Summary
The relationship between human skin pigmentation and protection from ultraviolet (UV) radiation is an important element underlying differences in skin carcinogenesis rates. The association between UV damage and the risk of skin cancer is clear, yet a strategic balance in exposure to UV needs to be met. Dark skin is protected from UV-induced DNA damage significantly more than light skin due to the constitutively higher pigmentation but an as yet unresolved and important question is what photoprotective benefit, if any, is afforded by facultative pigmentation (i.e. a tan induced by UV exposure). To address that and to compare the effects of various wavelengths of UV, we repetitively exposed human skin to suberythemal doses of UVA and/or UVB over 2 weeks after which a challenge dose of UVA&UVB was given. Although visual skin pigmentation (tanning) elicited by the different UV exposure protocols was similar, the melanin content and UV-protective effects against DNA damage in UVB-tanned skin (but not in UVA-tanned skin) were significantly higher. UVA-induced tans seem to result from the photooxidation of existing melanin and its precursors with some redistribution of pigment granules while UVB stimulates melanocytes to up-regulate melanin synthesis and increases pigmentation coverage, effects that are synergistically stimulated in UVA and UVB-exposed skin. Thus, UVA-tanning contributes essentially no photoprotection, although all types of UV-induced tanning result in DNA and cellular damage which can eventually lead to photocarcinogenesis.
Keywords: ultraviolet, skin, pigmentation, DNA damage, photoprotection
Introduction
The question of whether skin color imparts any biological advantage has been argued for decades and is fraught with controversy. Much of the existing evidence is based on studies in vitro and/or in animal models which often aren’t suitable to integrate all the physiological parameters found in human skin in situ. In this study, our goal was to evaluate whether UV damage (which correlates with increased risk of carcinogenesis in human skin) is attenuated by facultative skin pigmentation (commonly called a tan) induced by suberythemal UVA and/or UVB exposure (Johnson et al., 1972; Ortonne, 1990; Yamaguchi et al., 2006; Miyamura et al., 2007). While many individuals minimize their exposure to UV, others deliberately expose their skin to UV on the beach and/or in indoor tanning salons, despite its well known carcinogenic effects (Brash et al., 1996; Wikonkal & Brash, 1999; Agar et al., 2004; Besaratinia et al., 2005) and the increased risk of skin cancers including melanoma (Gilchrest et al., 1999; Halder & Ara, 2003; Bennett, 2008; Berwick, 2008; Tran et al., 2008; Abdel-Malek et al., 2010; von Thaler et al., 2010; Lazovich et al., 2010; Patton et al., 2010). One putative but unproven advantage of tanning might be an increased protection against subsequent UV exposure due to increased melanin levels (Wagner et al., 2002; Miller et al., 2008; Wolber et al., 2008) which might then protect the skin against further UV damage (Preston & Stern, 1992; Tadokoro et al., 2003; Halder & Ara, 2003; Tadokoro et al., 2005; Brenner & Hearing, 2008; Coelho et al., 2009b). However, arguments about skin color and the putative protective effects of melanin against UV damage still remain (Agar & Young, 2005; Caron-Schreinemachers et al., 2005). Studies using human skin exposed to ultraviolet (UV) radiation in situ are crucial to understand the biological effects of UV radiation on that tissue and the implications of varying levels of melanin pigment on those responses.
Due to the variability of solar output, let us consider the case of standard reference solar sunlight which includes ~94% UVA (320~400 nm) and ~6% UVB (290~320 nm)(CIE Technical Committee 2-17, 1989). Although UVA makes up a greater percentage of UV radiation that reaches the surface of the earth, UVB is the stronger partner, and the biological effects of UVA and UVB are quite different. UVB causes more direct DNA damage, principally in the form of cyclobutane pyrimidine dimers (CPD) or 6,4-photoproducts (64PP), than does UVA (Agar et al., 2004; Besaratinia et al., 2005; Mouret et al., 2006). However, UVA causes oxidative damage which induces reactive oxygen species (ROS) which in turn can damage DNA and other cellular constituents and increases photoaging of the skin (Berneburg et al., 1999; Krutmann, 2000). A few earlier attempts to evaluate the possible photoprotective effects of repetitive UVA- or UVB-induced tans were made some time ago, but suffered significantly from several limitations, including the induction of tans by erythemal doses, the use of high UVA doses, and the lack of analysis of melanin content or its distribution (Roser-Maass et al., 1982; Gange et al., 1985).
