Abstract
Elevated serum concentrations of follicle-stimulating hormone (FSH) are associated with diminished bone density in women, beginning years before menopause and the decline in estradiol. We hypothesized that FSH promotes development of myeloid cells toward the bone-resorbing osteoclast phenotype. This was tested by isolating peripheral blood mononuclear cells from nine healthy adults, incubating them in the presence of FSH at three different concentrations spanning the physiological range, and then measuring the expression of receptor activator for NF-κB (RANK, a surface marker for osteoclasts) on CD14+ cells by flow cytometry. In the absence of FSH, 3.3 ± 0.5% of the cells expressed high levels of the receptor (RANKhigh). Increasing concentrations of FSH caused a biphasic dose-response, with a maximal (1.5-fold) increase in RANKhigh cells achieved with 50 mIU/ml FSH (P = 0.02). Cytokines that influence development of osteoclasts were also measured in culture supernatants: Macrophage colony stimulating factor (M-CSF), osteoprotegerin (OPG) and tumor necrosis factor-α (TNFα) concentrations were not significantly influenced by FSH, whereas RANK-ligand was undetectable. This study supports the concept that the elevated circulating concentrations of FSH during perimenopause may contribute to the increased rate of bone loss by promoting the development of osteoclast precursor cells.
Keywords: human, monocytes, RANK, follicle-stimulating hormone, flow cytometry
1.0 Introduction
The development of osteoporosis in older women is often associated conceptually with the decline in circulating estradiol concentration that accompanies menopause. However, studies involving pre- and perimenopausal women have shown that elevated serum concentrations of follicle-stimulating hormone (FSH) correlate with diminished bone mineral density (BMD) beginning years before menopause and the decline in estradiol [1, 2]. In addition, a meta-analysis of ten prospective studies found that the rate of spinal BMD loss during perimenopause, when estradiol concentrations were still high, was greater than the rate of loss in the years following menopause, when estradiol concentrations were much lower [3].
Bone density depends on the balance between the activity of bone-forming osteoblasts and bone-resorbing osteoclasts. We hypothesized that FSH promotes development of osteoclast characteristics on circulating precursor cells, which comprise ~2% of the peripheral blood CD14+ monocytes in humans [4, 5]. These cells begin to express calcitonin and vitronectin receptors, produce tartrate-resistant acid phosphatase and become capable of bone resorption when cultured under appropriate in vitro conditions [4, 5]. A critical stimulus for osteoclast differentiation is transmitted via the receptor activator for NF-kB (RANK), a member of the tumor necrosis factor (TNF) receptor superfamily [6]. This receptor has been identified on circulating osteoclast precursor cells, making it a convenient surface marker for identifying osteoclast precursors by flow cytometry [7].
Therefore, the primary aim of this investigation was to determine if FSH increased RANK expression on CD14+ peripheral blood mononuclear cells isolated from healthy adults. Induction of RANK on CD14+ precursor cells requires macrophage colony stimulating factor (M-CSF) [8], therefore cell supernatants were assayed to determine if FSH influenced the secretion of this critical cytokine. Further differentiation of precursors to mature osteoclasts requires RANK ligand (RANKL) [6], which can be produced by activated T cells [9]. The binding of RANKL to RANK can be inhibited if it is bound by the decoy receptor osteoprotegerin (OPG) [6], which can be produced by B cells [10]. In addition, evidence suggests that FSH can promote murine osteoclast precursor differentiation via stimulation of TNFα [11]. Therefore the supernatants were also assayed to determine if FSH altered the secretion of soluble forms of these factors.
2.0 Materials and Methods
2.1 Blood Collection
Nine healthy adults donated blood for these experiments. These donors included two men (26 and 47 years of age), and seven women (22 to 57 years of age). All subjects provided informed consent, and the protocol was approved by the Human Assurance Committee at the Medical College of Georgia. The blood was drawn into three 10 ml heparinized vacutainer tubes for immediate mononuclear cell isolation and one K2EDTA tube was drawn for plasma, which was frozen at −70 °C for later analysis.
