Abstract
The phosphatidylinositol 3-kinase (PI3K) pathway has been shown to play a central role in regulating the host inflammatory response. Recent studies characterizing the downstream effector molecules within the PI3K pathway have identified that the serine/threonine kinase, glycogen synthase kinase 3 (GSK3), plays a pivotal role in regulating the production of pro- and anti-inflammatory cytokines. In innate immune cells, GSK3 inactivation augments anti-inflammatory cytokine production while concurrently suppressing the production of pro-inflammatory cytokines. The role of GSK3 in T cell biology has also been studied in detail and is involved in regulating multiple downstream signaling processes mediated by the T cell receptor (TCR), the co-stimulatory molecule CD28, and the IL-17 receptor. In vivo studies assessing the therapeutic properties of GSK3 inhibitors have shown that the inactivation of GSK3 can protect the host from immune-mediated pathology and death. This review will highlight the immunological importance GSK3 plays within different signal transduction pathways of the immune system, the cellular mechanisms regulating the activity of GSK3, the role of GSK3 in innate and adaptive immune responses, and the in vivo use of GSK3 inhibitors to treat inflammatory mediated diseases in animals.
Keywords: GSK3, PI3K, Dendritic cell, T cell, Inflammation
Background
GSK3 was originally isolated and characterized from skeletal muscle almost 30 years ago [1–3]. Since its initial characterization as a critical enzyme involved in glycogen biosynthesis, GSK3 has been demonstrated to be a point of convergence for numerous cell signaling pathways involved in a multitude of physiological processes. GSK3 regulates embryonic development, cell cycle control, cell differentiation, cell motility, microtubule function, apoptosis, cell adhesion, and inflammation (reviewed in [4–8]). The incredible number of cellular processes that are directly or indirectly controlled by GSK3 have been shown to be due, in large part, to its ability to post-translationally modify transcription factors via its ability to phosphorylate consensus site specific serine or threonine residues [5, 7, 9–11] (Figure 1A). Due to the ability of GSK3 to impact numerous intracellular signaling pathways, it is not surprising that the dysregulation of GSK3 has been shown to be involved in the initiation or progression of many diseases, including diabetes, Alzheimer's disease, bipolar disorder, and cancer [5, 7, 9–11].
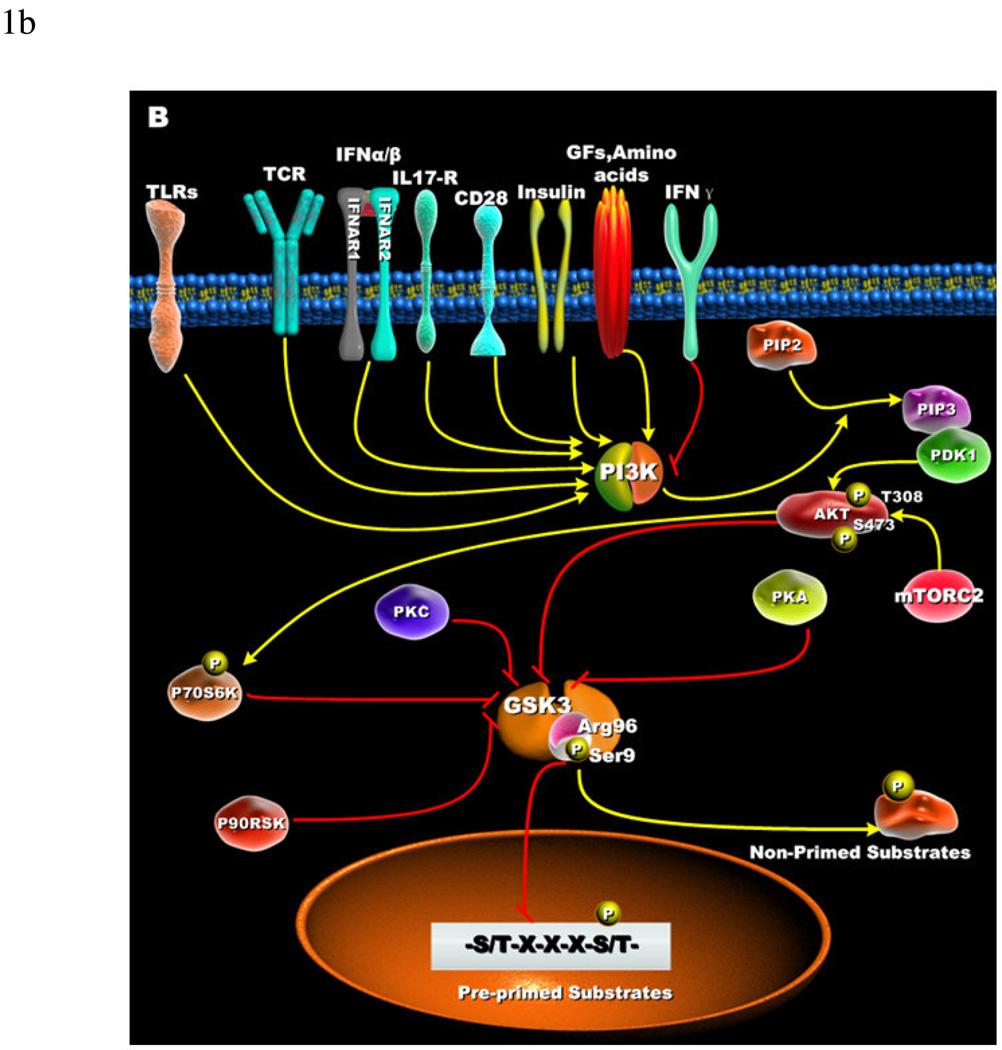
Figure 1. Cellular receptors involved in the phospho-inactivation of GSK3 and substrate specificity.
(A) Growth factors, amino acids, TLRs, TCR, CD28, and cytokine receptors have been shown to mediate the phospho-inactivation of GSK3-α (Ser21) and GSK3-β (Ser9). Activation of PI3K results in the generation of PIP3 that allows for the recruitment of Akt via its pleckstrin homology domain. Full activation of Akt occurs when phosphorylated at threonine 308 by PDK1 and serine 473 by mTORC2. Upon activation, Akt can phosphorylate GSK3-β (Ser9) that results in its inactivation. PKC, p70S6K, p90RSK, and PKA can phospho-inactivate GSK3. Inactivation of GSK3 results in the activation of transcription factors important for regulating the innate and adaptive inflammatory responses. (B) Active GSK3 exhibits a 100- to 1000-fold increase in substrate specificity for pre-primed substrates, as compared to non-primed substrates. N-terminal phosphorylation of GSK3-β (Ser9) acts as a pseudo-substrate for the phosphate-binding site and thus competes for the binding to arginine 96 with pre-primed, but not non-primed substrates.
In mammals, GSK3 exists as two major isoforms, GSK3-α and GSK3-β, and are encoded by distinct genes [12]. The gene encoding GSK3-α expresses a mature polypeptide of 51 Kilodaltons, whereas the gene encoding GSK3-β expresses a mature polypeptide of 47 Kilodaltons. The molecular weight differences between GSK3-α and GSK3-β are the result of a glycine-rich extension at the N-terminus of GSK3-α. Amino acid sequence comparisons of these two isoforms demonstrated that they exhibited an overall homology of 85%, with 98% homology within their kinase domains [12]. However, the last 76 amino acids within the C-terminus region of GSK3-α and GSK3-β exhibit only 36% homology [12]. Hoeflich et al. [13] first reported on the potential physiological consequences of these homology differences in that GSK3-β deficiency resulted in embryonic lethality and that the presence of GSK3-α was insufficient to compensate for the lack of GSK3-β. Whereas GSK3-β knockout (KO) mice die at approximately day 16 due to liver degeneration, GSK3-α KO mice are viable [14, 15]. Studies by Ruel et al. further demonstrated differences between GSK3 isoforms, since the over-expression of GSK3-β but not GSK3-α was able to rescue the absence of a homolog of GSK3 in Drosophila [16, 17]. Studies defining the role of GSK3 in axonal development and formation have clearly demonstrated that both isoforms of GSK3 are required [18]. In contrast, GSK3-α and GSK3-β isoforms have been shown to play redundant roles in the Wnt signaling pathway [14]. GSK3-α KO mice have been generated and reported to exhibit abnormalities within the structure of the brain that may affect behavior [15]. An alternative splice variant of GSK3-β has also been identified in the brains of mice, rats, and humans [19]. This splice variant, referred to as GSK3-β2, was shown to have an additional 13 amino acids in its catalytic domain, as compared to GSK3-β. Although the function of GSK3-β2 remains to be completely elucidated, immunohistochemistry staining identified that GSK3-β2 exhibited a different cellular localization pattern within the brain and had decreased kinase activity towards the GSK3 substrate tau, as compared to non-spliced GSK3-β [19].
Under basal cellular conditions, GSK3 is a constitutively active serine/threonine kinase. Initial studies assessing the cell signaling pathways activated by insulin identified that insulin receptor signaling resulted in the activation of the PI3K pathway and the subsequent phospho-inactivation of GSK3-α (Serine 21) or GSK3-β (Serine 9) by Akt [20–22]. Other cellular stimuli including growth factors [21, 23], phorbol esters [24], amino acids [25–27], Toll-like receptors (TLRs) [28, 29], T cell receptor (TCR) [30], CD28 [30], interleukin receptors [31], and KIT activation [32] have been shown to inactivate GSK3-α (Serine 21), GSK3-β(Serine 9), or both. The phospho-inactivation of GSK3 can be mediated by Akt [28, 33], P70S6K [25–27], p90RSK [34], PKC isoforms [35, 36], or PKA [37–39] (Figure 1A). To date, the phosphorylation of GSK3-α (Serine 21) or GSK3-β (Serine 9) has been the only mechanism reported to affect the activity of GSK3 in immune cells [10]. Nevertheless, GSK3 activity can be regulated by other cellular mechanisms including intracellular localization within the cell and the interaction with regulatory proteins and multi-protein complexes [5–7, 9, 11, 40, 41].
