Abstract
Atopic Dermatitis (AD) patients often acquire secondary skin infections resulting in increased inflammation. The increased inflammation occurs through the activation of multiple cell types including dendritic cells (DC). In this study, we investigated the activity of soluble products present in infected AD lesions by measuring the ability of patients’ wash fluids from a quantitative culture of lesions to activate DC. We found that wash fluid derived from AD lesions induced cytokine production by murine bone marrow-derived DC, including IL-1β, IL-6, IL-10, and tumor necrosis factor-α. The lipoprotein lipoteichoic acid (LTA) from Staphylococcus aureus was implicated as a potent stimulus in the wash fluids as only wash fluid samples that contained LTA exerted this activity, and exogenous LTA triggered similar DC cytokine activation. Wash fluid- and LTA-stimulated DC cytokine production required MyD88, but not the platelet-activating factor receptor (PAF-R), despite the ability of LTA to function through this receptor in keratinocytes. Thus, our results support a role for DC in the worsening of AD inflammation due to secondary bacteria infections.
Keywords: Dendritic cells, Staphylococcus aureus, atopic dermatitis, lipoteichoic acid, Interleukin-6, Tumor necrosis factor-alpha, platelet-activating factor
Introduction
Staphylococcus aureus infections are known triggers for skin inflammation and can modulate immune responses [1,2]. Patients with AD are particularly susceptible to staphylococcal skin infections, which associate with the worsening of their skin disease [3,4]. Although the mechanisms by which staphylococcal bacteria can worsen AD are not yet clear cytokine production following direct infection or interaction with bacterial products by keratinocytes or immune cells appears to play an important role [5,6]. Potential staphylococcal mediators that could worsen AD include the cell wall lipoprotein lipoteichoic acid (LTA) that can act as an agonist for the Toll-like receptor 2 (TLR2) [7] as well as the Platelet-activating factor receptor (PAF-R) [8,9].
Dendritic cells are a morphologically and functionally defined family of leukocytes that serve as highly specialized antigen-presenting cells in the skin [10, 11]. In keeping with their role in immune surveillance, DC express various pattern-recognition receptors including TLRs that allow them to interact with microbial products in their environment [7]. In response to microbial products, DC produce an array of pro-inflammatory cytokines including IL-1β, IL-6, and TNFα to activate an immune response. However, they also limit the immune response through production of anti-inflammatory cytokines such as IL-10. From their ability to regulate immune processes, DC have been implicated in the pathogenesis of AD [12].
Recently, it has been demonstrated that high levels of LTA can be found in secondarily impetiginized AD lesions [13]. Quantitative bacterial culture techniques revealed that almost one third of S. aureus-infected AD lesions expressed microgram/cm3 levels of LTA. High levels of inflammatory cytokines such as IL-6 and TNF-α were also found in these skin lesions. The amounts of LTA and pro-inflammatory cytokines correlated with both amounts of S. aureus bacteria found in AD lesions and levels of clinical inflammation [13].
In the current study, we investigated whether secondary bacteria infection produces biologically-active products in infected AD lesions which can modulate host immune responses by activating DC. We were able to demonstrate that small amounts of wash fluid derived from infected AD lesions can trigger cytokine production in murine bone marrow-derived DC (BMDC). The activation of BMDC was MyD88 dependent. The data suggest that lipoprotein LTA is an important component in AD lesion wash fluids inducing DC activation. Furthermore, MyD88 not the PAF-R plays an important role mediating signaling by soluble products in the wash fluids. These findings implicate the host immune response induced by DC in exacerbating AD during staphylococcal colonization and infection.
Methods
Mice and Cells
C57BL/6 mice (age 6–8 wk) were purchased from The Jackson Laboratory. Paf-r-/- mice were a kind gift from Professor Isshi (University of Tokyo). Myd88-/- mice were a kind gift of Dr. Kunkel (University of Michigan). Mice were housed under specific pathogen-free conditions at the Indiana University or University of Michigan's School of Medicine. All procedures were approved by the Animal Care and Use Committees of Indiana University and the University of Michigan Schools of Medicine. Murine BMDC were prepared as previously described [14].
Reagents
DC were treated with the following reagents: 100 ng/mL LPS (Sigma-Aldrich), 10 μg/mL LTA (or as described in figure legends; Invivogen), and 1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine (CPAF; Sigma-Aldrich). Polymyxin B was a kind gift of Dr. O'Roirdan (University of Michigan) and was originally obtained from Calbiochem. For DC maturation, 10 ng/mL recombinant GMCSF (Peprotech) was added as previously described [14].