2.2 Cell Isolation and Culture
Mononuclear cells were isolated from the heparinized blood by density gradient centrifugation using ficoll-hypaque (Sigma, St. Louis, MO), washed three times with sterile saline (0.9% NaCl) and resuspended in phenol red-free RPMI-1640 medium supplemented with 2 mM of L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1% (by volume) 1 M Hepes Buffer (all from Sigma), and 1% heat-inactivated autologous plasma. Cells were then distributed in sterile 12 × 75 mm polypropylene tubes (Fisher Scientific, Suwanee GA) at a density of 2.5 × 106 cells/tube and incubated with 0, 10, 50, or 100 mIU/ml of highly purified pharmaceutical grade human FSH (Bravelle, Ferring Pharmaceuticals, Suffern, NY) for 18 hours at 37°C in a humidified 5% CO2 atmosphere. After incubation, cell supernatants were collected, aliquotted and frozen at −70°C for later analyses. Receptor expression on the cells was determined by flow cytometry.
2.3 Flow Cytometry
The mononuclear cells were washed, resuspended to 1 × 107 cells/ml, blocked with 1 mg/ml human IgG (Sigma I8640), and then stained with phycoerythrin (PE)–conjugated anti-RANK (9A725, Abcam, Cambridge, MA). A PE-conjugated murine IgG1 isotype control (BD Biosciences, San Diego, CA) was used to test for nonspecific staining. Monocytes were identified with an allophycocyanin (APC)-conjugated anti-human CD14 (M5E2, BD Biosciences) which was added to the cells at the same time as the anti-RANK. After washing, the cells were fixed in 5% formalin/PBS, pH 7.3 and stored overnight at 4 °C. The following day, flow cytometric analysis was performed once per sample using a Becton Dickinson 2-laser, 4-detector FACSCalibur Flow Cytometer. Cell counts were restricted to intact cells identified by a dual parameter plot of forward scatter versus side scatter. Multi-color flow cytometry was performed with appropriate one-color controls for setting compensation for spectral overlap.
2.4 Immunoassays
Concentrations of M-CSF, OPG, TNFα and RANKL were measured once in each supernatant using a cytometric bead array (CBA, BD Biosciences, San Jose, CA)[12]. Separate bead populations were coated with murine capture antibodies raised against M-CSF (#840689, R&D Systems, Minneapolis, MN), OPG (#840369, R&D Systems), TNF (#51-9005468, BD Biosciences) and RANKL (# I1207, Peprotech, Rocky Hill, NJ). The biotinylated detection antibodies were goat anti-M-CSF (#840690, R&D Systems), anti-OPG (#840370, R&D Systems), anti-TNFα (#51-9004040, BD Biosciences) and rabbit anti-RANKL (#I1307, Peprotech). Phycoerythrin-conjugated streptavidin was purchased from Jackson Immunoresearch, West Grove, PA (#016-110-084). After incubation and washing, the beads were resuspended and analyzed with a BD FACSArray flow cytometer. Analytes were differentiated by bead fluorescence characteristics, and analyte concentration was determined by the fluorescence intensity of the labeled detection antibody, which was compared to standard curves generated with recombinant M-CSF (#840691, R&D Systems), OPG (#840371, R&D Systems), TNFα (#51-9003509) and RANKL (#310-01, Peprotech).
2.5 Data Analysis and Statistics
Values reported are means ± standard errors. Differences between control and FSH treatment conditions were assessed by repeated measures analysis of variance (ANOVA) followed by Dunnett’s post hoc test for multiple comparisons to a control using Statview software (SAS Institute, Cary NC). Statistical significance was accepted with a P value <0.05.