In order to better understand the molecular mechanisms responsible for the inactivation of GSK3 activity by serine phosphorylation, it is first important to define GSK3’s preference to phosphorylate substrates that are pre-primed (pre-phosphorylated) on a serine or threonine located around a five amino acid consensus sequence corresponding to serine/threonine-X-X-X-serine/threonine-P, where the first serine or threonine is the residue to be phosphorylated by GSK3, X can be any amino acid, and the last serine- or threonine-P is the pre-primed (pre-phosphorylated) residue [42](Figure 1B). The preferential phosphorylation of pre-primed (pre-phosphorylated) substrates by GSK3 has been shown to result in increasing substrate phosphorylation efficiency by more than 100 to 1000-fold, as compared to non-primed substrates [43]. This characteristic of GSK3 to prefer pre-primed (pre-phosphorylated) substrates was also demonstrated to play a role in its inactivation by serine phosphorylation. Studies by Frame et al. demonstrated that mutating arginine 96 to alanine abrogated the ability of GSK3 to phosphorylate pre-primed (pre-phosphorylated) but not non-primed substrates [44] (Figure 1B). Interestingly, although the arginine 96 mutant of GSK3-β could still phosphorylate non-primed substrates, this activity was not affected by Akt-mediated serine 9 phosphorylation of GSK3-β. These findings demonstrated that the N-terminal phosphorylation of GSK3-β on serine 9 acted as a pseudosubstrate for the phosphate-binding site (arginine 96) of GSK3-β and competed for the binding to arginine 96 with pre-phosphorylated substrates (Figure 1B). These studies also showed that a phospho-peptide mimetic of 11 residues that corresponded to the phosphorylated N-terminus of GSK3-β inhibited the ability of GSK3 to phosphorylate both pre-primed and nonprimed substrates. In contrast, a shortened phospho-peptide mimetic (NTptide-8) selectively inhibited the phosphorylation of pre-primed substrates by GSK3 [44]. These findings could have significant applications in the development of GSK3 inhibitors that exhibit pre-primed versus non-primed substrate selectivity.
GSK3 and its role in the innate immune system
The ability of the host immune system to recognize and respond to microbial components is largely mediated by the innate immune system via the expression of a family of type I transmembrane receptors, Toll-like receptors (TLRs) [45–47]. Activation of TLRs by microbial products leads to the engagement of a diverse number of intracellular signaling pathways that dictate qualitative and quantitative aspects of the host inflammatory response [48–50]. Although the production of inflammatory cytokines plays an important role in mediating the initial host defense against invading pathogens [51], an inability to regulate the nature, magnitude, or duration of the host inflammatory response can often mediate detrimental host effects, as observed in chronic inflammatory diseases. Thus, it is of critical importance to identify and characterize the cellular mechanisms that control the types and levels of inflammatory cytokines produced by immune cells.
The family of PI3K enzymes consist of three separate classes, including class I, II, and III [52]. In general, class I PI3Ks are responsible for converting PtdIns(4,5)P2 (PIP2) to PtdIns(3,4,5)P3 (PIP3) and consist of class IA and IB PI3Ks. Almost all studies to date analyzing the role of PI3K in controlling the inflammatory response in innate immune cells have dealt with class IA PI3Ks. The class IA PI3Ks are heterodimeric and have a p110 (p110α, p110β, and p110δ) catalytic subunit associated with a p85α, p55α, p50α, p85β, or p55γ regulatory subunit [52]. Direct evidence for the involvement of PI3K in TLR-signaling was initially shown by Arbibe et al. [33] in that site-directed mutagenesis of specific tyrosine residues within the cytosolic domain of TLR2 resulted in both a loss in the ability of p85 to associate with TLR2 and abrogated the ability of TLR2 to induce NF-κB transcriptional activity. Subsequent studies by Guha et al. [53] demonstrated that inhibition of PI3K resulted in the elevated production of several pro-inflammatory cytokines and this effect was associated with increased NF-κB p65 activity (Figure 2). However, it was not until the generation of PI3K KO mice that the impact of the PI3K pathway on the host inflammatory response was appreciated. Studies by Fukao et al. [54, 55] reported that mice deficient in the regulatory subunit of class IA PI3K (p85α) exhibited enhanced T helper 1 (Th1)-like immune responses to an intestinal nematode and were unable to clear the infection. Also, p85α KO mice on the BALB/c background were shown to be resistant to Leishmania major infection, unlike wild-type control mice, and exhibited augmented Th1-associated immunity (Figure 3). An assessment of the innate immune response in PI3K KO mice identified that the alterations in Th1 and Th2 immunity were likely the result of elevated IL-12 production by dendritic cells [54, 55] (Figure 3). It was subsequently demonstrated that the activation of the PI3K pathway by a TLR2-agonist exhibited differential effects on the production of the prototypical anti-inflammatory cytokine IL-10 and the pro-inflammatory cytokine IL-12 [56] (Figure 2). Specifically, these studies showed that the inhibition of PI3K activity reduced the production of IL-10, whereas IL-12 levels were enhanced. An assessment of mRNA levels further suggested that the ability of the PI3K pathway to differentially control these two key immunoregulatory cytokines was due to a transcriptional event [56] (Figure 2). Using both pharmacological and genetic approaches, it was shown that the inhibition of PI3K resulted in the loss of several downstream targets within the PI3K pathway that were activated in LPS-stimulated cells [28, 53, 56]. LPS was shown to induce the phosphorylation of Akt on both threonine 308 and serine 473 in which the blockade of PI3K attenuated the site-specific dual phosphorylation of Akt [28, 53, 56, 57]. The direct inhibition of Akt in monocytes resulted in a pro-inflammatory phenotype similar to that observed with PI3K inhibition [28]. The identification of which isoform of Akt was involved in regulating the downstream signaling properties of PI3K in innate immune cells has recently been elucidated by the findings of Androulidaki et al. using mice deficient in Akt1 or Akt2 [58]. Akt1 KO mice exhibited a hyper-inflammatory response to LPS and did not develop tolerance to endotoxin (Figure 3). Interestingly, while mice deficient in Akt1 produced elevated levels of inflammatory cytokines in response to LPS, mice deficient in Akt2 did not exhibit the same phenotype.
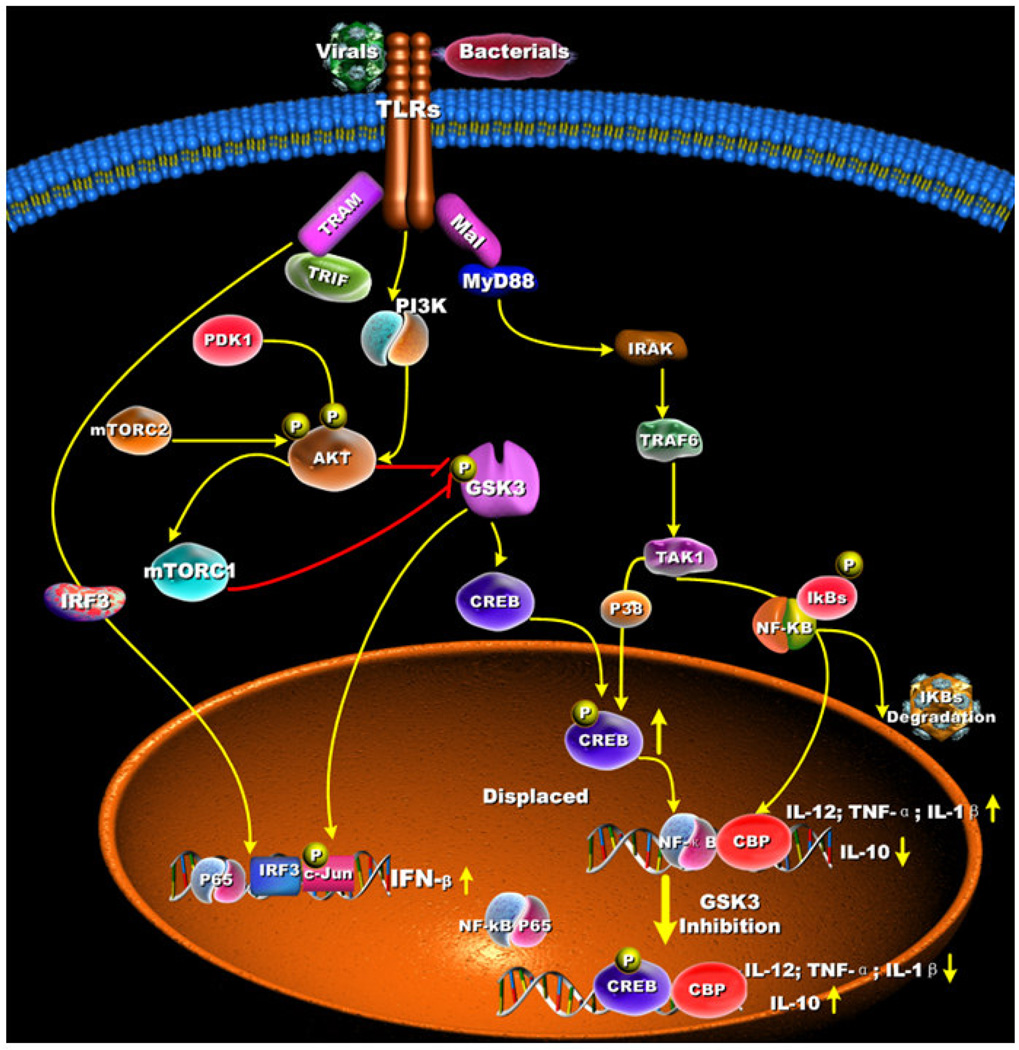
Figure 2. GSK3 regulation of MyD88-dependent and MyD88-independent cytokine production.
GSK3 differentially regulates the production of pro- and anti-inflammatory cytokine production by TLR-stimulated innate immune cells. For MyD88-dependent signaling, TLR-mediated inactivation of GSK3-β (Ser9) leads to the increased nuclear levels of 38 CREB (Ser133) that displaces NF-κB p65 from the co-activator of transcription CBP, which leads to lowered NF-κB p65 transcriptional activity. The increased transcriptional activity of CREB promotes IL-10 production. In contrast, the reduced transcriptional activity of NF-κB p65 results in the suppressed production of pro-inflammatory cytokines. For MyD88-independent signaling, the inactivation of GSK3-β (Ser9) results in increased levels of the transcription factor c-Jun that, in turn, promotes IFN-β production.