Atopic Dermatitis Subjects
Subjects with AD diagnosed using criteria of Hanifen and Rajka [15] were enrolled in these studies approved by the Indiana University Institutional Review Committee. The experimental procedures were previously described [13]. Subjects enrolled into the study underwent a clinical assessment of a clinically-infected lesion of dermatitis using the Eczema Area and Severity Index (EASI) [16]. Wash fluid was removed from the lesion and the subject treated with appropriate therapy, to include oral cephalexin antibiotic. Following two weeks, the subjects returned and the procedures were repeated.
Quantitative Analysis of Dermatitis Lesions
Wash fluid derived from lesions was removed from a 2.5 cm diameter polypropylene chamber as previously described [9,13] using the methodology established by Williamson and Kligman [17]. Briefly, a sterile 2.5 cm diameter ring of PVC tubing (Nalgene® Labware, Rochester, NY) was placed over the skin lesion of patient, then, 1 ml sterile rinse solution (0.069M Na2HPO4, 0.0064M NaH2PO4, and 0.1% Tx-100) was administered inside the ring chamber that was held tightly on the skin to prevent leakage. The rinse solution was stirred around in the chamber with a sterile Teflon® rod (Scientific Commodities Inc., Lake Havasu City, AZ) for 15-20 times and collected. This collection was repeated and 2 ml total rinse solution was obtained. This methodology has been shown to be 95% quantitative for aerobic surface bacteria [17]. The wash solution was then aliquotted for immunoblotting analysis of LTA, and bacterial quantitation. S. aureus and other bacteria including S. epidermidis (coagulase-negative staphylococcus) colonies were quantified in the microbiology lab in Indiana University Hospital by limiting dilution assay.
Measurement of LTA in wash fluid specimens
Quantitative measurements of LTA protein were as previously described [9,13]. Briefly, 32 μl rinse solution from each patient sample was separated on 18% Tris-HCl gradient gel (Bio-Rad Laboratories, Hercules, CA), along with standards of 10, 5, 2.5, and 1 ng LTA protein (Sigma-Aldrich, St. Louis, MO) dissolved in same rinse solution loaded on the same electrophoresis gel. LTA protein was determined by immunoblotting with LTA monoclonal antibody (QED Bioscience, San Diego, CA) and enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). The arbitrary optical densities were measured by Image J Software (NIH, Bethesda, MD). The quantification of LTA was determined according to the standard curve drawn. We estimate that the limit of detection of LTA in wash fluid in this assay is approximately 7 ng/ml.
ELISA
1.7% or 10% (v/v) of wash fluid from AD patient samples were incubated for 48 hr in the DC culture medium. Cytokine concentrations in supernatants were detected by ELISA using commercially available antibodies from eBiosciences.
Intracellular Calcium Mobilization Studies
Intracellular calcium mobilization response following the PAF-R agonist CPAF was used to assess DC for the presence of functional PAF-Rs. As previously described [9], DC were preloaded with the calcium-sensitive indicator FURA-2AM (3μM in HBSS; Molecular Probes, Eugene, OR) at 37 °C for 90 min, followed by washing, re-suspending and maintained in HBSS at room temperature before use. FURA-2-AM fluorescence was monitored in a Hitachi F-4010 spectrophotometer with excitation and emission wavelengths of 331 and 410 nm, respectively. Intracellular calcium levels were calculated as previously described [9].
Statistical Analysis
Statistical significance between groups was determined using a nonparametric t-test. Differences were considered significant when p < 0.05 (*).