3.0 Results
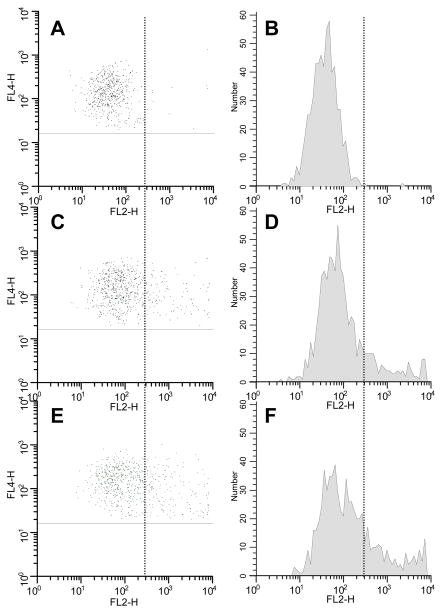
Illustrative flow cytometric dot plots and histograms of RANK expression on CD14+ monocytes are presented in Figure 1. Non-specific staining is illustrated in panels A and B, wherein cells incubated without FSH and stained with isotype control antibody exhibited relative fluorescence intensities (RFI) of <300. When stained with anti-RANK,10.0% of control cells (no FSH) exhibited positive RANK expression (RFI >300, panels C and D). Incubation with FSH (50 mIU/ml) resulted in 18.3% of cells expressing RANK, with much of the increase occurring at very high RFI (>3 000): from 2.4% in the absence of FSH to 7.6% with 50 mIU/ml FSH.
Figure 1.
Flow cytometry results from a single subject. A: Scatter plot for CD14+ gated cells (vertical axis) versus PE- conjugated isotype control (horizontal axis); B: Histogram of the PE fluorescence data of panel A; C: Scatter plot for CD14+ gated cells versus PE-conjugated anti-RANK under control conditions without FSH; D: Histogram of the PE fluorescence data of panel C; E and F: same as panels C and D, except cells were cultured with 50 mIU/ml FSH. The vertical line indicates the demarcation between nonspecific and specific staining (at an RFI of 300 for this subject).
Cumulative data for all subjects are presented in Figure 2. The total percentage of CD14+ cells exhibiting specific RANK expression was 8.5 ± 1.3% in control cultures, whereas maximal expression, 10.4 ± 1.6%, was observed on cells incubated with 50 mIU/ml of FSH (white bars, not statistically different from control). The expression of high levels of RANK (>3 000 RFI) was also analyzed: In the absence of FSH, 3.3 ± 0.5% of the cells were RANKhigh, whereas 4.9 ± 0.9% of the cells incubated with 50 mIU/ml were RANKhigh (black bars in Figure 2A, P = 0.02), a 1.5-fold increase. Essentially all of the increase in RANK+ cells was due to increased numbers of RANKhigh cells. No age or sex-dependent influences on FSH-induced mononuclear cell RANK expression were observed in the limited number of subjects tested.
Figure 2.
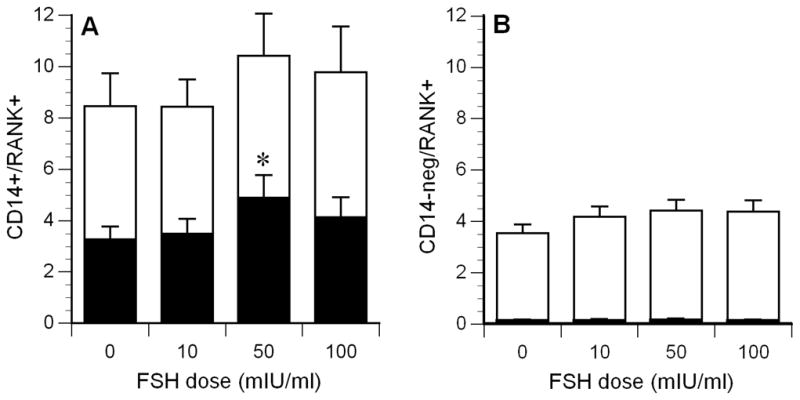
Mean RANK expression on CD14+ mononuclear cells (panel A) and CD14-negative cells (panel B). The top of the white bars indicates the total percentage of RANK positive cells, the black bars indicate the percentage of RANKhigh cells (RFI > 3000, extremely low in panel B). *P = 0.02, compared to control condition (0 FSH). N=9.
Basal RANK expression on CD14-negative cells was 3.5 ± 0.4%. FSH induced a small increase in RANK expression, with maximal expression observed for cells incubated with 50 mIU/ml of FSH (4.4 ± 0.4%, P = not significant, white bars, Figure 2B). The mean percentage of RANKhigh CD14-negative cells was less than 0.15% in any of the culture conditions.
