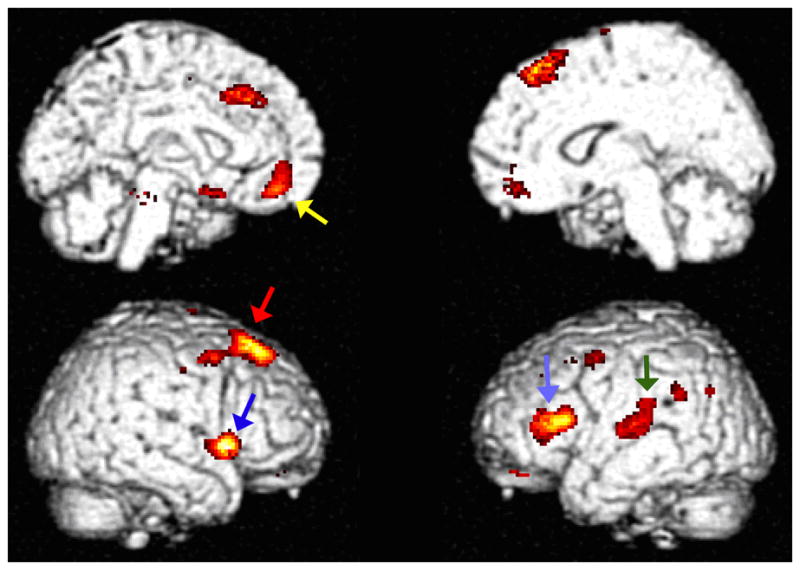
All voxels positively correlating with endogenous estrogen exposure at p<0.001 are shown in color. Years of endogenous estrogen exposure correlated with metabolism in areas within the right superior frontal gyrus (red arrow), right peri-insular region (dark blue), left inferior frontal gyrus (light blue), left parahippocampal gyrus (not visible from these surface rendering views), left subgenual anterior cingulate (yellow arrow), left superior lateral temporal gyrus (green arrow) (p<0.0005).
Hormone Replacement Therapy Formulations
17β-estradiol and Conjugated Equine Estrogen Formulations
Among 53 subjects, 35 subjects were taking formulations of 17β-estradiol (E) and 18 subjects were taking conjugated equine estrogen (CEE) at time of recruitment. Groups were similar with respect to years of education (av±SD; 17±2.0 vs. 16±1.9), age at menarche (13±1.6, 13±1.7), age at menopause (48±5.2, 46±6.7), age at the time of study recruitment (57±4.5, 58±5.3), and length of HT (9±5.7, 11±5.7). Approximately 2/3 of the subjects in the E and CEE groups had opposed therapy (24 of 35, and 11 of 18, respectively). The proportion of subjects taking E and CEE were also similar for the natural and surgical menopause groups, constituting 65% E for surgical group (13 of 20), and 67% E for the women who underwent natural menopause (22 of 33).
Group Differences in Cerebral Metabolism
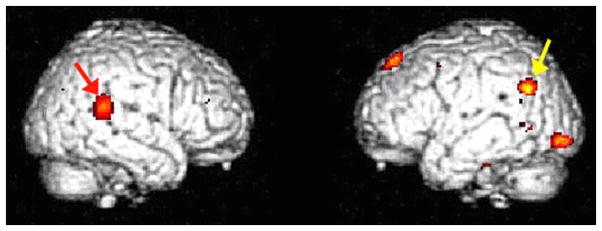
Analyses by statistical parametric mapping directly comparing E-users to CEE-users showed that levels of metabolism in parietotemporal cortex in the vicinity of Wernicke’s area of the left cerebral hemisphere, and the superior temporal gyrus in the auditory association area of the right cerebral hemisphere, were significantly greater in E-users (t=4.41, t=4.67, respectively; p<0.0005) (Figure 2).
Figure 2. Specific areas of Greater Metabolism in E-users Compared to CEE-users.
Metabolism in the (left) Wernicke’s area (yellow arrow) and right superior temporal gyrus (red arrow) was greater in E-users compared to CEE-users (p<0.0005). Voxels with significance p<0.005 are shown above in color.
Neuropsychologic performance and cerebral metabolic correlates
In examining associations with neuropsychologic performance, we found that E-users attained scores for the verbal memory cognitive domain that were 3 SD higher than those of CEE-users (p=0.007). We therefore examined associations of verbal memory performance with cerebral metabolism.