Although the focus of this study is on photoprotection due to pigmentation, it is important to note that skin thickening with increased keratinization, such as what is seen in palm skin, can contribute significantly to photoprotection. Skin thickness varies within an individual at different anatomical sites, and can also vary among individuals. It has been known for some time that the skin can undergo thickening with increasing stratum corneum and epidermal hyperplasia (Miescher, 1930; Johnson, 1983) upon exposure to UV. Large amounts of erythema-inducing acute UV radiation and repeated UV exposures give rise to this phenomenon. A previous study that evaluated the thickness of the epidermis and stratum corneum found increased thicknesses with the use of erythemal repetitive UV exposures (Bruls et al., 1984). That study found decreased transmission of UV with increasing thickness induced by erythemal UV, however the authors indicated that the variations in transmission at different regional areas of the body do not delineate the extent of UV sensitivity. Clearly, thicker skin, such as found on the palm of the hand, plays an important role in photoprotection when one considers its high UV absorption coefficient below 320 nm compared to the volar and dorsal forearm skin (Meinhardt et al., 2008). Evidence in the literature for different effects of erythemal vs suberythemal repetitive UV exposures on skin thickness is clear. In another study, individuals were irradiated 3 times weekly for 6 weeks with 0.5, 1 or 2 MED UVB (Pearse et al., 1987). In that study, UVB doses larger than 1 MED were needed to induce significant differences in epidermal and stratum corneum thickness.
However, data on suberythemal repetitive UV exposures are less conclusive due to the difficulty in interpreting results with different emission spectrums, irradiation doses and frequency of irradiations. In many studies investigating repetitive UV irradiations, such as suberythemal doses of UVA, there are conflicting reports in the literature. Part of the reason for this is that suberythemal doses in two different studies may vary greatly because of how much UVA is needed to produce erythema. Hence, daily suberythemal UVA doses of 2-4 J/cm2 will provide different responses compared to 20-40 J/cm2. Pearse et al. ( 1987) irradiated skin 3 times a week for 3 weeks with 6 J/cm2 UVA. Although the UVA irradiation was sufficient to increase epidermal and stratum corneum thickness to statistically significant levels, that daily dose was 2-3 times larger than the dose used in our study. In another study, daily UVA doses of 18 J/cm2 for 6 weeks (approximately 6-9 times larger than the dose used in our study) showed statistically significant increases in epidermal and stratum corneum thickness (Lavker et al., 1995). Further studies evaluating repeated UVA-rich sunbed exposures with non-invasive methods (i.e. confocal laser-scanning microscopy) show increased thickness with irradiation doses of 21 J/cm2 per session twice weekly for 3 weeks (Gambichler et al., 2004), yet much smaller UVA doses of 1.13-1.46 J/cm2 used in sunbeds twice weekly for 6 weeks (Ruegemer et al., 2002), similar to the ones used in our study, had no effect. Therefore, those earlier studies leave it inconclusive as to whether very low level suberythemal UVA and/or UVB exposures increase skin thickness to the point where it may be a contributing factor in photoprotection.
We recently reported that the effects of UVA or UVB on skin pigmentation are distinct, UVB eliciting dramatic increases in melanin synthesis, while UVA increases visible skin pigmentation but without any increase in melanin content (Wolber et al., 2008). Further, the gene expression patterns elicited by UVA or by UVB in the skin differ markedly, and synergistic effects are seen following exposure to UVA and UVB (Choi et al., 2010). The abilities of different UV wavelengths to elicit tumor formation is also distinct but well known (Black et al., 1997; Wikonkal & Brash, 1999; Matsumura & Ananthaswamy, 2002; de Gruijl, 2002; De Fabo et al., 2004); both types can elicit skin cancers, including melanoma, although the roles of UVA and UVB remain controversial (Setlow et al., 1993; Wood et al., 2006; Mitchell et al., 2010). However, the critical issue that remains unclear is whether UVA-induced tans, which do not increase melanin levels, are photoprotective against DNA damage from subsequent UV exposure (Swerdlow & Weinstock, 1998).