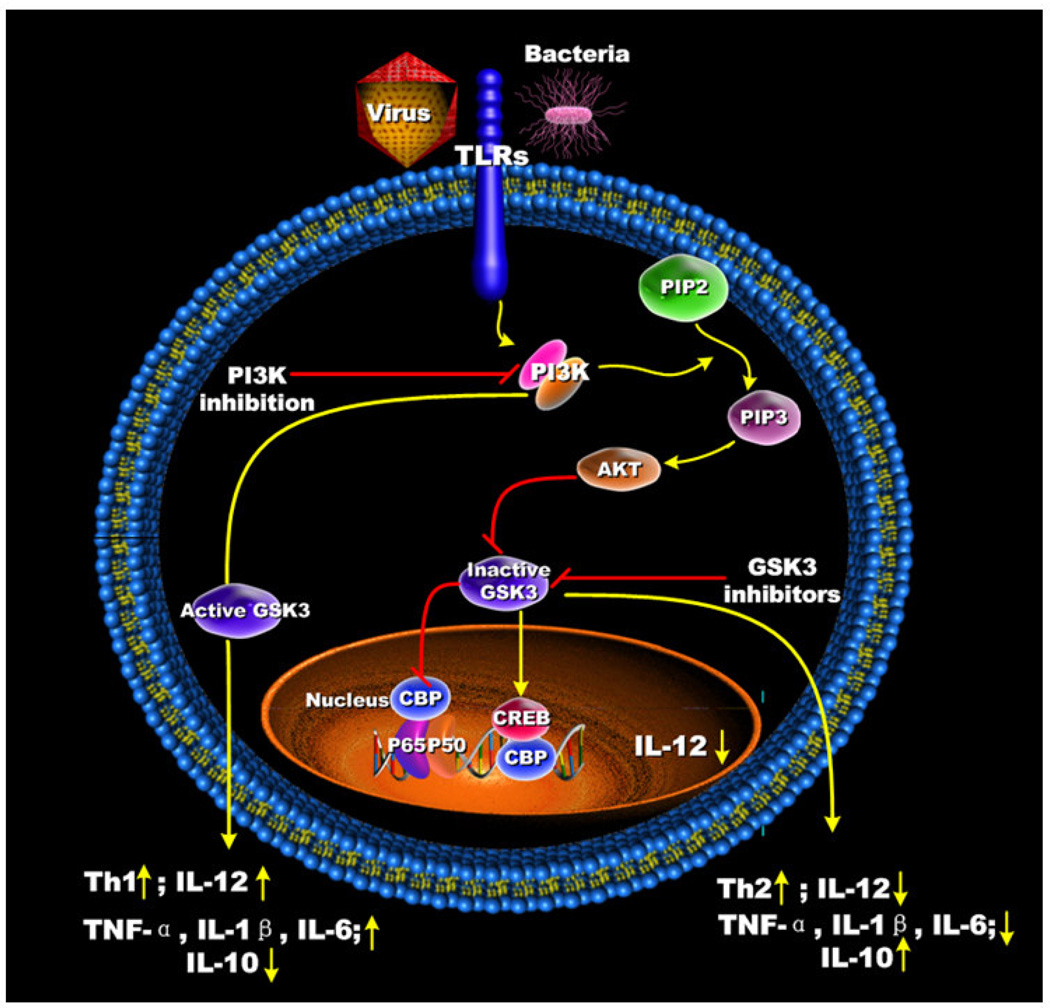
Figure 3. Regulation of PI3K and GSK3 mediates different outcomes on Th1- and Th2-type immune response.
PI3K/Akt phospho-inactivation of GSK3 augments Th2 type immune responses by attenuating IL-12 production and increasing IL-10 levels from TLR-stimulated innate immune cells. The enhanced Th1 type immune responses upon PI3K inhibition are the result of elevated IL-12 production from TLR-stimulated innate immune cells due to their inability to inactivate GSK3.
By comparing downstream targets of the PI3K pathway that were affected by either PI3K or Akt inhibition, it was identified that both of these molecules were involved in the ability of TLRs to mediate the phosphorylation of GSK3-β (serine 9) [53, 56] (Figure 3). siRNA-mediated knockdown of GSK3-β or pharmacological inhibition of GSK3 using a panel of different selective GSK3 inhibitors suppressed the production of IL-1β, IL-6, TNF, and IL-12, whereas the production of IL-10 was increased in TLR2-, TLR4-, TLR5- and TLR9-stimulated cells [28] (Figure 3). The importance of the GSK3-β isoform in controlling the inflammatory response to LPS was confirmed using mouse embryonic fibroblasts that were deficient in GSK3-β. Due to the capacity of GSK3 to differentially control the production of IL-10 and IL-12, Ohtani et al. set forth to determine the in vivo relevance of GSK3 inhibition by assessing the ability of GSK3 to alter Th1- and Th2-responses in mice using a Listeria major infection model [59]. For these studies, Ohtani et al. used PI3K (p85α) KO mice on the BALB/c background, which unlike their wild-type BALB/c littermate controls, are able to mount an effective Th1 response. In contrast to PI3K KO mice, inhibition of GSK3 in PI3K KO mice resulted in enhanced footpad swelling and these mice were unable to control the infection to L. major.
Studies by Hu et al. have identified the cellular mechanisms involved in the ability of IFN-γ to suppress TLR2-induced IL-10 production [10] (Figure 4). These studies reported that IFN-γ treatment of macrophages augmented the degradation of the inhibitory protein molecule IκB-α and increased NF-κB activity in TLR2-stimulated cells. In contrast, IFN-γ treatment suppressed the phosphorylated levels of GSK3-α/GSK3-β, increased the kinase activity of GSK3, and attenuated the IL-10-dependent phosphorylation of STAT3 in TLR2-stimulated macrophages. Inhibition of GSK3 abolished the ability of IFN-γto suppress the levels of IL-10 produced by TLR2-stimulated macrophages (Figure 4). Using siRNA targeting GSK3-β, or chimeric mice reconstituted with GSK3-β-deficient fetal liver cells, it was shown that the ability of IFN-γ to increase GSK3-β but GSK3-α activity was responsible for the reduced IL-10 levels produced by TLR2-stimulated cells. These findings identified GSK3-β as a key player involved in regulating the inflammatory properties of IFN-γ.
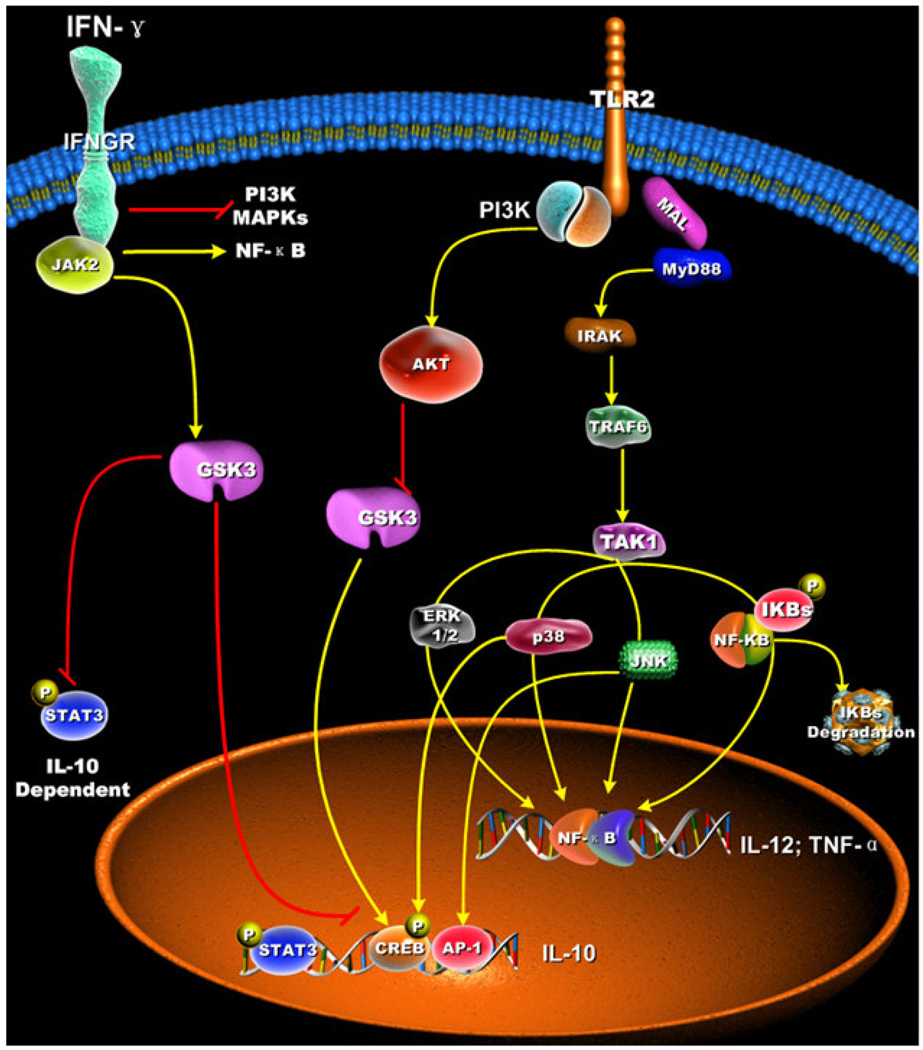
Figure 4. IFN-γ suppresses TLR2-mediated IL-10 production by increasing GSK3 activity.
IFN-γ treated macrophages stimulated with a TLR2 agonist produced elevated levels of TNF-α and IL-12, whereas IL-10 production was reduced. Treatment of macrophages with IFN-γ resulted in increased GSK3 activity, degradation of IκBα, and NF-κB transcriptional activity, whereas the activation of MAPKs (p38, JNK1/2, and ERK1/2), and IL-10-dependent STAT3 phosphorylation were reduced. The ability of IFN-γ to 39 suppress IL-10 and IL-10 dependent STAT3 activation was due to increased GSK3 activity.