Across all subjects, statistical parametric mapping analyses indicated that verbal memory performance positively correlated most strongly with the magnitude of cortical metabolism in the right superior frontal gyrus (t=3.60, p<0.0005 at voxels of peak significance: 10,32,54), in the (left) Wernicke’s area (t=2.86, p=0.003 at voxel of peak significance: −50,−80,34) and with metabolism of the right auditory association area (t=3.00, p=0.002 at voxel of peak significance: 70,−36,14). When adjusting for age, education, and years of endogenous estrogen exposure, similar correlations were seen (t=2.95, p=0.002; t=2.50, p=0.008; t=2.58, p=0.007, respectively). The correlations tended to be somewhat stronger among E-users with respect to metabolism in both the Wernicke’s (t=3.66, p<0.0005; t=3.48, p<0.001 after adjusting for age, education, and years of endogenous estrogen exposure) and auditory association (t=3.30, p=0.001; t=2.94, p=0.003 after adjusting for age, education, and years of endogenous estrogen exposure) areas, while CEE-users alone demonstrated the most significant correlation with metabolism of the superior frontal region (t=4.37, p<0.0005 after adjusting for age, education, and years of endogenous estrogen exposure)..
Estrogen-Progesterone versus Unopposed Estrogen Replacement Therapies
Among the 53 subjects taking estrogen-based hormone replacement therapy, 18 women were taking unopposed estrogen and 35 were taking progesterone-plus-estrogen replacement therapies, of whom 14 were taking MPA. Groups were similar in years of education (av±SD; 16±1.5 vs. 16±2.2), age at menarche (13±1.8, 13±1.5), age at recruitment (58±5.6, 58±4.4), and length of HT (10±5.7, 9±5.7) respectively. They differed in age at menopause (43±7.0, 49±3.7), (with the younger average age of the unopposed estrogen group primarily reflecting prematurely induced menopause secondary to surgical removal of uterus and/or ovaries in these subjects), and thus in mean number of years of endogenous estrogen exposure (31±6.4, 36±3.9). A similar proportion were taking E versus CEE: 69% were on estradiol, (24 of 35) in the progesterone-plus-estrogen group, and 61% were on estradiol in the unopposed group (11 of 18).
Analyses by statistical parametric mapping showed that postmenopausal women taking progesterone-plus-estrogen had lower metabolism than postmenopausal women taking unopposed estrogen within the right mesial temporal region (t=4.51, p<0.0005 at voxel of peak significance: 38,20,−36) and left inferior lateral temporal cortex (t=4.64, p<0.0005 at voxel of peak significance: −48,22,−34); the voxel of peak significance within the right mesial temporal region was in the largest cluster (3085 contiguous voxels at p<0.01, pcorrected=0.002) (Figure 3). After adjusting for years of endogenous estrogen exposure, the right and left temporal relative hypometabolism in progesterone-plus-estrogen users remained significant (t=4.01, p<0.0005; t=4.42, p<0.0005, respectively). The sVOI analyses corroborated SPM analyses, demonstrating that progesterone-plus-estrogen users had lower mean metabolism in corresponding temporal regions than unopposed estrogen users in the right mesial temporal cortex (rMT) t=2.61, p=0.01, and a trend towards lower metabolism in the left inferior lateral temporal cortex (liLT) t=1.72, p=0.09 (which became more significant after adjusting for endogenous estrogen exposure; see below.) Only 2 of 35 progesterone-plus-estrogen users had rMT metabolism levels greater than mean rMT metabolism of unopposed estrogen users and, conversely, only 5 of 18 unopposed estrogen users had rMT metabolism levels lower than mean rMT metabolism of progesterone-plus-estrogen users (Figure 4).
Figure 3. Posterior Temporal Areas of Decreased Metabolism in Progesterone-Plus-Estrogen Users Compared to Unopposed Estrogen Users.
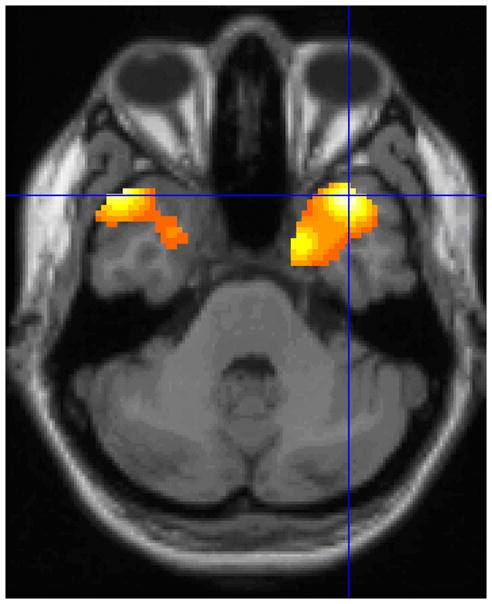
SPM analyses demonstrated right mesial temporal (t=4.51, p<0.0005) and left inferior lateral relative hypometabolism (t=4.64, p<0.0005) in progesterone-plus-estrogen users compared to unopposed estrogen users. Voxels with significance p<0.01 are shown above in color. The crosshairs intersect at voxel of peak significance (38,20,−36).