To address that topic, we generated tans in human skin by repetitive exposure to suberythemal doses of UVA and/or UVB and then challenged the skin with an erythemal dose of UV. Melanin contents in the tanned and in unexposed skin were assessed as were DNA damage levels to determine the putative photoprotective effects of those tans against the subsequent UV challenge to determine if they confer a biological advantage, i.e. increased photoprotection. Further, we assessed the photoprotection afforded by increased pigmentation in a 3-dimensional model of human skin and also analyzed the distribution patterns of melanin pigment granules in the skin following exposure to UVA and/or UVB. The results show clearly that the mechanisms of UVA- or UVB-induced skin tanning differ dramatically, and that the photoprotection afforded by UVB-induced tans are small while no significant photoprotection was afforded by UVA-induced tans compared to the control. This is an important consideration given that UVA-rich lamps are now frequently used in the indoor tanning industry to promote tanning with the implied potential benefit of reducing DNA damage and increasing protection against subsequent UV exposure. It is clear that such a claim is not warranted and might be responsible for the recent rapid increases in all types of skin cancers in subjects who frequent tanning parlors.
Results
Effect of Increased Melanin Content on Photoprotection of Human Skin Equivalents
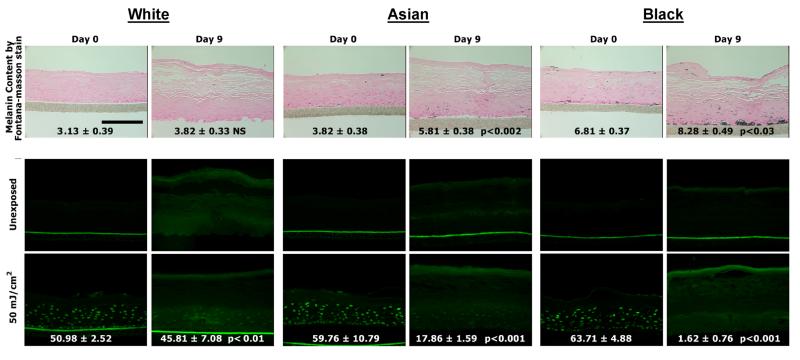
To assess the role of facultative pigmentation in photoprotection in skin with different levels of melanin content, we initially used 3-dimensional reconstructed human skin equivalents. Human skin equivalents of White (Caucasian), Asian, and Black origin produced increases in visual pigmentation with time in culture that are consistent with their racial/ethnic origin (Fig. 1, top). The pigmentation induced by supplementation of the medium with αMSH and bFGF (well-known melanogenic stimulators) produced increases in visible color as indicated by increases in MACR-assessed ΔL values, as previously reported (Coelho et al., 2009a). The human skin equivalents showed a 40 to 50% increase in melanin content from day 0 to day 9 (with the exception of the White), which is similar to the increased melanin content in the repetitively UVB-exposed skin (as shown below and as previously reported (Wolber et al., 2008)). Part of each human skin equivalent was exposed to a UV-challenge dose (50 mJ/cm2), and then was harvested and assessed for DNA damage (Fig. 1, bottom). All 3 types of human skin equivalents receiving the UV-challenge dose showed comparably high levels of DNA damage measured as levels of CPDs) in the day 0 specimens that had little pigmentation. However, the DNA damage in the more pigmented human skin equivalents at day 9 were significantly decreased in proportion to their relative increases in melanin content; damage was least in the Black skin, more in the Asian skin and highest in the White skin. While this model is a reasonable approximation for studying the effects of different pigment levels in the same type of human skin and demonstrates the photoprotective nature of increased melanin levels, it does not address the issue of whether increased pigmentation elicited by UV exposure provides a similar benefit. Thus, in subsequent experiments, we assessed the potential photoprotective nature of UV-induced facultative pigmentation in a clinical protocol.
Figure 1.
Increased melanin content and CPD damage of pigmented human skin equivalents after UV challenge. (Top) Images of melanin content in human skin equivalents from White, Asian, and Black were analyzed by Fontana-Masson staining and data are reported as mean density; scale bar = 50 μm. (Bottom) The unexposed control, or skin equivalents exposed to a UV challenge of 50 mJ/cm2 were harvested at day 0 (left column) and at day 9 (right column) immediately after the UV challenge. TDM2 was used as the primary antibody to detect CPD and is stained green; scale bar = 50 μm. Images were analyzed as noted in the Methods, and CPD levels are expressed as % CPD-positive area (mean ± SEM, n=10). Statistical significance of differences from the day 0 control are indicated (NS = not significant).