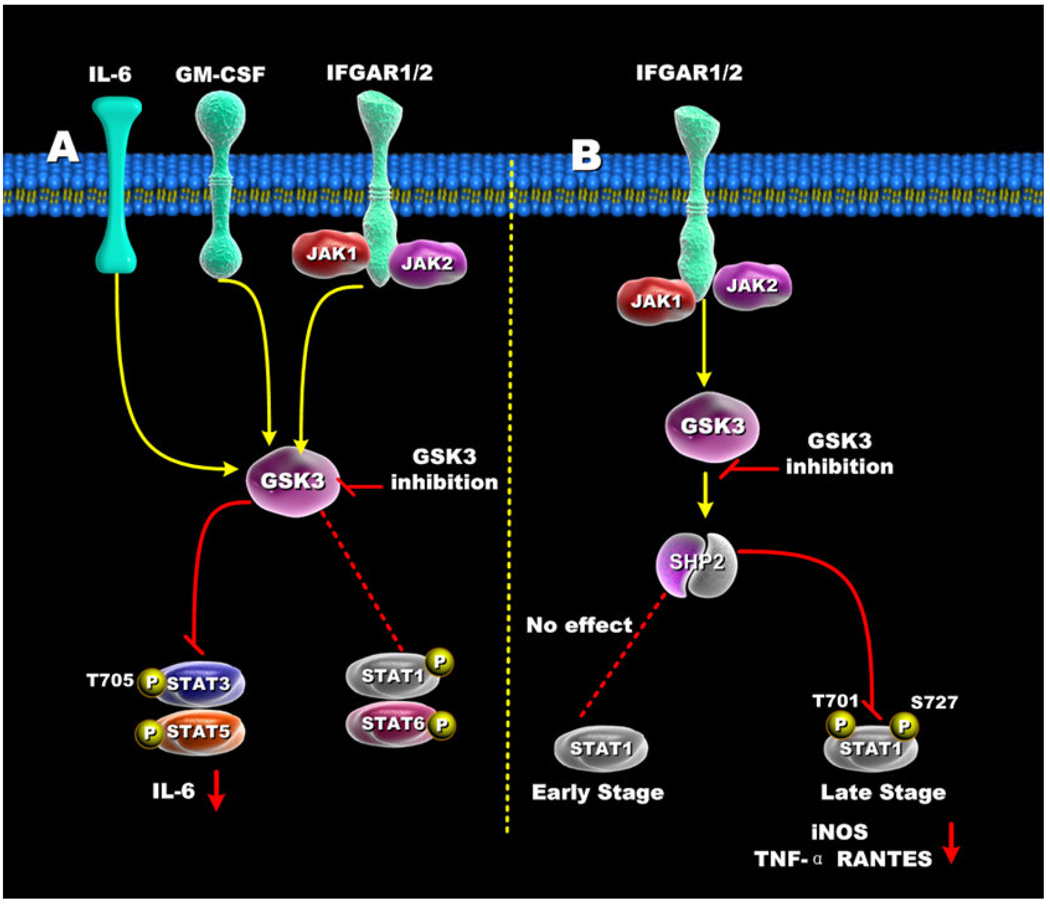
The studies of Hu et al. clearly defined a role for GSK3 within the IFN-γ- and TLR2-signaling pathways and demonstrated how GSK3 could indirectly affect STAT3 activation by its capacity to regulate IL-10 production [10] (Figure 5A). A direct interaction of GSK3 with a STAT family member was originally identified in Dictyostelium by showing that a homolog of GSK3 mediated the nuclear export of the Dictyostelium STAT protein Dd-STATα in cAMP-stimulated cells [60]. Beurel et al. also reported that GSK3 activity positively influenced the tyrosine phosphorylation and DNA-binding activity of STAT3 in response to several different cytokines, including both type I (IFN-α) and type II (IFN-γ) interferons, GM-CSF, and IL-6 [61] (Figure 5A). Interestingly, GSK3 activity appeared to strongly influence the tyrosine 705 phosphorylation of STAT3 but not the serine 727 phosphorylation of STAT3 in IFN-γ-stimulated murine astrocytes [61]. In this regard, it has recently been demonstrated by Samavati et al. that the phosphorylation of tyrosine 705 on STAT3 was important for the production of IL-1β and IL-6 production by LPS-stimulated cells [62]. Thus, the ability of GSK3 inactivation to strongly suppress the phosphorylation of STAT3 on tyrosine 705 may aid in understanding previous findings showing GSK3 inhibition potently suppressed IL-1β and IL-6 production by LPS-stimulated innate immune cells. A detailed comparison of STAT family members showed that GSK3 exhibited selectivity in its ability to influence STAT proteins. For example, whereas the activation of STAT3 and STAT5 were strongly reduced in the presence of GSK3 inhibition, STAT1 and STAT6 activity were minimally affected in astrocytes, microglia, and a murine macrophage cell-line [61] (Figure 5A). The selective knockdown of GSK3-β but not GSK3-α was demonstrated to be responsible for affecting STAT3 and STAT5 activation. The ability of GSK3 to positively affect STAT3 activation was shown to be critical for the production of IL-6 in the brain following LPS challenge, since the inhibition of GSK3 reduced the activation of STAT3 in the brain and reduced IL-6 levels in both the periphery and brain [63]. Tsai et al. have additionally reported that GSK3-β inhibition differentially affected the early and late phases of STAT1 phosphorylation [64] (Figure 5B). Although early STAT1 (1 hr or less post-stimulation) activation was not discernibly affected by GSK3 inhibition, later time points (> 1hr post-stimulation) of STAT1 activation were affected. GSK3 inhibition was shown to greatly diminish both serine 727 and tyrosine 701 phosphorylation of STAT1. These studies further elucidated the cellular mechanism by which GSK3 affected the phosphorylation of STAT1. Tsai et al. identified that the inhibition of GSK3 resulted in the activation of the Src homology-2 domain-containing phosphatase 2 (SHP2), which then mediated the dephosphorylation of STAT1 [64]. The functional significance of this was demonstrated by the findings that GSK3 inhibition suppressed the production of iNOS, nitrite, TNF, and RANTES by IFN-γ stimulated macrophages (Figure 5B). Taken together, these studies highlight the ability of GSK3 to differentially influence the activation of specific STAT family members and the importance this effect has on the inflammatory response. While it is clear that GSK3 inhibition negatively affected STAT3 activation, it will be interesting for future studies to elucidate the overall impact GSK3 plays in both the immediate and later stages of STAT3 activity. For example, GSK3 inhibition has been shown to augment the levels of IL-10 produced by LPS-stimulated innate immune cells. Since IL-10 receptor-signaling is a strong inducer of STAT3 activity, and STAT3 is required to mediate the anti-inflammatory properties of IL-10 [65], it will be interesting to determine how the ability of GSK3 to augment IL-10 production, but negatively regulate STAT3 activation, affects the overall anti-inflammatory properties of GSK3 inhibition. Nevertheless, it seems unlikely that the anti-inflammatory properties of GSK3 are a direct result of its capacity to augment IL-10 levels and affect the subsequent IL-10 mediated activation of STAT3 because it has been reported that the inactivation of GSK3 in macrophages still resulted in the suppression of pro-inflammatory cytokines in IL-10 deficient mice [59].
Figure 5. Regulation of STATs by GSK3.
(A) In IFN-γ, GM-CSF, and IL-6 stimulated cells, GSK3 inhibition suppressed both STAT3 and STAT5 activity, but only minimally affected STAT1 and STAT6 activity. (B) GSK3-β inhibition differentially affects early and late STAT1 activation in IFN-γ-treated cells. Inhibition of GSK3 had no effect on early STAT1 activity (<1hr) but diminished late STAT1 phosphorylation by increasing SHP-2 activity. The ability of GSK3 inhibition to reduce STAT1 activity resulted in reduced levels of iNOS, TNF, and RANTES by IFN-γ-stimulated macrophages.
Activation of TLRs can result in the recruitment of different downstream signaling adaptors that impart selectivity on the repertoire of cytokines produced. The TLR4-signaling pathway has been reported to activate two distinct cell-signaling pathways based on the recruitment of the adaptor molecules TIRAP-MyD88 or TRAM-TRIF [66–69] (Figure 2). The production of pro- and anti-inflammatory cytokines by TLR4-stimulated innate immune cells has been shown to be dependent upon signaling events initiated by TIRAP-MyD88, whereas the production of type I IFNs has been shown to be regulated, in large part, independently of MyD88 but does require the TRAM-TRIF-mediated pathway [66–69]. As the aforementioned studies have demonstrated, stimulation of TLR4 can activate the PI3K pathway, which restrains the MyD88-dependent production of pro-inflammatory cytokines by suppressing GSK3 activity (Figure 2). However, it still remained unclear if GSK3 played a functional role in MyD88-independent signaling. Studies by Wang et al. demonstrated that LPS-stimulated MyD88-deficient macrophages were still able to mediate the phosphorylation of GSK3-β (Serine 9) and that the magnitude and kinetics of GSK3-β (Serine 9) phosphorylation were similar to that observed in wild-type cells [29] (Figure 2). Further studies identified that GSK3-β negatively regulated the production of IFN-β through its ability to phosphorylate the transcription factor c-Jun [70, 71]. Thus, the molecular mechanisms by which GSK3-β regulates MyD88-dependent and MyD88-indpendent cytokine responses appear to involve different molecular pathways (Figure 2).
The roles of GSK3 in dendritic cell (DC) maturation, survival, and cytokine production have also been studied in detail by several laboratories. Studies by Rodionova et al. showed that GSK3 inhibited the maturation process of immature DC and suppressed the IL-4/GM-CSF-mediated differentiation of human monocytes into DC [72]. Flow cytometric analysis of these cells showed GSK3 inhibition resulted in a macrophage-like morphology/phenotype and that GSK3-inhibited monocytes cultured in IL-4 and GM-CSF lacked CD83 expression and exhibited low levels of the co-stimulatory molecules CD80 and CD86. Inhibition of GSK3 in DC stimulated with CD40L or E. coli suppressed their ability to produce pro-inflammatory cytokines. Ono et al. further reported that the inhibition of GSK3 impaired the capacity of GM-CSF-derived DC to induce Th2 but not Th1 responses and that these GSK3-inhibited cells lacked an ability to produce IL-6 in response to CD40L stimulation [73]. It has also recently been reported that probiotic bacteria mediate the inactivation of GSK3 and this effect was shown to play a key role in the ability of the probiotic bacterium, Bifidobacterium breve, to promote DC survival and induce high levels of IL-10 [74]. Due to the important role of IL-10 in maintaining immune homeostasis, it will be interesting for future studies to determine if these finding by Hoarau et al. [74] are indicative of probiotic bacterium in general and, if so, is this quality of probiotic bacteria to inactivate GSK3 and induce elevated levels of IL-10 involved in their therapeutic value to treat inflammatory disorders.
GSK3 has also been shown to be involved in the anti-inflammatory properties of the cholinergic pathway. The cholinergic anti-inflammatory pathway acts through the activation of the α7 nicotinic acetylcholine receptor (α7nAChR) on innate immune cells, particularly by vagus nerve–derived acetylcholine [75–77]. Indeed, vagotomized animals are exquisitely sensitive to bacterial-induced inflammatory shock [78]. The cellular mechanism by which α7nAChR activation dampens inflammation has been shown to involve multiple intracellular signalling pathways, including activation of the PI3K/Akt/GSK3-β pathway. The interaction of cotinine, the major metabolite of nicotine, with the α7nAChR leads to the activation of PI3K; the production of PIP3; the phosphorylation and activation of Akt; and, subsequently, the phosphorylation and inhibition of GSK3-β [79]. The ability of cotinine to induce the inactivation of GSK3-β does not affect the absolute or phosphorylated (Serine 276) levels of NF-κB p65 but does increase the levels of CREB (Serine133) [79]. Cotinine was also demonstrated to suppress the production of IL-1β, IL-6, TNF, and IL-12 while augmenting the production of IL-10 from LPS-stimulated monocytes. Thus, many of the anti-inflammatory properties of cotinine can be mimicked by direct GSK3-βinhibition, and alternatively, can be attenuated by preventing the activation of PI3K. Future studies will need to employ the use of constitutively active mutants of GSK3-β to directly determine the correlation between the anti-inflammatory properties of cotinine and its influence on GSK3-β activity.