Figure 4. Lower Right Mesial Temporal Metabolism in Progesterone-Plus-Estrogen Users Compared to Unopposed Estrogen Users.
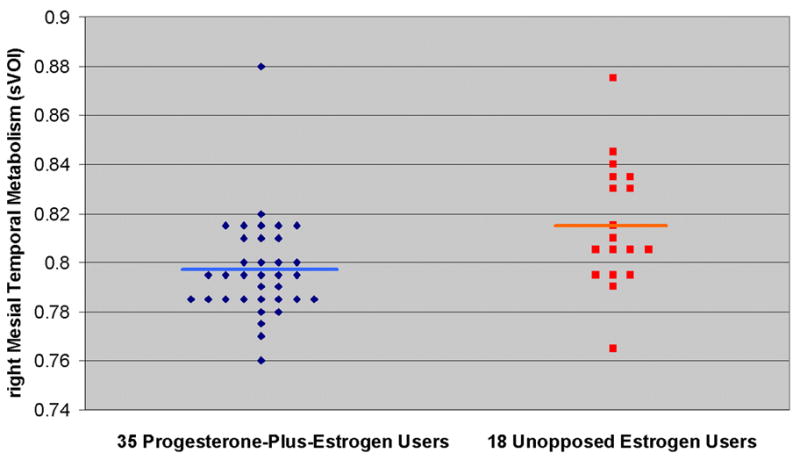
In the individual-subject plot above, 35 postmenopausal women on progesterone-plus-estrogen therapy are represented by blue diamonds and the average right mesial temporal metabolism, assessed by sVOI analysis, is shown in light blue (0.797). 18 postmenopausal women on unopposed estrogen therapy are represented by red squares and the average right mesial temporal metabolism is shown in orange (0.815). Progesterone-plus-estrogen users had a lower mean rate of metabolism than unopposed estrogen users (p=0.012), a difference that became more significant after correcting for years of endogenous estrogen exposure (p=0.007).
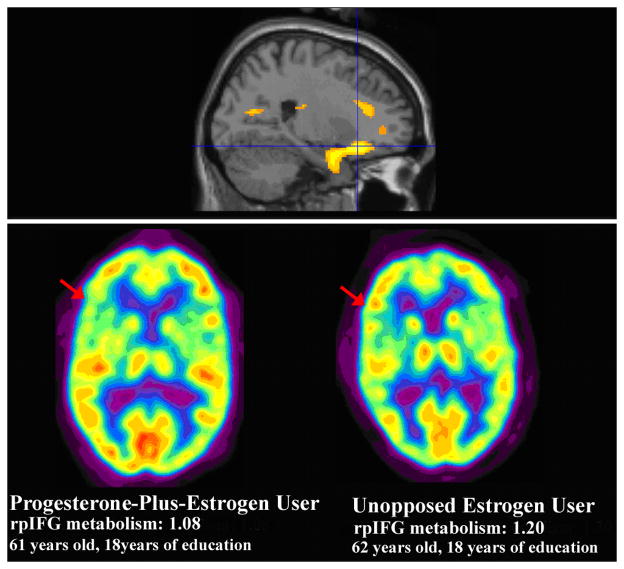
Analyses by statistical parametric mapping also demonstrated that women taking progesterone-plus-estrogen had lower metabolism than women taking unopposed estrogen in the right inferior frontal cortex (t=3.65, p<0.0005 at voxel of peak significance: 24,26,−14). The significance of this difference again persisted when statistical correction for the disparate number of years of endogenous estrogen exposure was taken into account (t=3.69, p<0.0005) and occurred in the largest regional cluster (1747 contiguous voxels at p<0.005, pcorrected=0.005) (Figure 5). The sVOI analyses corroborated SPM analyses, with progesterone-plus-estrogen users having lower mean metabolism than unopposed estrogen users in the right posterior inferior frontal gyrus (rpIFG), contralateral to Broca’s area in the left hemisphere (av±SD 1.14 ±0.03vs 1.16±0.03, t=2.29, p=0.026, a difference that became more significant following statistical correction for number of years of endogenous estrogen exposure: F=9.39, p=0.004). Only 3 of 18 unopposed estrogen users demonstrated levels of metabolism in this region that were lower than the mean metabolism of progesterone-plus-estrogen users and, conversely, only five of the 35 progesterone-plus-estrogen users had metabolic levels that were greater than the mean metabolism of unopposed estrogen users.