Characteristics of Tans Induced by Repetitive UV Exposure
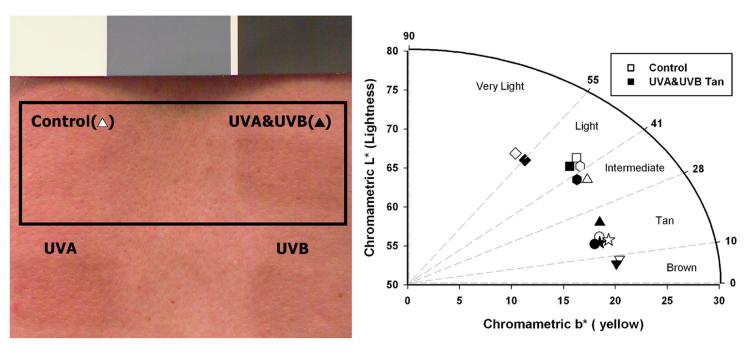
Details of the repetitive 2 week suberythemal UV exposure schedule used in this study are as previously reported (Choi et al., 2010). Briefly, subjects were exposed to 5 daily doses of 0.4 Minimal Erythemal Dose (MED) UVA and/or UVB in week 1, and then to 5 daily doses of 0.5 MED UVA and/or UVB in week 2; repetitive doses of UVA or UVB only were adjusted to provide equivalent tans as detailed in (Choi et al., 2010). On the Monday of the following week, part of each skin area was subjected to a 2 MED challenge with UVA&UVB (hereafter called the UV-challenge) and the UV-challenged and unchallenged areas were immediately biopsied to measure DNA damage. The exposure protocols used for UVA and/or UVB generate comparable tans at the doses used that are visually and quantifiably distinguishable by diffuse reflectance from the unexposed control (Fig. 2). Using the CIE L*a*b* color space system, we plotted the skin lightness (L*) versus the yellow component (b*) to discriminate even small pigmentation differences between the UVA&UVB-induced tans versus the unexposed controls across the distribution of subjects via the individual typology angle (ITA°) pigmentation categories. For each subject, there was a decrease in L*, i.e. a darkening of the skin, that while sometimes relatively minor, in some cases was sufficient to increase the visible pigmentation to cross into the next skin pigmentation level. Although the changes in ITA are not dramatic as assessed by reflection spectroscopy, they do reflect statistically significant increases in melanin content and changes in distribution that correspond to considerable visual changes in skin pigmentation.
Figure 2.
Tanning responses in the skin following UV exposure. (Left) Representative image of tanning responses induced in subject 2 after 2 weeks of suberythemal repetitive exposure to UVA & UVB. (Right) Vector representation of the UVA&UVB exposed skin versus the unexposed control in the L* and b* plane of the CIE L*a*b* color space system. These parameters allow for calculation of the individual typology angle (ITA°) as defined in the Methods and allow for segmentation into several pigmentation categories.
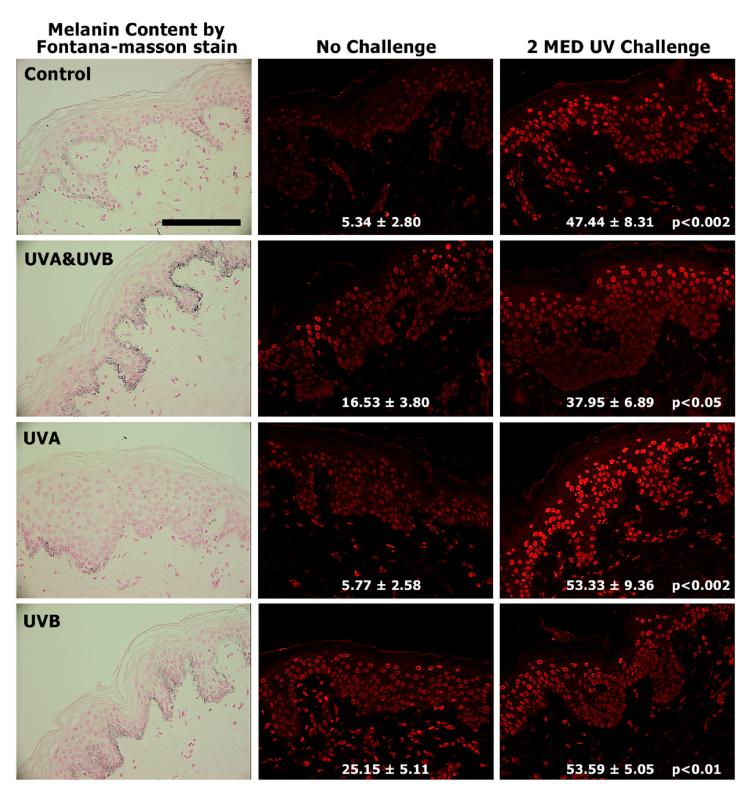