GSK3 and its role in the adaptive immune system
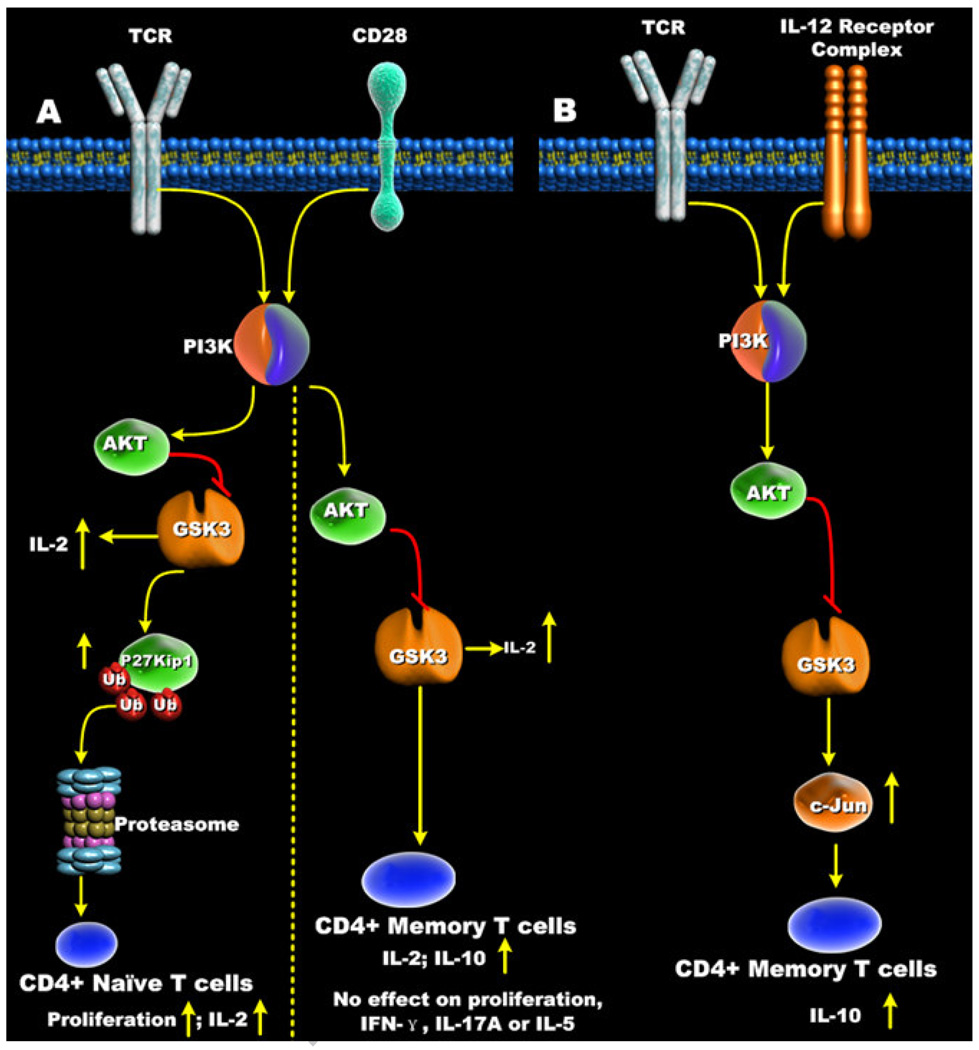
Studies by Welsh et al. were one of the first reports that identified that the activity of GSK3 was affected in mitogen-stimulated human T cells [80]. The role of GSK3 in regulating antigen-specific CD8+ T cells was subsequently reported by Ohteki et al. and showed that antigen-specific stimulation of CD8+ T cells resulted in the inactivation of GSK3 [81]. GSK3 inactivation in CD8+ T cells was shown to enhance IL-2 production and increase cellular proliferation (Figure 6A). Other studies identified that GSK3 was an important effector molecule involved in the B7-CD28 co-stimulatory signaling pathway, in which the B7 isoforms, B7-1 (CD80) and B7-2 (CD86), interact with CD28 expressed on T cells. CD28 co-stimulation of T cells was shown to increase PI3K activity and mediate the inactivation of GSK3 [82–86]. Interestingly, pharmacological inactivation of GSK3 in human T cells has been shown to closely mimic the CD28 co-stimulatory signal for T cell proliferation [87, 88] (Figure 6A). Although it is not completely understood how GSK3 controls T cell proliferation, retroviral-mediated expression of a constitutively active GSK3-β mutant in murine TCR-transgenic CD8+ T cells resulted in decreased proliferation and suppressed IL-2 production upon TCR stimulation [81]. Moreover, both CD28 co-stimulation and pharmacological inhibition of GSK3 have been reported to promote the degradation of the cyclin-dependent kinase inhibitor p27kip1 in human CD4+ T cells, an event favoring the G1-S phase transition of TCR-stimulated T cells [87] (Figure 6A). Taken together, these findings demonstrate that GSK3 plays a prominent role in regulating IL-2 production and proliferation of T cells.
Figure 6. Effects of GSK3 inhibition on CD4+ T cell responses.
(A) Inactivation of GSK3-β in naïve CD4+ T cells increased both proliferation and IL-2 production. In contrast, inhibition of GSK3-β in memory CD4+ T cells increased IL-2 and IL-10 production but had no effect on memory CD4+ T cell proliferation or production of IL-5, IFN-γ, or IL-17. (B) IL-12 receptor signaling results in the phospho-inactivation of GSK3-β. Inactivation of GSK3-β by IL-12 increased total c-Jun levels that promoted IL-10 production by CD3/IL-12-stimulated memory CD4+ T cells.
Studies defining the impact of B7-CD28-signalling on naïve and memory CD4+ T cell responses identified several important differences [89]. Naïve CD4+ T cell proliferation was shown to be dependent on CD28 co-stimulation whereas memory CD4+ T cell proliferation could occur in its absence (Figure 6A). However, several laboratories reported that the CD28 signaling pathway still influenced memory CD4+ T cell function [90, 91], thus raising the question of whether CD28 triggers distinct signaling pathways in naïve and memory CD4+ T cells. Since previous studies investigating the functional role of GSK3 in human T cells utilized un-fractionated T cells or un-fractionated CD4+ T cells [87, 88], it was unclear if CD28-mediated inactivation of GSK3 was also differentially influencing the cellular responses of naïve and memory CD4+ T cells. To address this issue, naïve and memory CD4+ T cells were cell-sorted, and the role of GSK3 in CD28-signaling was evaluated. CD28 co-stimulation enhanced the frequency of naïve and memory CD4+ T cells displaying phosphorylated GSK3-β (Serine 9) when stimulated with anti-CD3 [30] (Figure 6A). The inhibition of GSK3 exhibited similar effects as CD28 co-stimulation in naïve cells, since the proliferative capacity of anti-CD3-stimulated naïve CD4+ T cells was similarly augmented in the presence of either GSK3 inhibition or CD28 activation. In contrast, the ability of GSK3 inactivation or CD28 co-stimulation to enhance memory CD4+ T cell proliferation was only observed at low TCR (anti-CD3) signal strengths [30]. Analyzing the number of cellular division cycles influenced by GSK3 also yielded similar results. Inactivation of GSK3 in naïve CD4+ T cells increased the frequency of cells undergoing at least one cellular division, as well as increasing the mean cycle number of cells displaying at least one cellular division. In contrast, no discernible differences in the frequency of cells undergoing at least one cellular division or in the mean cycle number was observed in memory CD4+ T cells stimulated in the presence of the GSK3 inhibitor, lithium, or CD28 co-stimulation. These differences in proliferation between naïve and memory CD4+ T cells upon GSK3 inhibition correlated with the degradation of the cyclin dependent kinase inhibitor p27kip1, as the pharmacological inhibition of GSK3 decreased the cellular levels of p27kip1 in naïve, but not memory CD4+ T cells upon TCR (anti-CD3) cross-linking (Figure 6A). Despite these findings showing the decreased dependence on GSK3 inactivation for memory CD4+ T cell proliferation, the production of IL-2 by both naïve and memory CD4+ T cells was shown to be strongly dependent on GSK3 [30].
One of the many phenotypic differences between naive and memory CD4+ T cells is their capacity to produce effector cytokines, including IL-2, IL-4, IL-5, IL-10, IFN-γ, and IL-17. Previous studies analyzing the role of GSK3 in regulating IL-2 production by naïve CD4+ T cells, memory CD4+ T cells, and CD8+ T cells clearly demonstrated that the GSK3-β isoform negatively regulated the ability of these cells to produce this important immunomodulatory cytokine (Figure 6A). Previous studies by Okamoto et al. demonstrated that the production of the anti-inflammatory cytokine IL-10 by human memory CD4+ T cells was dependent upon the activation of the PI3K-signaling pathway [92]. Since CD28 co-stimulation of CD4+ T cells was previously shown to repress GSK3 activity in a PI3K-dependent manner, Garcia et al. set forth to determine if the reported ability of the CD28-signaling pathway to augment IL-10 production by CD4+ T cells was due to the ability of CD28 to inactivate GSK3. Inhibition of PI3K or Akt led to a reduction in the levels of phosphorylated GSK3-β (Serine 9), and reduced the levels of IL-10, IFN-γ, IL-17A, and IL-5 produced after anti-CD3/CD28 stimulation (Figure 6A). These findings demonstrated that PI3K and Akt do not selectively regulate IL-10 levels despite being involved in the phosphorylation of GSK3-β (Serine 9). However, it was subsequently shown that the specificity for IL-10 regulation within the PI3K-Akt pathway was mediated by GSK3-β. Moreover, inhibition of GSK3-β in anti-CD3/CD28-stimulated memory CD4+ T cells rescued the deficiencies in IL-10 production observed upon PI3K inhibition but did not influence the production of IL-5, IFN-γ, or IL-17A. Garcia et al. also showed that the inactivation of GSK3-β in anti-CD3-stimulated memory CD4+ T cells selectively increased the production of IL-10 and that the ability of anti-CD3-stimulated memory CD4+ T cells to produce IL-10, but not IL-5, IFN-γ, or IL-17A, required the inactivation of GSK3-β [30] (Figure 6A). These findings identified the fundamental importance of GSK3 in controlling the levels of IL-10 produced by anti- CD3-or anti-CD3/CD28-stimulated human memory CD4+ T cells.
