Abstract
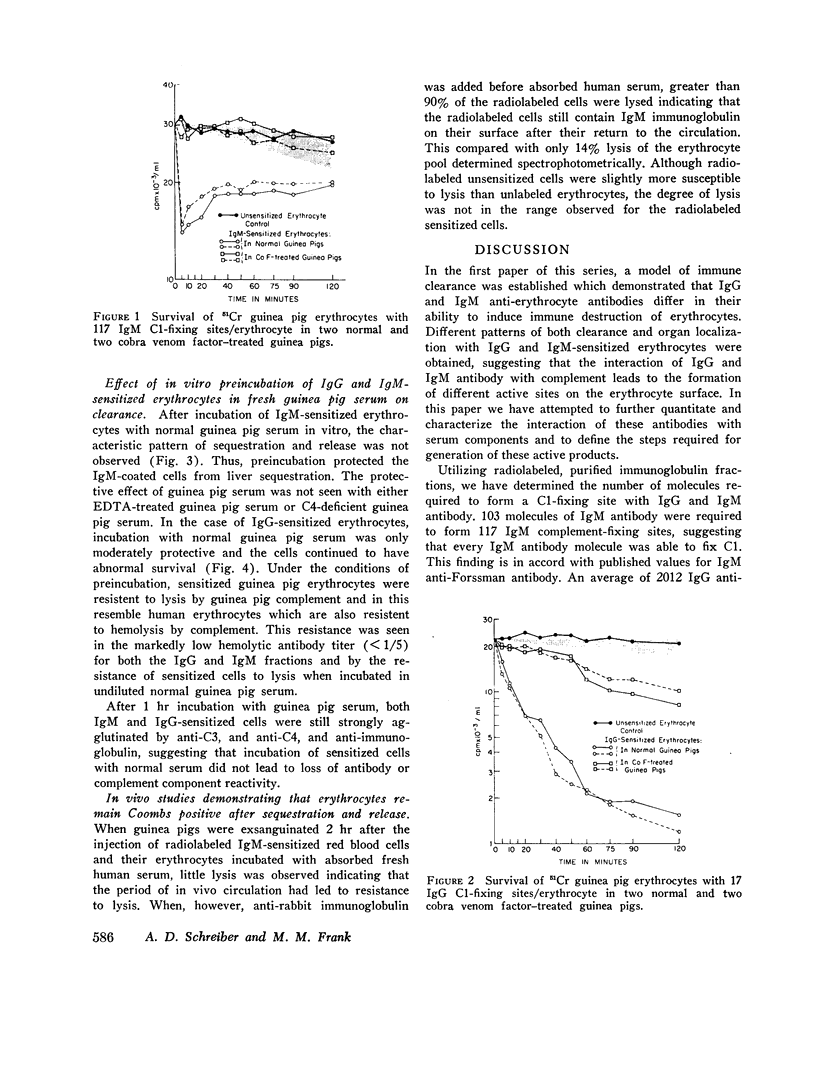
A model for the immune clearance and destruction of homologous erythrocytes has been further explored. In this model, every IgM anti-erythrocyte antibody molecule in an antibody preparation was shown to fix Cl. About 2000 IgG antibody molecules were required to form a Cl-fixing site on the guinea pig erythrocyte surface. 60 IgM complement-fixing sites per erythrocyte were required for the immune clearance of IgM-sensitized erythrocytes. This number of sites could be detected by a direct agglutination test. 1.4 complement-fixing sites were required for immune clearance of IgG-sensitized cells, a number of molecules which could not be detected by direct agglutination. This number could, however, be detected with the use of a Coombs antiglobulin reagent.