We have previously reported that UVA alone has no effect on melanin content (as also seen in Fig. 3, left) or melanocyte density while both of those parameters were increased significantly by UVB with or without UVA (Wolber et al., 2008; Choi et al., 2010). The 2 MED UV-challenge caused no immediate significant change in melanin content in terms of melanin positive area (Fig. 4, top), or melanin content (Table 1) in any of the specimens. In addition, the thickness of the stratum corneum and the number of cells in the epidermis did not change significantly in any of the specimens, although the stratum corneum thickness did show an increasing trend with UVA or UVB exposure (Table 1). However, given the lack of statistical significance in the thickness under the irradiation conditions of our study, and the fact that similar small increases were seen in UVA- and in UVB-exposed skin, it seems unlikely that skin thickness was a significant factor in any potential photoprotection, although it cannot be entirely excluded. Identification of melanocytes using antibodies to tyrosinase, DCT or MITF, revealed that UVB with or without UVA caused significant increases in melanocyte density after 2 weeks, while UVA alone had no effect, as previously reported (Choi et al., 2010).
Figure 3.
Melanin content and CPD damage in the skin following UV exposure. (Left) Representative sections from subject 5 were stained using the Fontana-Masson method to evaluate melanin content. (Center) Unexposed (control), UVA-, UVB- and UVA&UVB-exposed skin specimens as noted were biopsied before or after (Right) the 2 MED UV challenge; images from subject 5 are shown as an example. TDM2 was used as the primary antibody to detect CPD and is stained red due to the emission of the Alexa 568 conjugated secondary antibody; scale bar = 50 μm. Images were analyzed as noted in the Methods, and CPD levels are expressed as % CPD-positive area (mean ± SEM, n=7). CPD levels are expressed as mean fluorescence intensity as follows: Control – No Challenge 0.19 ± 0.05, Control – UV Challenge 0.87 ± 0.10, p < 0.0005; UVA&UVB – No Challenge 0.52 ± 0.09, UVA&UVB – UV Challenge 0.68 ± 0.09, p < 0.05; UVA – No Challenge 0.29 ± 0.04, UVA – UV Challenge 0.78 ± 0.09, p < 0.002; UVB – No Challenge 0.51 ± 0.06, UVB – UV Challenge 0.80 ± 0.06, p < 0.01. In addition CPD statistical significance of differences from the corresponding unchallenged areas is indicated.
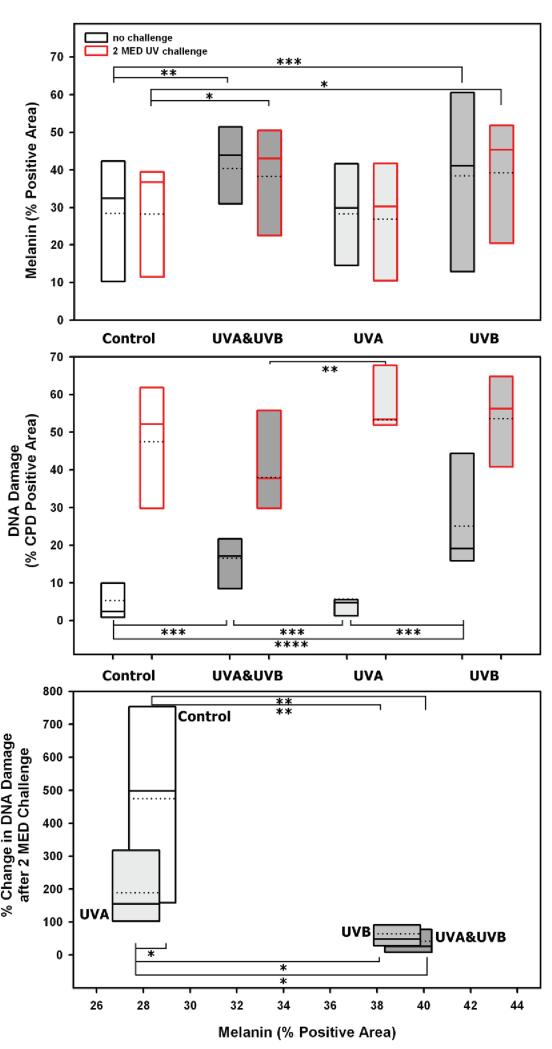
Figure 4.