IL-12p70 is an important immunomodulatory cytokine that can induce IFN-γ production from multiple cell types, such as NK cells, B cells, APCs, and T cells. IL-12 can also influence the gene expression profiles of a variety of other genes, including the production of IL-10 by T cells [93–96]. The IL-12-mediated production of IL-10 from T cells has been suggested to function as a negative feedback mechanism, as IL-10 can suppress the production of IL-12 from APCs [97–101]. In fact, studies by Chang et al. identified that the magnitude of the IL-10 recall response of memory CD4+ T cells is dependent on the presence of IL-12 [97]. Studies using human or murine T cells have also demonstrated that IL-12 can condition differentiated CD4+ T cells into producing IL-10 [98, 101]. Yoo et al. identified that the activation of the IL-12 receptor increases PI3K activity [102]. Due to the importance of PI3K and GSK3-β in controlling IL-10 production [30], it seemed likely that the ability of IL-12 to promote IL-10 production from human memory CD4+ T cells was dependent upon the PI3K signaling pathway. Garcia et al. reported that the ability of IL-12 to enhance IL-10 production from memory CD4+ T cells required an intact PI3K signaling pathway [31] (Figure 6B). Moreover, these studies showed that the PI3K-mediated inactivation of GSK3-β was required for IL-12 to promote IL-10 production from memory CD4+ T cells. The inactivation of GSK3-β in anti-CD3/IL-12-stimlated memory CD4+ T cells was shown to lead to the increased levels of the transcription factor c-Jun, and this effect was required for IL-12 to promote IL-10 production (Figure 6B). An interesting finding from these studies was the identification that siRNA-mediated knockdown of c-Jun not only reduced the levels of IL-10 produced by anti-CD3/IL-12-stimulated CD4+ T cells, but also attenuated the levels of IL-10 produced by anti-CD3-stimulated memory CD4+ T cells. These findings suggest that c-Jun likely plays a broader role in controlling IL-10 production by memory CD4+ T cells. Since human CD4+ T cells capable of producing IL-10 are present within the memory CD4+ T cell population [103], and can be produced by a variety of CD4+ T cell subpopulations such as Th1, Th2, Foxp3−, and FoxP3+, it will be interesting to determine if GSK3 and its ability to control c-Jun levels plays a role in the capacity of these CD4+ T cell subpopulations to produce IL-10.
In contrast to the role of GSK3 inhibition in promoting the production of the anti-inflammatory cytokine IL-10 by IL-12-stimulated memory CD4+ T cells, studies by Shen et al. have identified that the inactivation of GSK3 augmented the levels of multiple target genes induced by IL-17 [104]. Moreover, these studies showed that the increased expression of GSK3-β reduced the C/EBP-dependent transcription of IL-6 and 24p3 induced by IL-17-stimulated cells. Since the IL-17 target genes, IL-6 and 24p3, require C/EBP-dependent transcription, these studies also determined how GSK3 was influencing this process. With the aid of GSK3-β deficient cells, it was demonstrated that GSK3 was involved in the phosphorylation of C/EBP-β at threonine 179. The GSK3-β dependent phosphorylation of C/EBP-β, in conjunction with ERK1/2-dependent phosphorylation of C/EBP-β at threonine 188, was demonstrated to suppress the production of inflammatory genes induced by IL-17 receptor signaling. These findings are extremely exciting in that they have highlighted another role for GSK3 in T cell biology. Moreover, inactivation of GSK3 in CD4+ T cells is likely to simultaneously increase their production of IL-10 and the ability of IL-17 to increase the transcription of inflammatory target genes. Although the overall suppressive capacity of IL-10 on Th17 responses remains to be resolved, studies by Fitzergerald et al. have shown that IL-10 production can reduce the levels of IL-17 produced by Th17 cells [105]. The impact these two seemingly opposing pathways would have on dictating the overall CD4+ T cell inflammatory response remains a question that will likely only be answered by assessing the role of GSK3 in vivo. Clearly, additional studies will be required to resolve the overall impact GSK3 has on the inflammatory potential of Th17 cells and Th17-mediated inflammatory diseases.
The ability of GSK3-β to suppress the production of pro-inflammatory cytokines while increasing the levels of IL-10 produced by LPS-stimulated monocytes was shown to involve a molecular competition between the transcription factors CREB and NF-κB p65 for the co-activator of transcription CBP [28] (Figure 2). The nuclear levels of CBP are limiting whereas the nuclear levels of CREB (Serine 133) and NF-κB p65 (Serine 276) can increase upon TLR-stimulation [10, 28, 106–109]. Since CREB (Serine 133) and NF-κB p65 (Serine 276) have the same binding site on CBP, the nuclear amounts of CREB and NF-κB p65 dictate their ability to displace the alternative transcription factor and initiate transcription of CREB or NF-κB p65 responsive genes [107–109]. GSK3 inhibition in LPS-stimulated monocytes was shown to augment the nuclear levels of CREB (Serine 133) while having no discernible effect on NF-κB p65 (Serine 276) levels. As a result, GSK3 inhibition promoted the association of CREB (Serine 133) to CBP while simultaneously reducing the amount of NF-κB p65 (Serine 276) associated with CBP [28]. Due to the importance of CREB in positively influencing the transcriptional regulation of IL-10 [110], and the predominant pro-inflammatory role NF-κB p65 plays in innate immune cells, these findings were in agreement with the capacity of GSK3 inhibition to suppress the inflammatory response. Indeed, siRNA-mediated knockdown of CREB abrogated the ability of GSK3 inhibition to increase the levels of IL-10 and suppress the levels of IL-12 produced by LPS-stimulated monocytes [28].
The suppressive properties of IFN-γ on TLR2-mediated IL-10 production have also been shown to involve GSK3 and its ability to affect the overall levels and transcriptional activity of CREB and AP-1 family members (c-Fos/c-Jun) [10]. These studies identified that the phosphorylated levels of CREB (Serine 133) were reduced when TLR2-stimulated macrophages where cultured in the presence of IFN-γ. Hu et al. reported that inhibition of the mitogen-associated protein kinase (MAPK) p38 mimicked the inhibitory effects of IFN-γ on phosphorylated CREB (Serine 133) levels [10]. Since the transcriptional activity of CREB is largely regulated by phosphorylation at serine 133 [106, 107], p38 is likely involved in the ability of GSK3 to affect IL-10 production due to both GSK3 and p38 influencing the transcriptional activity of CREB. Considering the findings by Thornton et al. [111] demonstrating that p38 can directly phosphorylate and inactivate GSK3, and that downstream substrates of p38, i.e. MAPKAPK-1, can also inactivate GSK3, it will be interesting to dissect the direct and indirect effects of p38 on the inhibition of GSK3 and further define if the ability of p38 to affect GSK3 activity plays a role in the capacity of p38 to regulate the inflammatory response.
Although the cellular mechanisms identifying how GSK3 mediates its anti-inflammatory properties, i.e. IL-10, have been demonstrated to involve CREB and AP-1 family members, GSK3 has been reported to affect a multitude of intracellular signaling pathways that influence the activation of other kinases and transcription factors. Studies by Hoeflich et al. [13] were the first to implicate GSK3-β in regulating NF-κB p65 in which the absence of GSK3-β severely affected the TNF-induced transcriptional activation of NF-κB p65 without altering upstream regulatory events, such as the degradation of IκB-α that masks the nuclear localization signal of NF-κB. The proteolytic processing of NF-κB p105 to generate NF-κB p52 was shown by Demarchi et al. [112] to also involve GSK3-β. Specifically, GSK3-β was shown to phosphorylate NF-κB p105 and this phosphorylation event was shown to prime NF-κB p105 for degradation in cells stimulated with TNF. GSK3 inhibition has also been reported to affect the phosphorylation of NF-κB p65 (Serine 536) in a rat model assessing the protective role of the GSK3 inhibitors SB216763 and TDZD-8 [113–119]. Several studies have also identified that GSK3-β can influence the activation of the MAPK-signaling pathways. Rehani et al. [120] identified that active GSK3-β negatively affected the activation of the MAPK ERK1/2 in LPS-stimulated cells by blunting the activation of ERK1/2 in a rac1-dependent mechanism. This cell-signaling pathway was shown to suppress the production of the anti-inflammatory cytokine IL-1Ra by monocytes stimulated with LPS [120]. GSK3-β has also been reported to affect ERK1/2 activity due to GSK3 negatively regulating PKC-δ [121]. In these studies, inhibition of GSK3 up-regulated the phosphorylated levels of ERK1/2 that, in turn, increased the production of COX-2 and IL-8 in human colon cancer cell-lines. Studies using fibroblasts deficient in GSK3-β have also reported that TNF was unable to activate any of the three classical MAPKs, including ERK1/2, JNK1/2, and p38 [122]. However, unlike the findings by Wang et al. [121], the studies by Takada et al. [122] reported that GSK3-β deficiency attenuated the induction of COX-2. GSK3-β also was recently reported to be required for KIT-SCF signaling in human mast cells [32]. shRNA-mediated knockdown of GSK3-β resulted in blunting the KIT-dependent activation of p38, JNK1/2, c-Jun, ATF-2, and NF-κB. These studies by Radinger et al. [32] identified that GSK3 activity was essential for KIT-mediated cytokine production and chemotaxis by mast cells. Taken together, these studies highlight some of the intracellular signaling pathways that GSK3 has been demonstrated to regulate. Given that GSK3 is ubiquitously expressed and has the ability to phosphorylate such a large number of kinases and transcription factors, the impact of GSK3 in immune cell signaling is likely only beginning to be identified and characterized.
The anti-inflammatory properties of GSK3 inhibition have also been evaluated in different animal models of inflammation. The anti-inflammatory properties of GSK3 inhibition were first evaluated in vivo using the high-dose endotoxin shock model [28]. Mice were administered a GSK3 inhibitor either prophylactically (2 hours before LD100 of LPS) or therapeutically (2 hours after LD100 of LPS) and administered an LD100 of LPS. Survival rates were greater than 70% and 50% in mice given the GSK3 inhibitor SB216763 prophylactically or therapeutically, respectively. In contrast, the survival rates of control mice were 0%. GSK3 inhibition also greatly suppressed (50 to 90%) the levels of the pro-inflammatory cytokines, IL-6, IL-12 p40, and IFN-γ whereas IL-10 levels were increased by more than two-fold [28]. Cuzzocrea et al. [113–118] have also shown that the non-ATP competitive GSK3 inhibitor TDZD-8 suppressed the renal, pancreatic, and neuromuscular damage induced by LPS (with or without peptidoglycan) in a rat model. GSK3 inhibition has also been shown to exhibit protection against other septic associated events, i.e. hemorrhage and resuscitation, by reducing both renal dysfunction and liver injury [119]. Recent work by Zhang et al. [123] has also demonstrated the importance of GSK3 in protecting the host against infection using intact, live bacterium in that lithium treatment of mice reduced the levels of pro-inflammatory cytokines and increased survival rates to F. tularensis infection. The anti-inflammatory properties of GSK3 inhibition were also evaluated in a TLR2-mediated mouse model for collagen-induced arthritis and peritonitis [10]. Mice given the GSK3 inhibitor lithium had greatly reduced joint swelling and peritoneal cell numbers, as compared to control mice [117, 124]. GSK3-β haploinsufficiency also resulted in greatly diminished peritoneal cells numbers, as compared wild-type control mice [124]. Mouse chimeras in which irradiated mice were reconstituted with GSK3-β (+/−) or GSK3-β (−/−) fetal liver cells had greater than a 50% reduction in the number of peritoneal cells induced by the TLR2-agonist Pam3-Cys, as compared to GSK3-β (+/+) mice [10]. Cuzzocrea and colleagues have reported similar findings in a collagen-induced arthritis model using type II collagen and complete Freund’s Adjuvant [117]. Mice given a GSK3 inhibitor exhibited reduced paw edema, reduced weight loss, attenuated production of inflammatory mediators, and suppressed bone erosion, as compared to control mice [116]. The broad-spectrum influence of GSK3 inhibition on the inflammatory response was demonstrated using an EAE mouse model [125]. Inhibition of GSK3 reduced the clinical score of EAE in mice regardless of whether lithium was administered before or after clinical symptoms appeared. Histological analysis of the spinal cord also demonstrated that GSK3 inhibition suppressed spinal cord demyelination and reduced the infiltration of both CD4+ T cells and neutrophils [125]. These studies also showed that lithium treatment of mice reduced the proliferation and production of IFN-γ, IL-6, and IL-17 by MOG(35–55)-peptide stimulated splenocytes [125]. Other reports have implicated an immunoprotective role for GSK3 in the treatment of asthma [126], TNB-induced colitis [127], acute ischemia-reperfusion [128], enhanced survival in NZB/W lupus mice [129], and the reduction in secondary damage in response to spinal cord trauma [115]. Taken together, these studies demonstrate the importance of GSK3 in regulating a vast number of inflammatory responses and the potential therapeutic value of GSK3 inhibitors for the treatment of inflammatory diseases.