Figure 5. Frontal Areas of Decreased Metabolism in Progesterone-Plus-Estrogen Users Compared to Unopposed Estrogen Users.
SPM analyses with correction show right inferior frontal relative hypometabolism in progesterone-plus-estrogen users (n=35) compared to unopposed estrogen users (n=18) (top row, sagittal view) (t=3.69, p<0.0005). All voxels shown in color have p<0.005. In the second row of the figure, metabolism in the right posterior inferior frontal gyral (rpIFG) area of a 61 year old postmenopausal woman taking progesterone-plus-estrogen (left) is compared to metabolism of a 62 year old postmenopausal woman taking unopposed estrogen (right).
Potential interactions of opposed versus unopposed estrogen with the other major variables discussed above (years of endogenous estrogen exposure and E vs. CEE) relative to rates of metabolism in the standardized VOI’s were examined with ANOVA. Taking into account endogenous estrogen exposure, sVOI analyses corroborated significantly lower metabolism in right mesial temporal, and left inferior lateral temporal cortex, in women taking opposed estrogen regimens (F=7.788, p=0.007; F=4.199, p=0.046, respectively). Simultaneously taking into account years of endogenous estrogen exposure and E vs CEE use, sVOI analyses again corroborated significantly lower metabolism in these regions (F=7.624, p=0.008; F=4.122, p=0.048).
Looking at the other direction, women taking unopposed estrogen did not have any areas demonstrating significantly lower metabolism after correction, than women taking progesterone-plus-estrogen formulations.
DISCUSSION
Relative regional cerebral hypometabolism of 53 postmenopausal women on HT selected to be at heightened risk of eventually developing dementia was associated with a presumptive historical risk factor for cognitive decline: shorter endogenous estrogen exposure. Years of endogenous estrogen exposure positively correlated most robustly with metabolism in the right superior frontal gyrus, and also in right peri-insular area, left inferior frontal gyrus, left parahippocampus, subgenual anterior cingulate, and left superior lateral temporal cortex. Additionally, in the present analysis E-users had greater metabolism than CEE-users in the Wernicke’s area and the auditory association area. Verbal memory was better in postmenopausal women taking E than CEE, and cognitive differences were associated with metabolic differences in these receptive language and auditory association areas for E-users and in right superior frontal gyrus for CEE-users. Postmenopausal women taking estrogen-plus-progesterone users had lower metabolism than postmenopausal women taking unopposed estrogen in the right mesial temporal region, left inferior lateral temporal cortex, and right inferior frontal cortex. Overall, particular areas of relatively preserved metabolism were seen in women with more years of endogenous estrogen exposure, as well as in women taking estradiol-based formulations or estrogen therapies unopposed by progesterone, together suggesting regionally specific neuroprotective estrogenic effects. The results presented here provide support for estrogen having a neuroprotective role in brain regions affected during aging.
Several of the regions in the current study have also been implicated in a number of previous studies on hormonal influences involving neuroimaging. Relevant to our identification of hormone related activity in superior and inferior frontal gyri, similar or overlapping regions of frontal cortical involvement have been identified as positively correlating with HT: orbital gyrus of the frontal cortex (Eberling et al., 2004), right superior frontal gyrus (Ottowitz et al., 2008), left middle/superior frontal cortex (Persad et al., 2009) and left medial frontal cortex (Maki and Resnick, 2000; Persad et al., 2009). Initiation of E/HT has also been shown to result in more effective activation of the prefrontal cortex during fMRI visual working memory tasks, suggesting functional plasticity in these frontocortical working memory systems among postmenopausal women that can be altered by E/HT (Smith et al., 2006). In a recent double-blind, placebo-controlled study, the inferior frontal region was also an area that increased during fMRI tests of verbal and spatial working memory in E/HT users, suggesting improved executive function (Joffe et al., 2006). In addition to increases in inferior frontal activation, Joffe and colleagues also found parietal activation (bordering Brodman’s area 40, near the Wernicke’s region) in E/HT users (40–60yrs) during a verbal recall task (2006). This finding is pertinent to our observation of hormonal effects in Wernicke’s area and its correlation with verbal memory in all subjects, especially E-users. In a recent, double-blind placebo-controlled study, postmenopausal women (56–60yrs) randomized to hormone therapy had increased activation in the frontal cortex and left inferior parietal cortex (Brodman’s Area 40) during memory encoding (Persad et al., 2009). These findings suggest that estrogen may provide cognitive benefits and protect language-related brain areas when given to younger women shortly after or during the menopausal transition.