Results
Wash fluid from AD lesions induces cytokine production in DC
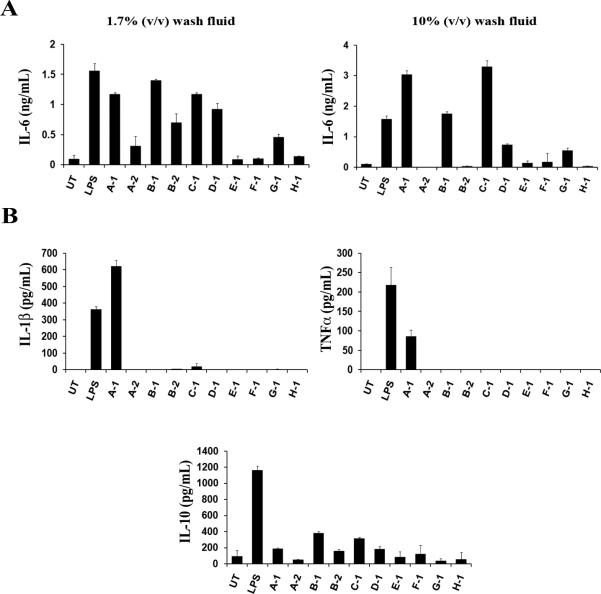
Bacterial infection is a known trigger for worsening of AD. To assess whether infected AD skin lesions contain agents that can activate DC cells, we tested the ability of wash fluid obtained by quantitative culture technique from children with inflamed and potentially infected AD lesions. As shown in Table I, ten samples derived from eight patients were used in these studies. Two of the subjects (A-2, B-2) represent follow-up visits 14 days following treatment with topical steroids and oral antibiotic, cephalexin. Of the ten samples, six contained S. aureus indicative of secondary infection. Also, six of the samples contained other strains of bacteria and four contained detectible levels of LTA. We then prepared BMDC and cultured them with low levels (final concentration 1.7% v/v) of patient wash fluid samples followed by ELISA to measure the amounts of cytokines produced by DC. We first assessed the pro-inflammatory cytokine IL-6. Seven of the ten samples resulted in IL-6 production that was above the amount from untreated (UT) cells (Fig. 1A). An increase in concentration to the final concentration 10% v/v of patient wash fluids resulted in significant increases in IL-6 production suggesting the dose-dependent activation by a soluble factor in AD lesion wash fluid (Fig. 1A).
Table I. Comparison of clinical assessment of inflammation, amounts of S. aureus, and levels of LTA in AD lesions.
The visit number, EASI score of the tested lesion, concentration of S. aureus or other bacteria (in CFU/ml), and LTA levels are listed for the 10 wash fluid specimens derived from 8 subjects.
| Sample # | Visit | EASI-les | Log SA (CFU/ml) | Other bacteria (log CFU/ml) | LTA (ng/ml) |
|---|---|---|---|---|---|
| A-1 | 1 | 8 | 6.5 | None | 236 |
| A-2 | 2 | 3 | 2.0 | None | ND |
| B-1 | 1 | 12 | 5.6 | S. epi (4.1) | 16 |
| B-2 | 2 | 6 | ND | S. epi (2.8); Strep viridans (3.3) | ND |
| C-1 | 1 | 9 | 5.6 | S. epi (3.2) | 18 |
| D-1 | 1 | 9 | 5.9 | None | 83 |
| E-1 | 1 | 10 | 7.0 | None | ND |
| F-1 | 1 | 7 | ND | S. epi (2.8) | ND |
| G-1 | 1 | 7 | ND | S. epi (3.4) | ND |
| H-1 | 1 | 9 | ND | S. epi (2.6); Pseudomonas aeruginosa (2.3) | ND |
Fig. 1. Wash fluid from infected AD lesions stimulates cytokine production in DC.
Murine DC from C57BL/6 mice were treated with LPS (100 ng/ml) or wash fluid derived from infected AD lesions of subjects in Table I. UT; untreated. Supernatants were collected after 48 hours and the amounts of indicated cytokines were measured by ELISA. (A) DC were cultured at two different concentrations of wash fluid. 17μl of patient samples was added to 1ml of culture medium (1.7% (v/v)) or 100μl of patient samples was added to 1ml (10% (v/v)). IL-6 cytokine production was quantified from both. (B) IL-1β, TNF-α, and IL-10 cytokine production was quantified for patient samples cultured at 10% (v/v). The data represent mean +/- SD from triplicate samples.
Samples from E1, F-1 and H-1 did not produce meaningful levels of IL-6 even at the high concentration of 10% suggesting that the three lacked the component inducing cytokine production in DC. Samples from A-2 and B-2 induced less DC cytokine response than their counterparts, A-1 and B-1, suggesting that the two-week treatment regimen including antibiotic course was capable of removing the cytokine inducing factor(s) from the two patients’ wash fluids. Although 7 patients’ samples were able to activate IL-6 production only one patient wash fluid (A-1) was capable of inducing IL-1β and TNFα production (Fig. 1B). All patients’ samples produced anti-inflammatory IL-10 in various amounts. High IL-10 producers such as B-1 and C-1 also secreted more IL-6 than the rest suggesting a correlation in the production of these two cytokines.