Mean M-CSF concentrations in control cultures were 500 ± 110 pg/ml, and incubation with FSH had no influence on the M-CSF concentrations in the other cell culture supernatants (black bars in Figure 3). The basal concentrations of OPG were low (6.8 ± 0.6 pg/ml), and the concentrations did not change significantly in the supernatants of cells incubated with FSH (dark gray bars in Figure 3). Basal TNFα concentrations were 470 ± 221 pg/ml, and although the mean concentration was 47% higher in supernatants incubated with 100 mIU/ml of FSH, this increase was not statistically significant (light gray bars in Figure 3). RANKL was undetectable (< 2 pg/ml) in any of the supernatants.
Figure 3.
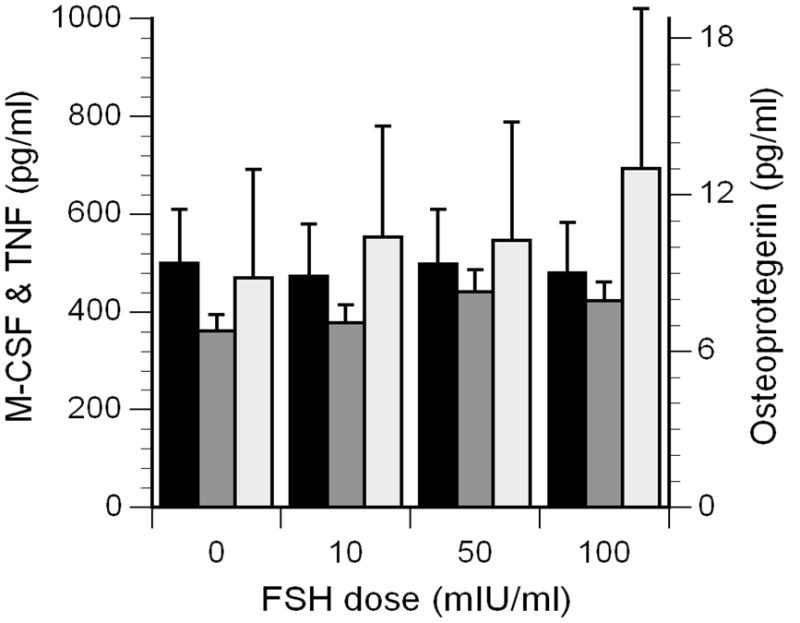
Concentrations of M-CSF (black bars, scale on left), OPG (dark gray bars, scale on right), and TNFα (light gray bars, scale on left) in the supernatants of mononuclear cells cultured with FSH.
4.0 Discussion
Previous studies have shown that FSH enhanced RANKL-stimulated osteoclastogenesis from human peripheral blood mononuclear cells over 8 days of incubation [13]. The present data indicate that one mechanism contributing to this effect is an increase in FSH-induced RANK expression, which occurs within 18 hours.
FSH had no significant influence on RANK expression at a concentration of 10 mIU/ml, which is in the range of the circulating follicular-phase FSH concentrations of women during the majority of their reproductive years. However, FSH caused a significant increase in RANK expression at 50 mIU/ml, concentrations typical during perimenopause that are associated with increased bone loss [14]. At higher concentrations reached after menopause (100 mIU/ml), FSH had less of an influence on RANK expression. These results are consistent with clinical observations that the rate of spinal BMD loss is greater during perimenopause than in the years following menopause [3].
FSH preferentially increased the numbers of CD14+ cells expressing high levels of RANK (MFI >3 000). A previous study has shown that such CD14+ cells, with relatively high expression of RANK, are more likely to differentiate into mature osteoclasts than are cells expressing low or moderate levels of RANK [7]. This is consistent with the concept that osteoclast precursors may be part of a distinct subpopulation of monocytes: It has also been reported that monocytes capable of proliferation yield more osteoclasts than monocytes that are incapable of proliferation [15].
In the present study, CD14-negative cells also expressed RANK. These results are consistent with previous studies that have detected RANK on lymphocytes, primarily B cells and natural killer cells [7]. The functional consequences of RANK expression on these cells are not presently understood. RANK has also been identified on dendritic cells, and stimulation by RANKL augments their ability to stimulate T cells [16].