We also observed hormonal effects in the mesial temporal structures including hippocampal and parahippocampal regions. Functional neuroimaging studies utilizing PET and fMRI in healthy aging women, have consistently reported greater metabolic activity in mesial temporal structures, including the hippocampus, amygdala, and entorhinal cortex, among users of E/HT vs. non-users (Resnick et al., 1998; Shaywitz et al., 1999; Eberling 2000; Maki and Resnick, 2000; Maki and Resnick, 2001; Rasgon et al., 2001; Gleason et al., 2006). Regional brain volumes were recently assessed in structural MRI images of the brains of women who had been previously enrolled in WHIMS (Resnick et al., 2009b). Overall, mean hippocampal volumes tended to be smaller among women randomized to hormone therapy, by an average of −0.10cm3 (p=0.05); in the group with at least 2cm3 ischemic burden, the effect was more pronounced, with hippocampal volumes smaller in the HT arm by an average of −0.16cm3 (p=0.005).. Hence, those data are consistent with the WHIMS findings of detrimental cognitive effects associated with opposed CEE therapy. The fact that both types of HT were associated with increase in hippocampal neuronal firing could be due to predominant effects of the estrogen component, rather than the presence of the progesterone. In addition, Ottowitz and colleagues found that estradiol increased connectivity between the right hippocampus and right prefrontal cortex suggesting that estradiol may enhance verbal memory performance by means of recruiting a bilateral cooperation between prefrontal and hippocampal systems during verbal memory tasks (Ottowitz et al., 2008).
Overall, basic science analyses using both in vitro and in vivo model systems have also indicated that estrogen — typically 17β-estradiol but also in some instances conjugated equine estrogens — protect neurons against insults associated with AD (Brinton, 2005). Moreover, these same estrogens in the same model systems can activate biochemical, genomic, cellular and behavioral mechanisms of memory (Singh et al., 1994; Toran-Allerand, 2000; Frye et al., 2007). An important aspect of these studies, and of virtually all of the basic science in vitro and in vivo analyses, is that neurons were healthy prior to estrogen exposure and prior to exposure to neurodegenerative insults or lesions (Brinton, 2005). In human studies, beyond the neuroimaging results discussed above, data on the effects of estrogen or postmenopausal cognition have been consistent. Recent reviews of human observational studies and clinical trials have emphasized lack of benefits or likely harm of hormonal therapies in older postmenopausal women and/or insufficient evidence for benefit in younger women. (Genazzani et al., 2007; Barrett-Connor and Laughlin, 2009) Of particular note, results from WHIMS indicated significant increases in risk for probable dementia in the combination HT subtrial alone and in the combined analysis of combination HT and CEE-alone subtrials (Shumaker et. al, 2003; Shumaker et. al, 2004) and poorer performance on the 3MS (Rapp et. al, 2003; Espeland et. al, 2004), after initiation of HT in postmenopausal women aged 65 and older. On the other hand, in the Multi-Institutional Research in Alzheimer Genetic Epidemiology case-control study, the protective association of HT was modified by age and was seen among younger, but not older, postmenopausal women (Henderson et al., 2005).
It remains an open question, whether the neuroprotective effects of estrogen documented in preclinical studies can be replicated in randomized controlled trials performed with the most suitable patient group under appropriate conditions. On a path to accomplish these goals, variables to be examined as our data suggest, include both the formulations of estrogen and progestogens (progesterone versus MPA) employed in human studies and clinical trials, dosage and levels of exposure, timing of hormone therapy exposure, duration of hormone therapy, as well as substrate differences (i.e., neuronal health status and risk for or presence of degenerative disease). One clinical trial which takes these variables in the account is on its way (KEEPS) (Miller et al., 2009), but results are not yet being made available. Other limitations of the current work that future studies will be able to address, include assessing the potential effect of testosterone in hormone replacement regimens (present in the HT of 5 of the 53 subjects in the study, too small a subgroup to separately assess) and any differences in effects of MPA vs micronized progesterone that may exist.