In regards to the potential factors, it was noted in the small number of specimens that the amount of LTA seems to correlate strongly with the potential to induce DC activation. Patient sample A-1 contains almost three-fold higher LTA than any other sample and was the only specimen able to activate other pro-inflammatory responses, IL-1β and TNFα. Together, these data suggest that AD lesions contain soluble factor(s) with biological activity capable of activating DC to produce cytokines.
Wash fluid-induced DC cytokine production involves MyD88
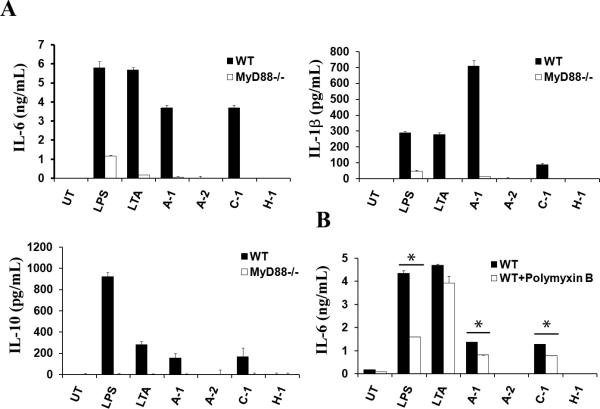
The lipoprotein LTA is a known inducer of DC cytokine production [18-20]. Since all wash fluids containing measurable amounts of LTA had the ability to stimulate DC cytokine production, we asked if LTA in wash fluid is responsible for the biological activity. Because LTA is a potent TLR2 agonist, and TLR2 requires the MyD88 adapter protein for signaling [7], we tested whether MyD88 participates in LTA-mediated activation of DC. To do this, we prepared BMDC from wild type and MyD88-deficient mice (Myd88-/-), cultured BMDC in the presence of wash fluids, and then measured the amount of cytokines present in the supernatant. For this experiment, we used wash fluid from two high-responders (A-1, C-1) and two low responders (A-2, H-1). Figure 2A illustrates that the cytokine production was abolished in Myd88-/- (Fig. 2A). As expected, treating BMDC with LPS or LTA, both of which depend on MyD88, showed compromised cytokine production in the absence of MyD88.
Fig. 2. Wash fluid-mediated cytokine production is MyD88-dependent.
Murine DC from C57BL/6 (WT) or Myd88-/- mice were treated with LPS (100 ng/ml), LTA (10 μg/ml), or wash fluid derived from infected AD lesions of subjects in Table I (final concentration 10 % v/v). UT; untreated. Supernatants were collected after 48 hours and analyzed by ELISA. (A) IL-6, IL-1β, and IL-10 production was quantified from two high-responders (A-1, C-1) and two low responders (A-2, H-1). (B) Wash fluids (final volume 10% (v/v)), LPS (100ng), or LTA (10μg) were pre-incubated with Polymyxin B (2.5μg) for 15 minutes at room temperature and then added to the DC culture. Samples not treated with Polymyxin B were cultured in the same way. The data represent mean +/- SD from triplicate samples.
In addition to LTA, lipopolysaccharide (LPS) is a potent activator of DC, which also requires MyD88. Wash fluid contains bacterial products including possibly LPS and therefore it is possible that the results seen with the deficiency of MyD88 could be a compound effect due to the lack of both LTA- and LPS-mediated signaling. To test this, we employed Polymyxin B, an antibiotic capable of binding LPS components [21], to inhibit a potential effect of LPS in wash fluids. We treated wash fluid with Polymyxin B prior to adding them to the DC culture. The results showed a significant difference in IL-6 production (Fig. 2B). Together, the data suggest that LTA and to a lesser extent LPS in patient samples are the primary components contributing to the biological activity of wash fluid which activates DC cytokine production in a MyD88-dependent manner.