No FSH-induced changes in soluble OPG or RANKL were observed in the present study. However, it is not certain that the OPG and RANKL assays detected all possible forms of these proteins since OPG monomers, dimers and OPG/RANKL complexes exist [17]. Furthermore, it is possible that FSH might influence RANKL signaling by affecting its membrane-bound expression on CD14-negative mononuclear cells. RANKL-mediated signaling is transmitted primarily in a juxtacrine manner via the membrane-bound form of RANKL, rather than in a paracrine manner as a soluble mediator [6].
Although a previous study found that recombinant FSH induced a significant increase in TNFα secretion from murine bone marrow-derived macrophages [11], we found no significant FSH-induced increase in TNFα secretion from human peripheral blood mononuclear cells. In addition to the species and cell differences, the previous study used a higher dose of FSH, 100 ng/ml, which would correspond to 1000 mIU/ml [18].
In summary, the present study indicates that FSH induces expression of RANK on CD14+ cells, indicating the acquisition of osteoclast precursor cell characteristics. These findings may contribute to a mechanistic explanation for the increased rate of bone loss that occurs during the perimenopausal period.
Acknowledgments
This work was supported by AG027714 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sowers MF, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF., Jr Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endo Metab. 2008;93:3958–3964. doi: 10.1210/jc.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sowers MR, Finkelstein JS, Ettinger B, Bondarenko I, Neer RM, Cauley JA, Sherman S, Greendale GA. The association of endogenous hormone concentrations and bone mineral density measures in pre-and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int. 2003;14:44–52. doi: 10.1007/s00198-002-1307-x. [DOI] [PubMed] [Google Scholar]
- 3.Prior JC. Perimenopause: The complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 4.Fujikawa Y, Quinn JMW, Sabokbar A, McGee JOD, Athanasou NA. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology. 1996;137:4058–4060. doi: 10.1210/endo.137.9.8756585. [DOI] [PubMed] [Google Scholar]
- 5.Massey HM, Flanagan AM. Human osteoclasts derive from CD14-positive monocytes. Br J Haematol. 1999;106:167–170. doi: 10.1046/j.1365-2141.1999.01491.x. [DOI] [PubMed] [Google Scholar]
- 6.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Ann Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 7.Atkins GJ, Kostakis P, Vincent C, Farrugia AN, Houchins JP, Findlay DM, Evdokiou A, Zannettino ACW. RANK expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J Bone Miner Res. 2006;21:1339–1349. doi: 10.1359/jbmr.060604. [DOI] [PubMed] [Google Scholar]
- 8.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor κB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 10.Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM, Pascual V, Hood LE, Clark EA. OPG/FDCR-1, TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. 1998;161:6113–6121. [PubMed] [Google Scholar]
- 11.Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci USA. 2006;103:14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Cytometric bead array: A multiplexed platfform with applications in various areas of biology. Clin Immunol. 2004;110:252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu L-L, Yaroslavskiy, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Sowers MF, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endo Metab. 2006;91:1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 15.Lari R, Kitchener PD, Hamilton JA. The proliferative human monocyte subpopulation contains osteoclast precursors. Arthritis Res Ther. 2009;11:R23. doi: 10.1186/ar2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leibbrandt A, Penninger JM. RANK/RANKL: Regulators of immune responses and bone physiology. Ann NY Acad Sci. 2008;1143:123–150. doi: 10.1196/annals.1443.016. [DOI] [PubMed] [Google Scholar]
- 17.Rogers A, Eastell R. Review: Circulating osteoprotegerin and receptor activator for nuclear factor κB ligand: Clinical utility in metabolic bone disease assessment. J Clin Endo Metab. 2005;90:6323–6331. doi: 10.1210/jc.2005-0794. [DOI] [PubMed] [Google Scholar]
- 18.Bergh C. Recombinant follicle stimulating hormone. Hum Reprod. 1999;14:1418–1419. doi: 10.1093/humrep/14.6.1418. [DOI] [PubMed] [Google Scholar]