Understanding the intra-cellular signaling networks that dictate the host’s inflammatory response are critical for the identification of potential therapeutic targets. The ability of GSK3 to regulate the inflammatory response in both innate and adaptive immune cells shows the central importance this kinase plays in controlling inflammation. Animal models have demonstrated that GSK3 inhibitors can suppress inflammation and protect the host against inflammatory-mediated pathology and death. The fact that GSK3 is at the junction of so many intracellular signaling pathways involved in controlling inflammation makes it an attractive candidate for the treatment of inflammatory diseases. However, due to the involvement of GSK3 in many other physiological processes outside of the immune system, future studies will be needed to determine the short- and long-term effects of GSK3 inhibition on the host.
Abbreviations Used in This Paper
- α7NaChR
alpha7 nicotinic acetylcholine receptors
- AP-1
activator protein 1
- APC
Antigen presenting cells
- ATF-2
Activating transcription factor 2
- C/EBP
CCAAT/enhancer-binding protein
- CBP
CREB-binding protein
- Cox-2
cyclooxygenase-2
- CREB
cAMP response element-binding
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- ERK
extracellular-signal-regulated kinases
- Foxp3
forkhead box P3
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GSK3
Glycogen synthase kinase-3
- IFN
Interferons
- IL-17
Interleukin-17
- iNOS
Inducible nitric oxide synthase
- IRF-3
Interferon regulatory factor-3
- IκB
Inhibitor of κB
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- MAPK
mitogen-activated protein kinases
- MyD88
myeloid differentiation factor 88
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK cells
Natural killer cells
- PI3K
Phosphoinositide 3-kinase
- PIP3
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3)
- PKA
cAMP-dependent protein kinase or cAPK
- PKC
Protein kinase C
- RANTES
regulated upon activation, normal T cell expressed and secreted
- RSK
Ribosomal S6 kinases
- S6K
S6 kinase
- SCF
Stem cell factor
- STAT
Signal Transducers and Activator of Transcription
- TCR
T cell receptor
- TIRAP
TIR-associated protein
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- TRAM
TRIF-related adaptor molecule
- TRIF
TIR-domain-containing adapter-inducing interferon-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 2.Rylatt DB, Aitken A, Bilham T, Condon GD, Embi N, Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur J Biochem. 1980;107:529–537. [PubMed] [Google Scholar]
- 3.Woodgett JR, Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5) Biochim Biophys Acta. 1984;788:339–347. doi: 10.1016/0167-4838(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 4.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3--an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 9.Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions, and regulation. Chem. Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, Woodgett JR, Ivashkiv LB. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Patel S, Woodgett J. Glycogen synthase kinase-3 and cancer: good cop, bad cop? Cancer Cell. 2008;14:351–353. doi: 10.1016/j.ccr.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. Embo J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 14.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberte C, Khan M, Okamoto K, Chambers JW, Fletcher PJ, Macaulay K, Doble BW, Henkelman M, Miyakawa T, Roder J, Woodgett JR. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol Brain. 2009;2:35. doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulou D, Bianchi MW, Bourouis M. Functional studies of shaggy/glycogen synthase kinase 3 phosphorylation sites in Drosophila melanogaster. Mol Cell Biol. 2004;24:4909–4919. doi: 10.1128/MCB.24.11.4909-4919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruel L, Stambolic V, Ali A, Manoukian AS, Woodgett JR. Regulation of the protein kinase activity of Shaggy(Zeste-white3) by components of the wingless pathway in Drosophila cells and embryos. J Biol Chem. 1999;274:21790–21796. doi: 10.1074/jbc.274.31.21790. [DOI] [PubMed] [Google Scholar]
- 18.Garrido JJ, Simon D, Varea O, Wandosell F. GSK3 alpha and GSK3 beta are necessary for axon formation. FEBS Lett. 2007;581:1579–1586. doi: 10.1016/j.febslet.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Mukai F, Ishiguro K, Sano Y, Fujita SC. Alternative splicing isoform of tau protein kinase I/glycogen synthase kinase 3beta. J Neurochem. 2002;81:1073–1083. doi: 10.1046/j.1471-4159.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 20.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 21.Cross DA, Alessi DR, Vandenheede JR, McDowell HE, Hundal HS, Cohen P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303(Pt 1):21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross DA, Watt PW, Shaw M, van der Kaay J, Downes CP, Holder JC, Cohen P. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. 1997;406:211–215. doi: 10.1016/s0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- 23.Brady MJ, Bourbonais FJ, Saltiel AR. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3-L1 cells. J Biol Chem. 1998;273:14063–14066. doi: 10.1074/jbc.273.23.14063. [DOI] [PubMed] [Google Scholar]
- 24.Shaw M, Cohen P. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett. 1999;461:120–124. doi: 10.1016/s0014-5793(99)01434-9. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong JL, Bonavaud SM, Toole BJ, Yeaman SJ. Regulation of glycogen synthesis by amino acids in cultured human muscle cells. J Biol Chem. 2001;276:952–956. doi: 10.1074/jbc.M004812200. [DOI] [PubMed] [Google Scholar]
- 26.Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of system A amino acid transport. Biochem J. 2000;350(Pt 2):361–368. [PMC free article] [PubMed] [Google Scholar]
- 27.Krause U, Bertrand L, Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur J Biochem. 2002;269:3751–3759. doi: 10.1046/j.1432-1033.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- 28.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Garcia CA, Rehani K, Cekic C, Alard P, Kinane DF, Mitchell T, Martin M. IFN-beta production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-beta. J Immunol. 2008;181:6797–6802. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia CA, Benakanakere MR, Alard P, Kosiewicz MM, Kinane DF, Martin M. Antigenic experience dictates functional role of glycogen synthase kinase-3 in human CD4+ T cell responses. J Immunol. 2008;181:8363–8371. doi: 10.4049/jimmunol.181.12.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia CA, Wang H, Benakanakere MR, Barrett E, Kinane DF, Martin M. c-jun controls the ability of IL-12 to induce IL-10 production from human memory CD4+ T cells. J Immunol. 2009;183:4475–4482. doi: 10.4049/jimmunol.0901283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radinger M, Kuehn HS, Kim MS, Metcalfe DD, Gilfillan AM. Glycogen synthase kinase 3beta activation is a prerequisite signal for cytokine production and chemotaxis in human mast cells. J Immunol. 2010;184:564–572. doi: 10.4049/jimmunol.0902931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 34.Saito Y, Vandenheede JR, Cohen P. The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem J. 1994;303(Pt 1):27–31. doi: 10.1042/bj3030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol. 2002;22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballou LM, Tian PY, Lin HY, Jiang YP, Lin RZ. Dual regulation of glycogen synthase kinase-3beta by the alpha1A-adrenergic receptor. J Biol Chem. 2001;276:40910–40916. doi: 10.1074/jbc.M103480200. [DOI] [PubMed] [Google Scholar]
- 37.Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheridan CM, Heist EK, Beals CR, Crabtree GR, Gardner P. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J Biol Chem. 2002;277:48664–48676. doi: 10.1074/jbc.M207029200. [DOI] [PubMed] [Google Scholar]
- 39.Tanji C, Yamamoto H, Yorioka N, Kohno N, Kikuchi K, Kikuchi A. A-kinase anchoring protein AKAP220 binds to glycogen synthase kinase-3beta (GSK-3beta) and mediates protein kinase A-dependent inhibition of GSK-3beta. J Biol Chem. 2002;277:36955–36961. doi: 10.1074/jbc.M206210200. [DOI] [PubMed] [Google Scholar]
- 40.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 41.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 42.Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW, Roach PJ. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987;262:14042–14048. [PubMed] [Google Scholar]
- 43.Thomas GM, Frame S, Goedert M, Nathke I, Polakis P, Cohen P. A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of axin and beta-catenin. FEBS Lett. 1999;458:247–251. doi: 10.1016/s0014-5793(99)01161-8. [DOI] [PubMed] [Google Scholar]
- 44.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 45.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue ofthe Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 46.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 47.Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 48.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 50.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 51.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 52.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 53.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 54.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 55.Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata JJ, Koyasu S. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 56.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J. Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 57.Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 58.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginger RS, Dalton EC, Ryves WJ, Fukuzawa M, Williams JG, Harwood AJ. Glycogen synthase kinase-3 enhances nuclear export of a Dictyostelium STAT protein. Embo J. 2000;19:5483–5491. doi: 10.1093/emboj/19.20.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J Biol Chem. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samavati L, Rastogi R, Du W, Huttemann M, Fite A, Franchi L. STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol. 2009;46:1867–1877. doi: 10.1016/j.molimm.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation. 2009;6:9. doi: 10.1186/1742-2094-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai CC, Kai JI, Huang WC, Wang CY, Wang Y, Chen CL, Fang YT, Lin YS, Anderson R, Chen SH, Tsao CW, Lin CF. Glycogen synthase kinase-3beta facilitates IFN-gamma-induced STAT1 activation by regulating Src homology-2 domain-containing phosphatase 2. J Immunol. 2009;183:856–864. doi: 10.4049/jimmunol.0804033. [DOI] [PubMed] [Google Scholar]