PAF-R in DC is functional
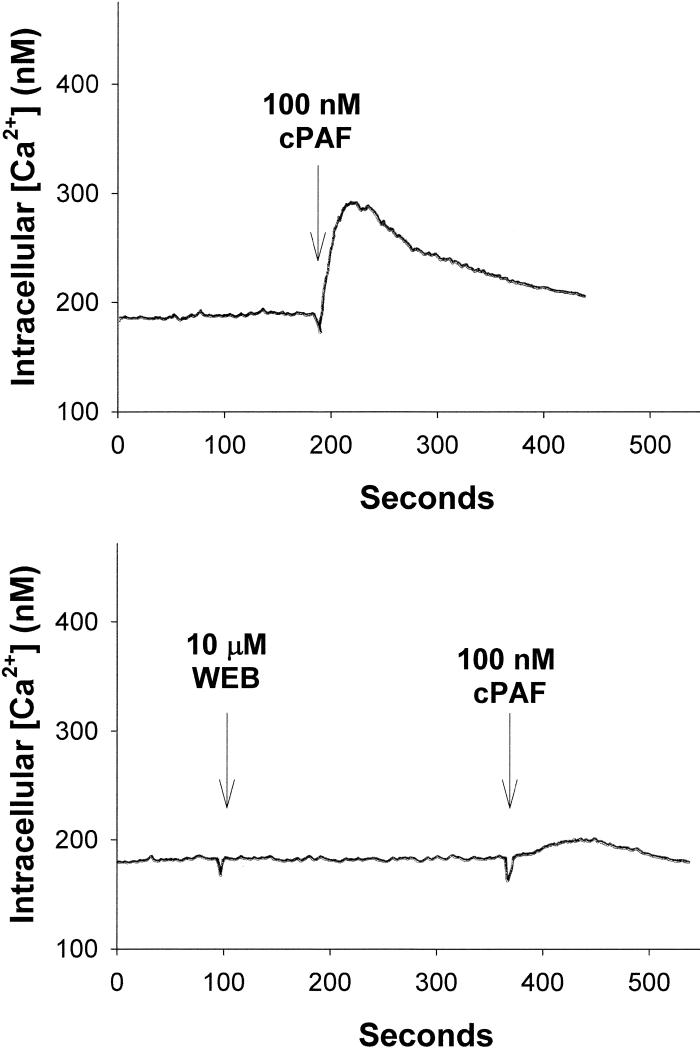
LTA can also act as an agonist for the PAF-R in several model systems [8,9]. Because LTA levels correlate with the potency of wash fluid to activate DC, we examined whether LTA exerts its effect through the PAF-R in DC. Although human DC have been reported to respond to PAF-R agonists [22-24] it is necessary to test whether the PAF-R is expressed and functional in murine DC. To do this, we loaded BMDC with FURA-2AM, treated with the PAF-R agonist carbamoyl PAF (CPAF), and measured the response by the intracellular calcium mobilization. DC responded to CPAF, confirming that murine DC express functional PAF-R (Fig. 3). The CPAF triggered response was blocked by pretreatment with 10 μM of the PAF-R antagonist WEB2086. These studies indicate that BMDC express functional PAF-Rs.
Fig 3. Murine DC have functional PAF-R.
Murine DC from C57BL/6 mice were loaded with calcium sensitive fluorescent dye FURA-2AM, and treated with 100 nM of the PAF-R agonist CPAF, and B. 10 μM of the PAF-R antagonist WEB2086 (WEB) prior to 100nM CPAF. Intracellular calcium levels were measured over time. These data are representative of at least three separate experiments.
PAF-R does not play a role for cytokine production by DC
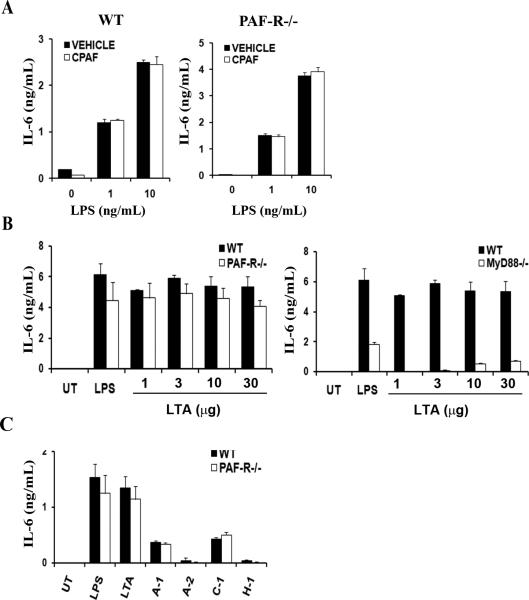
Having observed the presence of functional PAF-R in DC and because triggering PAF-R in other cell types induces the production of IL-6 and TNF-α [25-27], we next investigated whether PAF-R is involved in cytokine production. In particular, we designed these experiments to evaluate whether the DC PAF-R alone could stimulate cytokine production, or could prime DC for exaggerated cytokine production, or exerted an inhibitory effect. To do this, we first examined the ability of DC to produce cytokines in response to CPAF by treating DC with LPS alone or together with CPAF. Adding CPAF did not result in an increased IL-6 compared to that of LPS without CPAF (Fig. 4A). Similarly, CPAF treatment of DC did not result in the production of TNF-α, nor was TNF-α production augmented by co-treatment of CPAF and LPS in wild-type DC (data not shown). Together, the data suggest that CPAF does not augment LPS responses.