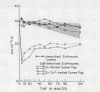
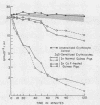
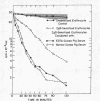
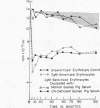
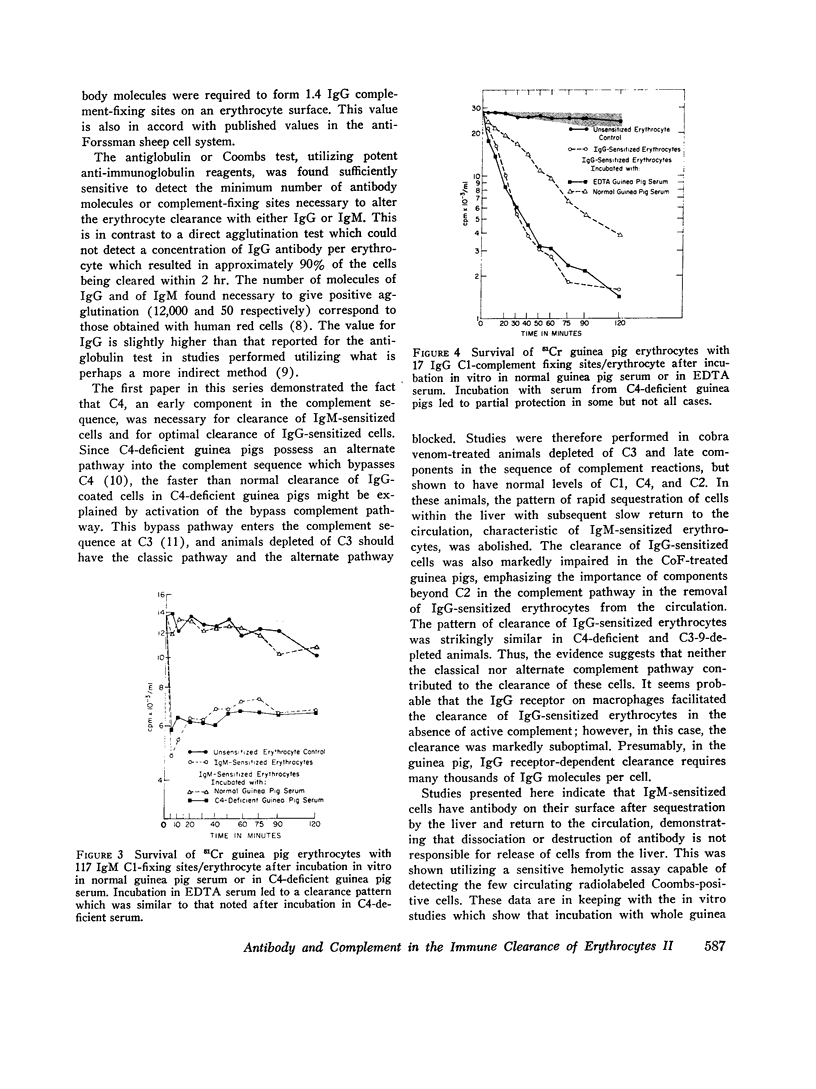
Depletion of the late components of complement (C3-9) with cobra venom was associated with the loss of ability to clear IgM-sensitized cells and a marked deficit in the ability to clear IgG-coated cells. Thus, late (C3-9) components of complement as well as an early component (C4) were required for normal clearance of sensitized erythrocytes. There was no evidence that activation of the alternate pathway of complement action could lead to accelerated erythrocyte clearance.
In vitro incubation of IgG and IgM-sensitized erythrocytes in fresh serum led to deposition of C3 and C4 on the erythrocyte surface. IgM-sensitized cells treated in this way had a normal survival. IgM-sensitized cells also were shown to remain Coombs positive after their release from the liver. The evidence suggests that the interaction of an IgM site with fresh serum in vitro and in vivo leads to formation of a site which allows for sequestration of cells in the liver. With continued exposure to serum components, this site is destroyed or inactivated. This serum-dependent inactivation is complement-dependent as shown by the use of EDTA-treated and C4-deficient serum. IgG complement-fixing sites are only partially inactivated by incubation in fresh serum, further emphasizing the differences in the biologic activity of IgM and IgG antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Lo Buglio A. F., Jandl J. H., Cotran R. S. The interaction between human monocytes and red cells. Binding characteristics. J Exp Med. 1970 Dec 1;132(6):1191–1206. doi: 10.1084/jem.132.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Complement fixation on cell surfaces by 19S and 7S antibodies. Science. 1965 Oct 22;150(3695):505–506. doi: 10.1126/science.150.3695.505. [DOI] [PubMed] [Google Scholar]
- Brown D. L., Lachmann P. J., Dacie J. V. The in vivo behaviour of complement-coated red cells: studies in C6-deficient, C3-depleted and normal rabbits. Clin Exp Immunol. 1970 Sep;7(3):401–421. [PMC free article] [PubMed] [Google Scholar]
- Economidou J., Hughes-Jones N. C., Gardner B. The functional activities of IgG and IgM anti-A and anti-B. Immunology. 1967 Sep;13(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Ellman L., Green I., Judge F., Frank M. M. In vivo studies in C4-deficient guinea pigs. J Exp Med. 1971 Jul 1;134(1):162–175. doi: 10.1084/jem.134.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. S., Turner E., Bingham M. Chronic hemolytic anemia due to cold agglutinins: the mechanism of resistance of red cells to C' hemolysis by cold agglutinins. J Clin Invest. 1967 Sep;46(9):1461–1474. doi: 10.1172/JCI105638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. S., Turner E., Bingham M., Woods R. Chronic hemolytic anemia due to cold agglutinins. II. The role of C' in red cell destruction. J Clin Invest. 1968 Apr;47(4):691–701. doi: 10.1172/JCI105764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland B. C., Leddy J. P., Vaughan J. H. The detection of cell-bound antibody on complement-coated human red cells. J Clin Invest. 1970 May;49(5):898–906. doi: 10.1172/JCI106309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H. Haemolytic efficiency of rabbit IgG anti-Forssman antibody and its augmentation by anti-rabbit IgG. Nature. 1967 Dec 30;216(5122):1295–1296. doi: 10.1038/2161295a0. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Ca++-dependent binding of antigen-19 S antibody complexes to macrophages. J Immunol. 1969 May;102(5):1172–1178. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr A new concept of immunosuppression in hypersensitivity reactions and in transplantation immunity. Surv Ophthalmol. 1966 Aug;11(4):498–505. [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972 Mar;51(3):575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]