- 65.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–576. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 66.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 67.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 70.Morton S, Davis RJ, McLaren A, Cohen P. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. Embo J. 2003;22:3876–3886. doi: 10.1093/emboj/cdg388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Rodionova E, Conzelmann M, Maraskovsky E, Hess M, Kirsch M, Giese T, Ho AD, Zoller M, Dreger P, Luft T. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood. 2007;109:1584–1592. doi: 10.1182/blood-2006-06-028951. [DOI] [PubMed] [Google Scholar]
- 73.Ono T, Yanagawa Y, Iwabuchi K, Nonomura K, Onoe K. Glycogen synthase kinase 3 activity during development of bone marrow-derived dendritic cells (DCs) essential for the DC function to induce T helper 2 polarization. Immunology. 2007;122:189–198. doi: 10.1111/j.1365-2567.2007.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoarau C, Martin L, Faugaret D, Baron C, Dauba A, Aubert-Jacquin C, Velge-Roussel F, Lebranchu Y. Supernatant from bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS One. 2008;3:e2753. doi: 10.1371/journal.pone.0002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 76.Oke SL, Tracey KJ. The inflammatory reflex and the role of complementary and alternative medical therapies. Ann N Y Acad Sci. 2009;1172:172–180. doi: 10.1196/annals.1393.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ulloa L, Tracey KJ. The "cytokine profile": a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rehani K, Scott DA, Renaud D, Hamza H, Williams LR, Wang H, Martin M. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim Biophys Acta. 2008;1783:375–382. doi: 10.1016/j.bbamcr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Welsh GI, Miyamoto S, Price NT, Safer B, Proud CG. T-cell activation leads to rapid stimulation of translation initiation factor eIF2B and inactivation of glycogen synthase kinase-3. J Biol Chem. 1996;271:11410–11413. doi: 10.1074/jbc.271.19.11410. [DOI] [PubMed] [Google Scholar]
- 81.Ohteki T, Parsons M, Zakarian A, Jones RG, Nguyen LT, Woodgett JR, Ohashi PS. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J Exp Med. 2000;192:99–104. doi: 10.1084/jem.192.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kapeller R, Prasad KV, Janssen O, Hou W, Schaffhausen BS, Rudd CE, Cantley LC. Identification of two SH3-binding motifs in the regulatory subunit of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:1927–1933. [PubMed] [Google Scholar]
- 83.Pages F, Ragueneau M, Klasen S, Battifora M, Couez D, Sweet R, Truneh A, Ward SG, Olive D. Two distinct intracytoplasmic regions of the T-cell adhesion molecule CD28 participate in phosphatidylinositol 3-kinase association. J Biol Chem. 1996;271:9403–9409. doi: 10.1074/jbc.271.16.9403. [DOI] [PubMed] [Google Scholar]
- 84.Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 85.Prasad KV, Cai YC, Raab M, Duckworth B, Cantley L, Shoelson SE, Rudd CE. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc Natl Acad Sci U S A. 1994;91:2834–2838. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wood JE, Schneider H, Rudd CE. TcR and TcR-CD28 engagement of protein kinase B (PKB/AKT) and glycogen synthase kinase-3 (GSK-3) operates independently of guanine nucleotide exchange factor VAV-1. J Biol Chem. 2006;281:32385–32394. doi: 10.1074/jbc.M604878200. [DOI] [PubMed] [Google Scholar]
- 87.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 88.Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci U S A. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Howland KC, Ausubel LJ, London CA, Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–4470. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 90.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 91.Waldrop SL, Davis KA, Maino VC, Picker LJ. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J Immunol. 1998;161:5284–5295. [PubMed] [Google Scholar]
- 92.Okamoto N, Tezuka K, Kato M, Abe R, Tsuji T. PI3-kinase and MAP-kinase signaling cascades in AILIM/ICOS- and CD28-costimulated T-cells have distinct functions between cell proliferation and IL-10 production. Biochem Biophys Res Commun. 2003;310:691–702. doi: 10.1016/j.bbrc.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 93.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 94.Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 95.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 96.Yoshimoto T, Okamura H, Tagawa YI, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc Natl Acad Sci U S A. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang HD, Helbig C, Tykocinski L, Kreher S, Koeck J, Niesner U, Radbruch A. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur J Immunol. 2007;37:807–817. doi: 10.1002/eji.200636385. [DOI] [PubMed] [Google Scholar]
- 98.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peritt D, Aste-Amezaga M, Gerosa F, Paganin C, Trinchieri G. Interleukin-10 induction by IL-12: a possible modulatory mechanism? Ann N Y Acad Sci. 1996;795:387–389. doi: 10.1111/j.1749-6632.1996.tb52701.x. [DOI] [PubMed] [Google Scholar]
- 100.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 101.Windhagen A, Anderson DE, Carrizosa A, Williams RE, Hafler DA. IL-12 induces human T cells secreting IL-10 with IFN-gamma. J Immunol. 1996;157:1127–1131. [PubMed] [Google Scholar]
- 102.Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival signals to murine CD4+ T cells through phosphatidylinositol 3-kinase/Akt signaling pathway. J Immunol. 2002;169:3637–3643. doi: 10.4049/jimmunol.169.7.3637. [DOI] [PubMed] [Google Scholar]
- 103.Yssel H, De Waal Malefyt R, Roncarolo MG, Abrams JS, Lahesmaa R, Spits H, de Vries JE. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992;149:2378–2384. [PubMed] [Google Scholar]
- 104.Shen F, Li N, Gade P, Kalvakolanu DV, Weibley T, Doble B, Woodgett JR, Wood TD, Gaffen SL. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci Signal. 2009;2 doi: 10.1126/scisignal.2000066. ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 106.Parker D, Ferreri K, Nakajima T, LaMorte VJ, Evans R, Koerber SC, Hoeger C, Montminy MR. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol Cell Biol. 1996;16:694–703. doi: 10.1128/mcb.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- 108.Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol Cell Biol. 1999;19:6367–6378. doi: 10.1128/mcb.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 110.Platzer C, Fritsch E, Elsner T, Lehmann MH, Volk HD, Prosch S. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur. J. Immunol. 1999;29:3098–3104. doi: 10.1002/(SICI)1521-4141(199910)29:10<3098::AID-IMMU3098>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 111.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability. J Biol Chem. 2003;278:39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- 113.Cuzzocrea S, Crisafulli C, Mazzon E, Esposito E, Muia C, Abdelrahman M, Di Paola R, Thiemermann C. Inhibition of glycogen synthase kinase-3beta attenuates the development of carrageenan-induced lung injury in mice. Br J Pharmacol. 2006;149:687–702. doi: 10.1038/sj.bjp.0706902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cuzzocrea S, Di Paola R, Mazzon E, Crisafulli C, Genovese T, Muia C, Abdelrahman M, Esposito E, Thiemermann C. Glycogen synthase kinase 3beta inhibition reduces the development of nonseptic shock induced by zymosan in mice. Shock. 2007;27:97–107. doi: 10.1097/01.shk.0000235084.56100.71. [DOI] [PubMed] [Google Scholar]
- 115.Cuzzocrea S, Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Collin M, Esposito E, Bramanti P, Thiemermann C. Glycogen synthase kinase-3 beta inhibition reduces secondary damage in experimental spinal cord trauma. J Pharmacol Exp Ther. 2006;318:79–89. doi: 10.1124/jpet.106.102863. [DOI] [PubMed] [Google Scholar]
- 116.Cuzzocrea S, Genovese T, Mazzon E, Esposito E, Muia C, Abdelrahman M, Di Paola R, Bramanti P, Thiemermann C. Glycogen synthase kinase-3beta inhibition attenuates the development of bleomycin-induced lung injury. Int J Immunopathol Pharmacol. 2007;20:619–630. doi: 10.1177/039463200702000320. [DOI] [PubMed] [Google Scholar]
- 117.Cuzzocrea S, Mazzon E, Di Paola R, Muia C, Crisafulli C, Dugo L, Collin M, Britti D, Caputi AP, Thiemermann C. Glycogen synthase kinase-3beta inhibition attenuates the degree of arthritis caused by type II collagen in the mouse. Clin Immunol. 2006;120:57–67. doi: 10.1016/j.clim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 118.Cuzzocrea S, Mazzon E, Esposito E, Muia C, Abdelrahman M, Di Paola R, Crisafulli C, Bramanti P, Thiemermann C. Glycogen synthase kinase-3beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007;33:880–893. doi: 10.1007/s00134-007-0595-1. [DOI] [PubMed] [Google Scholar]
- 119.Dugo L, Abdelrahman M, Murch O, Mazzon E, Cuzzocrea S, Thiemermann C. Glycogen synthase kinase-3beta inhibitors protect against the organ injury and dysfunction caused by hemorrhage and resuscitation. Shock. 2006;25:485–491. doi: 10.1097/01.shk.0000209545.29671.31. [DOI] [PubMed] [Google Scholar]
- 120.Rehani K, Wang H, Garcia CA, Kinane DF, Martin M. Toll-like receptor-mediated production of IL-1Ra is negatively regulated by GSK3 via the MAPK ERK1/2. J Immunol. 2009;182:547–553. doi: 10.4049/jimmunol.182.1.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Q, Zhou Y, Wang X, Evers BM. Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene. 2006;25:43–50. doi: 10.1038/sj.onc.1209004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- 123.Zhang P, Katz J, Michalek SM. Glycogen synthase kinase-3beta (GSK3beta) inhibition suppresses the inflammatory response to Francisella infection and protects against tularemia in mice. Mol Immunol. 2009;46:677–687. doi: 10.1016/j.molimm.2008.08.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2008;181:338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bao Z, Lim S, Liao W, Lin Y, Thiemermann C, Leung BP, Wong WS. Glycogen synthase kinase-3beta inhibition attenuates asthma in mice. Am J Respir Crit Care Med. 2007;176:431–438. doi: 10.1164/rccm.200609-1292OC. [DOI] [PubMed] [Google Scholar]
- 127.Whittle BJ, Varga C, Posa A, Molnar A, Collin M, Thiemermann C. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3beta. Br J Pharmacol. 2006;147:575–582. doi: 10.1038/sj.bjp.0706509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gao HK, Yin Z, Zhou N, Feng XY, Gao F, Wang HC. Glycogen synthase kinase 3 inhibition protects the heart from acute ischemia-reperfusion injury via inhibition of inflammation and apoptosis. J Cardiovasc Pharmacol. 2008;52:286–292. doi: 10.1097/FJC.0b013e318186a84d. [DOI] [PubMed] [Google Scholar]
- 129.Lenz SP, Izui S, Benediktsson H, Hart DA. Lithium chloride enhances survival of NZB/W lupus mice: influence of melatonin and timing of treatment. Int J Immunopharmacol. 1995;17:581–592. doi: 10.1016/0192-0561(95)00032-w. [DOI] [PubMed] [Google Scholar]