Fig. 4. PAF-R mediated signaling does not play a role in cytokine production by DC.
(A) Murine DC from C57BL/6 or Paf-r-/- mice were treated with the PAF-R agonist CPAF (100nM) alone, or as co-activation with 1 or 10 ng/ml of LPS. Forty-eight hours later, the amount of IL-6 in the supernatant was measured by ELISA. The data represent mean +/- SD from triplicate samples. (B) Murine DC from PAF-R-/- or MyD88-/- mice together with C57BL/6 mice were treated with LPS (100 ng/ml) various doses of LTA (1-30 μg/ml), or left untreated. IL-6 was measured as in (A). (C) Murine DC from C57BL/6 or PAF-R-/- mice were treated with LPS (100ng/ml), LTA (10μg/ml), or patient wash fluids (final volume 10% (v/v)). Forty-eight hours later, IL-6 was measured in supernatants by ELISA. The data represent mean +/- SD from triplicate samples.
To further examine the role of PAF-R in DC activation and cytokine production, we tested DC lacking PAF-R. As seen with the wild type cells in Figure 3, Paf-r-/- cells showed little difference in IL-6 levels regardless of CPAF addition (Fig. 4A). Next, we treated wild-type and Paf-r-/- DC with various concentrations of LTA and found that the amount of IL-6 was comparable between the two (Fig. 4B). In contrast, the absence of MyD88 severely reduced IL-6 production, yet at high concentrations Myd88-/- cells were still capable of producing small amounts of IL-6 (Fig. 4B). Production of cytokines IL-1β, TNFα, and IL-10 showed similar patterns as with IL-6 (data not shown). Because DC rely on MyD88 to produce cytokines upon treatment with wash fluid we asked whether the PAF-R signaling pathway is also involved in making cytokines. When wild-type and PAF-R-/- DC were treated with wash fluid samples from four patients, both showed equivalent IL-6 (Fig. 4C). Together, these data demonstrate that TLR pathways, but not PAF-R play a role in DC cytokine production induced by LTA or wash fluid samples from infected AD lesions.
Discussion
Bacterial infection with S. aureus is a known trigger for worsening of AD [1-4]. The demonstration that active AD lesions have decreased levels of antimicrobial peptides in comparison to normal skin or other inflammatory diseases such as psoriasis has provided a mechanism for the increased incidence of staphylococcal infections in this population [28]. The exact mechanism(s) by which staphylococcal impetiginization can worsen AD is unknown at present. Though the keratinocyte has been implicated in this process, other immune cells such as DC could certainly be participants. The present studies demonstrate that wash fluid derived from a small group of clinically infected AD lesions possessed soluble factors capable of activating murine DC. It has been previously demonstrated that high levels of these same cytokines, especially IL-6, are found in wash fluid samples derived from infected AD lesions [13].
The exact soluble factor(s) found in the skin lesions which trigger DC cytokine production remain somewhat unknown. However, our current study provides several lines of evidence implicating LTA as a major player in this process. Wash fluid samples that activated DC cytokine production also contained measureable amounts of LTA. The use of MyD88-/- DC confirmed that the activation of DC is TLR-dependent. Inasmuch as inhibition of LPS with polymixin decreased the biological activity of the patient samples, endotoxin is also playing a role in the biological activity of the wash fluid samples. There have been several studies examining the role of TLR2 in AD, though questions remain [4]. Some groups have linked TLR2 to recurring bacterial infections, similar to that of S. aureus in AD [29]. Since treatment of human DC with LTA also results in cytokine production [30,31] the present study lends support to the role TLR2 in the worsening of AD through LTA-induced activation of pro-inflammatory cytokines from DC.