Further along these lines, since many demographic factors and other types of patient characteristics may potentially affect cognitive performance and corresponding patterns of cerebral metabolism, greater homogeneity among subjects can be expected to produce greater statistical power, in analysis of cognitive and metabolic data from a subject pool of given size. In the present investigation, all of our subjects are postmenopausal women with well-documented pharmacologic status with respect to hormonal (and other) therapies, all are middle age (between 50 and 65 years old), and initiated hormone therapy perimenopausally. In addition, the detailed information obtained by self-report and recorded from the medical records with respect to estrogen status - both historical (ages at menarche and menopause, etc.) and current (specifics regarding types of estrogen used, and whether they were progesterone-containing or unopposed, etc.) – allows for subgroup and correlational analyses of cerebral metabolism that is generally not feasible in analysis of brain PET data obtained in other investigations. These advantages are purchased at the cost of an inevitable corresponding disadvantage: an inherent limitation is that the findings cannot be assumed to be generalizable beyond the particular kind of populations comprising our subject pool. In the current study, evidence is presented for relative preservation of metabolism in specific brain regions in middle age women at increased risk for future development of AD being associated with greater endogenous estrogen exposure, and unopposed or estradiol-based HT formulations initiated in the perimenopausal period. While these findings suggest a potential neuroprotective role for estrogen under these conditions, future studies including other subpopulations will be needed to examine the degree of generalizability of the findings reported here.
Acknowledgments
This study was funded by a grant from the National Institute on Aging (R01 AG22008) and supported in part by grant M01 RR-00070 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archer JS. Relationship between estrogen, serotonin, and depression. Menopause. 1999;6(1):71–8. [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19(4):469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Laughlin GA. Endogenous and exogenous estrogen, cognitive function, and dementia in postmenopausal women: evidence from epidemiologic studies and clinical trials. Semin Reprod Med. 2009;27(3):275–82. doi: 10.1055/s-0029-1216280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeusz CF, Wesnes KA, Kulkarni J, Vitetta L, Croft RJ, Nathan PJ. Estradiol treatment and its interaction with the cholinergic system: effects on cognitive function in healthy young women. Horm Behav. 2008;54(5):684–93. doi: 10.1016/j.yhbeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Bishop J, Simpkins JW. Estradiol enhances brain glucose uptake in ovariectomized rats. Brain Res Bull. 1995;36(3):315–20. doi: 10.1016/0361-9230(94)00208-i. [DOI] [PubMed] [Google Scholar]
- Bora SH, Liu Z, Kecojevic A, Merchenthaler I, Koliatsos VE. Direct, complex effects of estrogens on basal forebrain cholinergic neurons. Exp Neurol. 2005;194(2):506–22. doi: 10.1016/j.expneurol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann NY Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Jalali C, de Lacalle S. Morphological effects of estrogen on cholinergic neurons in vitro involves activation of extracellular signal-regulated kinases. J Neurosci. 2004;24(4):982–90. doi: 10.1523/JNEUROSCI.2586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Reed BR, Coleman JE, Jagust WJ. Effect of estrogen on cerebral glucose metabolism in postmenopausal women. Neurology. 2000;55(6):875–7. doi: 10.1212/wnl.55.6.875. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21(1):364–71. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. the UPRDS Development Committee. In: Recent developments in Parkinson’s disease. Fahn S, Marsden CD, Calne D, Goldstein M, editors. Vol. 2. Macmillan; New Jersey: 1987. pp. 153–163. [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. II Retrieval Brain. 1998;121(PT7):1249–56. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith C, et al. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Friston K, Holmes A, Worsley K, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88(2):208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276(5310):264–6. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Pluchino N, Luisi S, Luisi M. Estrogen, cognition and female ageing. 2007;13(2):175–87. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- Gleason C, Schmitz T, Hess T, et al. Hormone effects on fMRI and cognitive measures of encoding: importance of hormone preparation. Neurology. 2006;67(11):2039–41. doi: 10.1212/01.wnl.0000247277.81400.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Rojansky N, Palter S, Tworek H, Hissin P, Wang K. Estrogen augments serotonergic activity in postmenopausal women. Biol Psychiatry. 1995;37(7):434–41. doi: 10.1016/0006-3223(94)00181-2. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen T, Hallikainen M, Koivisto K, Helkala EL, Reinikainen KJ, Soininen H, Mykkänen L, Laakso M, Pyörälä K, Riekkinen PJ., Sr A follow-up study of age-associated memory impairment: neuropsychological predictors of dementia. J Am Geriatr Soc. 1995;43(9):1007–15. doi: 10.1111/j.1532-5415.1995.tb05565.x. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA MIRAGE Study Group. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. Am J Psychiatry. 2005;162(4):683–90. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, Rapoport SI, Schapiro MB, Horwitz B. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology. 1998;50(6):1585–93. doi: 10.1212/wnl.50.6.1585. [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall J, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–22. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Kendall DA, Stancel GM, Enna SJ. Imipramine: effect of ovarian steroids on modifications in serotonin receptor binding. Science. 1981;211(4487):1183–5. doi: 10.1126/science.6258229. [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Chu Y, Polish A, Roberts J, McKay H, Mufson EJ, Leurgans S, Morrison JH, Kordower JH. Effects of estrogen replacement therapy on cholinergic basal forebrain neurons and cortical cholinergic innervation in young and aged ovariectomized rhesus monkeys. J Comp Neurol. 2004;472(2):193–207. doi: 10.1002/cne.20050. [DOI] [PubMed] [Google Scholar]
- Lasiuk GC, Hegadoren KM. The effect of estradiol on central serotonergic systems and its relationship to mood in women. Biol Res Nurs. 2007;9(2):147–60. doi: 10.1177/1099800407305600. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89(2):484–90. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized double-blind trial. Neurology. 2007;69(13):1322–30. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21(2):373–83. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Effects of estrogen on patterns of brain activity at rest and during cognitive activity: a review of neuroimaging studies. Neuroimage. 2001;14(4):789–801. doi: 10.1006/nimg.2001.0887. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138(3):1027–30. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Martin A, Fedio P. Word production and comprehension in Alzheimer’s disease: the breakdown of semantic knowledge. Brain Lang. 1983;19(1):124–41. doi: 10.1016/0093-934x(83)90059-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Coirini H, Danielsson A, Frankfurt M, Gould E, Mendelson S, Schumacher M, Segarra A, Woolley C. Steroid and thyroid hormones modulate a changing brain. J Steroid Biochem Mol Biol. 1991;40(1–3):1–14. doi: 10.1016/0960-0760(91)90160-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Neural estrogen receptors in the life cycle of the albino rat. Exp Brain Res. 1981;(Suppl3):61–79. doi: 10.1007/978-3-642-45525-4_5. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meusburger SM, Keast JR. Testosterone and nerve growth factor have distinct but interacting effects on structure and neurotransmitter expression of adult pelvic ganglion cells in vitro. Neuroscience. 2001;108(2):331–40. doi: 10.1016/s0306-4522(01)00420-1. [DOI] [PubMed] [Google Scholar]
- Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, Lobo RA, Manson JE, Merriam GR, Naftolin F, Santoro N, Taylor HS, Harman SM. Using basic science to design a clinical trial: Baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) J Cardiovasc Transl Res. 2009;2(3):228–239. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, Greer PJ, Lopresti B, Loucks TL, Berga SL. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry. 2000;48(8):854–60. doi: 10.1016/s0006-3223(00)00967-7. [DOI] [PubMed] [Google Scholar]
- Namba H, Sokoloff L. Acute administration of high doses of estrogen increases glucose utilization throughout brain. Brain Res. 1984;291(2):391–4. doi: 10.1016/0006-8993(84)91276-9. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47(1):29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Porrino LG, Crane AM, Sokoloff L. Local cerebral glucose utilization in normal female rats: variations during the estrous cycle and comparison with males. J Cereb Blood Flow Metab. 1985;5(3):393–400. doi: 10.1038/jcbfm.1985.54. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Siedlecki KL, Lindquist MA, Dougherty DD, Fischman AJ, Hall JE. Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion: an FDG-PET study. Psychoneuroendocrinology. 2008;33(10):1419–25. doi: 10.1016/j.psyneuen.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad CC, Zubieta JK, Love T, Wang H, Tkaczyk A, Smith YR. Enhanced neuroactivation during verbal memory processing in postmenopausal women receiving short-term hormone therapy. Menopause. 2009;92(1):197–204. doi: 10.1016/j.fertnstert.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17(5):485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Ping SE, Trieu J, Wlodek ME, Barrett GL. Effects of estrogen on basal forebrain cholinergic neurons and spatial learning. J Neurosci Res. 2008;86(7):1588–98. doi: 10.1002/jnr.21609. [DOI] [PubMed] [Google Scholar]
- Polkowski K, Mazurek AP. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol Pharm. 2000;57(2):135–55. [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Geist CL, Kenna H, Powers B, Wroolie TE, Silverman DH. Decline of inferior parietal brain metabolism in postmenopausal women who discontinue hormone therapy. Journal of Nuclear Medicine Meeting Abstracts. 2008;49:226. [Google Scholar]