The present studies confirm that DC express functional PAF-R. The role of the DC PAF-R is unclear, though it has been reported that PAF or oxidized high density lipoproteins that exert PAF-R agonistic activity inhibit the migration of DC [32]. Though LTA has been shown to act as an agonist of the PAF-R [8,9], the present studies demonstrate that PAF-R activation does not induce appreciable cytokine production from DC. Moreover, PAF-R deficiency had little impact on the ability of DC to produce cytokines in response to LTA and AD wash fluid. Interestingly, however, PAF-R ligation induced Ca++ mobilization indicating that the DC PAF-R is functional. Nonetheless, neither PAF-R-mediated signaling nor intracellular Ca++ signaling alone is sufficient for DC to produce cytokines. Moreover, the DC PAF-R does not exert augmentative or inhibitory effects [33] on cytokine production induced by LTA or LPS.
The present studies suggest that DC could be involved in the worsening of AD associated with S. aureus infection. Evaluation of wash fluid samples using quantitative bacterial culture methodology from clinically impetiginized AD lesions revealed that relatively small amounts of the wash fluid (even 1.7% v/v) was found to exert potent cytokine producing effects on murine DC. We propose that staphylococcal products such as LTA are involved in this process. Further studies are necessary to elicit other soluble factors in AD lesions and their respective effects on the pathogenesis of AD in humans.
Acknowledgements
This research was supported in part by grants from the Riley Memorial Association, and the National Institutes of Health grants HL62996 (JBT), U19 AI070448 (CHC, MHK, JBT) and Veteran's Administration Merit Award (JBT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leung DY. Infection in atopic dermatitis. Curr. Opin. Pediatr. 2003;15:399–404. doi: 10.1097/00008480-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Baker BS. The role of microorganisms in atopic dermatitis. Clin. Exp. Immunol. 2006;144:15–43. doi: 10.1111/j.1365-2249.2005.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sehra S, Barbé Tuana FM, Holbreich M, Mousdicas N, Chang C-H, Travers JB, Kaplan MH. Scratching the surface: Towards understanding the pathogenesis of atopic dermatitis. Crit. Rev. Immunol. 2008;28:15–43. doi: 10.1615/critrevimmunol.v28.i1.20. [DOI] [PubMed] [Google Scholar]
- 4.Bieber T. Atopic dermatitis. New. Engl. J. Med. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 5.Mandi Y, Endresz V, Krenacs L, Regely K, Degre M, Beladi I. Tumor necrosis factor production by human granulocytes. Internat. Arch. Allergy & Applied Immunology. 1991;96:102–6. doi: 10.1159/000235479. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki T, Kano R, Sato H, Nakamura Y, Watanabe S, Hasegawa A. Effects of staphylococci on cytokine production from human keratinocytes. Br. J. Dermatol. 2003;148:46–50. doi: 10.1046/j.1365-2133.2003.05017.x. [DOI] [PubMed] [Google Scholar]
- 7.Michelsen KS, Aicher A, Mohaupt M, Hartung T, Dimmeler S, Kirschning CJ, Schumann RR. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 2001;276:25680–86. doi: 10.1074/jbc.M011615200. [DOI] [PubMed] [Google Scholar]
- 8.Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nature. Med. 2002;8:41–46. doi: 10.1038/nm0102-41. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Mousdicas N, Yi Q, Al-Hassani M, Billings S, Perkins SM, et al. Staphylococcal Lipoteichoic Acid Inhibits Delayed-Type Hypersensitivity Reactions via the Platelet-activating Factor Receptor. J. Clin. Invest. 2005;115:2855–2861. doi: 10.1172/JCI25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell E. Dendritic cells: New DCs found deep in skin. Nat. Rev. Immunol. 2008;8:2256. [Google Scholar]
- 11.Rupec RA, Boneberger S, Ruzicka T. What is really in control of skin immunity: lymphocytes, dendritic cells, or keratinocytes? facts and controversies. Clinics in Dermatology. 2010;28:62–6. doi: 10.1016/j.clindermatol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Novak N, Koch S, Allam JP, Bieber T. Dendritic cells: bridging innate and adaptive immunity in atopic dermatitis. Journal of Allergy & Clinical Immunology. 2010;125:50–9. doi: 10.1016/j.jaci.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Travers JB, Kozman A, Mousdicas N, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J. Aller. & Clin. Immunol. 2010;125:146–52. doi: 10.1016/j.jaci.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promoted IL-12 production by dendritic cells. J. Exp. Med. 2005;201:1899–1903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanifen JM, Rajka G. Diagnostic features of atopic dermatitis. Acta. Derm. Venereol. 1980;92:44–47. [Google Scholar]