- Rasgon NL, Kenna HA, Geist C, Small G, Silverman D. Cerebral metabolic patterns in untreated postmenopausal women with major depressive disorder. Psychiatry Res. 2008;164(1):77–80. doi: 10.1016/j.pscychresns.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang SC, Phelps ME, Small GW. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol Aging. 2005;26(2):229–35. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Small GW, Siddarth P, Miller K, Ercoli LM, Bookheimer SY, Lavretsky H, Huang SC, Barrio JR, Phelps ME. Estrogen use and brain metabolic change in older adults: A preliminary report. Psychiatry Res. 2001;107(1):11–8. doi: 10.1016/s0925-4927(01)00084-1. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–8. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Resnick S, Maki P, Golski S, Kraut M, Zonderman A. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34(2):171–82. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, An Y, Maki PM, Coker LH, Jackson R, Stefanick ML, Wallace R, Rapp SR Women’s Health Initiative Study of Cognitive Aging Investigators. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab. 2009a;94(11):4152–61. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009b;72(2):135–42. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA Women’s Health Initiative Study of Cognitive Aging Investigators. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91(5):1802–10. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839–50. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Sar M, Stumpf WE. Central noradrenergic neurons concentrate 3H-oestradiol. Nature. 1981;289(5797):500–2. doi: 10.1038/289500a0. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368(6472):633–5. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Shaywitz S, Shaywitz B, Pugh K, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281(13):1197–202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanisk ML, Hendrix SL, Lewis CE, Masaki K, Coker LH Women’s Health Initiative Memory Study. Conjugated equine estrogens and incidence of probably dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, ThalLWallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Morley Kotchen J, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103(3):303–11. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644(2):305–12. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Small GW, Kuhl DE, Riege WH, Fujikawa DG, Ashford JW, Metter EJ, Mazziotta JC. Cerebral glucose metabolic patterns in Alzheimer’s disease. Effect of gender and age at dementia onset. Arch Gen Psychiatry. 1989;46(6):527–32. doi: 10.1001/archpsyc.1989.01810060047008. [DOI] [PubMed] [Google Scholar]
- Small GW, La Rue A, Komo S, Kaplan A, Mandelkern MA. Predictors of cognitive change in middle-aged and older adults with memory loss. Am J Psychiatry. 1995;152(12):1757–64. doi: 10.1176/ajp.152.12.1757. [DOI] [PubMed] [Google Scholar]
- Smith GS, de Leon MJ, George AE, Kluger A, Volkow ND, McRae T, Golomb J, Ferris SH, Reisberg B, Ciaravino J, et al. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer’s disease. Pathophysiologic implications. Arch Neurol. 1992;49(11):1142–50. doi: 10.1001/archneur.1992.00530350056020. [DOI] [PubMed] [Google Scholar]
- Smith Y, Love T, Persad C, Tkaczyk A, Nichols T, Zubieta J. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab. 2006;91(11):4476–81. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Minoshima S, Kuhl DE, Zubieta JK. Effects of long-term hormone therapy on cholinergic synaptic concentrations in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86(2):679–84. doi: 10.1210/jcem.86.2.7222. [DOI] [PubMed] [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: a function anatomical study of memory. Proc Natl Acad Sci USA. 1992;89(5):1837–41. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93(16):8660–5. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YC, Lin KP, Hoh CK, Huang SC, Hoffman EJ. Utilization of 3-D Elastic Transformation in Registration of Chest X-ray CT and Whole Body PET. IEEE Trans Nucl Sci. 1997;44:1606–1612. [Google Scholar]
- Toran-Allerand CD. Novel sites and mechanisms of oestrogen action in the brain. Novartis Fond Symp. 2000;230:56–69. doi: 10.1002/0470870818.ch6. discussion 69–73. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6(1):71–9. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Ungar S, Makman MH, Morris SA, Etgen AM. Estrogen uncouples beta-adrenergic receptor from the stimulatory guanine nucleotide-binding protein in female rat hypothyalamus. Endocrinology. 1993;133(6):2818–26. doi: 10.1210/endo.133.6.8243309. [DOI] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Katz MJ, Sliwinski M, Crystal HA, Bushke H, Lipton RB. Cognitive performance in surgically menopausal women on estrogen. Neurology. 2000;55(6):872–4. doi: 10.1212/wnl.55.6.872. [DOI] [PubMed] [Google Scholar]
- Vincent A, Fitzpatrick LA. Soy isoflavones: are they useful in menopause. Mayo Clin Proc. 2000;75(11):1174–84. doi: 10.4065/75.11.1174. [DOI] [PubMed] [Google Scholar]
- Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res. 2006;1094(1):163–78. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1999;37(1):103–18. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10(12):4035–9. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]