- 16.Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp. Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 17.Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J. Invest. Dermat. 1965;45:498–503. doi: 10.1038/jid.1965.164. [DOI] [PubMed] [Google Scholar]
- 18.Roses RE, Xu S, Xu M, Koldovsky U, Koski G, Czerniecki BJ. Differential production of IL-23 and IL-12 by myeloid-derived dendritic cells in response to TLR agonists. J. Immunol. 2008;181:5120–5127. doi: 10.4049/jimmunol.181.7.5120. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HJ, Yang JS, Woo SS, Kim SK, Yun CH, Kim KK, Han SH. Lipoteichoic acid and muramyl dipeptide synergistically induce maturation of human dendritic cells and concurrent expression of proinflammatory cytokines. J. Leuk. Biol. 2007;81:983–989. doi: 10.1189/jlb.0906588. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso LS, Araujo MI, Góes AM, Pacífico LG, Oliveira RR, Oliveira SC. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb. Cell Fact. 2007;6:1. doi: 10.1186/1475-2859-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dichmann S, Rheinen H, Panther E, Herouy Y, Czech W, Termeer C, et al. Downregulation of platelet-activating factor responsiveness during maturation of human dendritic cells. J. Cell. Physiol. 2000;185:394–400. doi: 10.1002/1097-4652(200012)185:3<394::AID-JCP9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Sozzani S, Longoni D, Bonecchi R, Luini W, Bersani L, D'Amico G, et al. Human monocyte-derived and CD34+ cell-derived dendritic cells express functional receptors for platelet activating factor. FEBS Lett. 1997;418:98–100. doi: 10.1016/s0014-5793(97)01358-6. [DOI] [PubMed] [Google Scholar]
- 24.Au BT, Williams TJ, Collins PD. Zymosan-induced IL-8 release from human neutrophils involves activation via the CD11b/CD18 receptor and endogenous platelet-activating factor as an autocrine modulator. J. Immunol. 1994;152:5411–5419. [PubMed] [Google Scholar]
- 25.Roth M, Nauck M, Yousefi S, Tamm M, Blaser K, Perruchoud AP, et al. Platelet-activating factor exerts mitogenic activity and stimulates expression of interleukin 6 and interleukin 8 in human lung fibroblasts via binding to its functional receptor. J. Exp. Med. 1996;184:191–201. doi: 10.1084/jem.184.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley SW, Dy LC, et al. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J. Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- 27.Travers JB, Edenberg HJ, Zhang Q, Al-Hassani M, Yi Q, Baskaran S, et al. Augmentation of UVB radiation-mediated early gene expression by the epidermal platelet-activating factor receptor. J. Invest. Dermatol. 2008;128:455–460. doi: 10.1038/sj.jid.5701083. [DOI] [PubMed] [Google Scholar]
- 28.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 29.Mrabet-Dahbi S, Dalpke AH, Markus Frey MN, Draing C, Brand S, Heeg K, Werfel T, Renz H. The Toll-like receptor 2 R753Q mutation modifies cytokine production and Toll-like receptor expression in atopic dermatitis. American Academy of Allergy, Asthma & Immunology. 2008;121:1013–9. doi: 10.1016/j.jaci.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Critical Care. 2009;13(4):R119. doi: 10.1186/cc7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller JF, Carrouel F, Colomb E, et al. Toll-like receptor 2 activation by lipoteichoic acid induces differential production of pro-inflammatory cytokines in human odontoblasts, dental pulp fibroblasts and immature dendritic cells. Immunobiology. 2010;215:53–9. doi: 10.1016/j.imbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Jeong YI, Jung ID, Lee CM, et al. The novel role of platelet-activating factor in protecting mice against lipopolysaccharide-induced endotoxic shock. PLoS ONE. 2009;4:e6503. doi: 10.1371/journal.pone.0006503. [DOI] [PMC free article] [PubMed] [Google Scholar]