Relationship of UV exposure to CPD damage and melanin content. (Top) Interindividual distribution of % melanin positive area and (Middle) CPD positive area graphed as box plots for unexposed (control), UVA-, UVB- and UVA&UVB-exposed skin as well as the areas receiving the 2 MED UV challenge denoted with a red boundary (the dashed line in each box plot represents the mean, the solid line the median value). (Bottom) The % change in DNA damage after the 2 MED UV challenge was calculated and shows an inverse correlation relative to the mean % melanin positive area. * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.0005, not significant comparisons are absent from graphs.
Table 1.
Melanin content and stratum corneum thickness of skin specimens.
| without UV challenge | with UV challenge | |||||
|---|---|---|---|---|---|---|
| Melanin content (a.u.) |
Stratum corneum (μm) |
Epidermal cells (count/field) |
Melanin content (a.u.) |
Stratum corneum (μm) |
Epidermal cells (count/field) |
|
| Control | 9.03 ± 1.62 | 16.6 ± 2.1 | 225 ± 20 | 8.49 ± 1.48 | 16.6 ± 1.8 | 247 ± 26 |
| UVA | 8.62 ± 1.59 NS |
19.5 ± 2.9 NS |
239 ± 21 NS |
8.65 ± 1.37 NS |
17.9 ± 2.3 NS |
244 ± 25 NS |
| UVB | 12.70 ± 2.10 p<0.01 |
20.7 ± 3.6 NS |
226 ± 18 NS |
12.13 ± 1.27 p<0.05 |
19.0 ± 2.9 NS |
245 ± 15 NS |
| UVA + UVB | 12.50 ± 1.43 NS |
20.0 ± 2.3 NS |
234 ± 28 NS |
12.07 ± 0.63 p<0.05 |
22.3 ± 3.7 NS |
268 ± 23 NS |
| n = 6 | n = 6 | n = 6 | n = 6 | n = 6 | n = 6 | |
Data are reported as means ± SEM; NS = not significant. Statistical significance of differences from the corresponding control is indicated.
Effect of Skin Tanning by UVA and/or UVB on DNA Damage Elicited by the UV-Challenge
Repetitive exposure of human skin to UVB with or without UVA resulted in CPD damage in the nuclei of epidermal cells that remained for at least 3 days after the last UV exposure (Fig. 3, center; Fig. 4, middle). Repetitive exposure to UVA alone produced minor but statistically insignificant amounts of CPD compared to unirradiated skin.
To test whether the facultative tans were photoprotective, an area in each study site was subjected to a 2 MED UV-challenge (Fig. 3, right). All UV-challenged areas had obvious increases in DNA damage compared with the unchallenged areas (Fig. 4, middle), although levels of CPDs in the UVB-tanned areas (with or without UVA) were clearly lower than in the other areas. This was particularly evident in the basal layers of the epidermis where the UVB-induced tans had dramatically less DNA damage compared to the UVA-induced tan and the unexposed control. When the DNA damage in the unchallenged sites is subtracted from that in the UV-challenged sites (control Δ = 42.1, UVA & UVB Δ = 21.4, UVA Δ= 47.6 and UVB Δ = 28.4), the CPD damage was much greater in the unexposed area and in the UVA-tanned area compared to the unchallenged control, but the UVB-tanned skin, and to a greater extent the UVA&UVB-tanned skin, had much less further DNA damage (Fig. 4, middle).
To further assess the relationship between melanin content and its distribution after each type of UV exposure and the putative protective effect(s), the % change in DNA (CPD) damage after the 2 MED UV-challenge was assessed (Fig. 4, bottom). The melanin content in skin tanned by UVB with or without UVA, showed an inverse correlation with CPD damage following the 2 MED UV-challenge, while no such correlation was found between melanin content and CPD damage in the UVA only and in the unirradiated control skin.
Mechanism of UV Effects on Skin Pigmentation
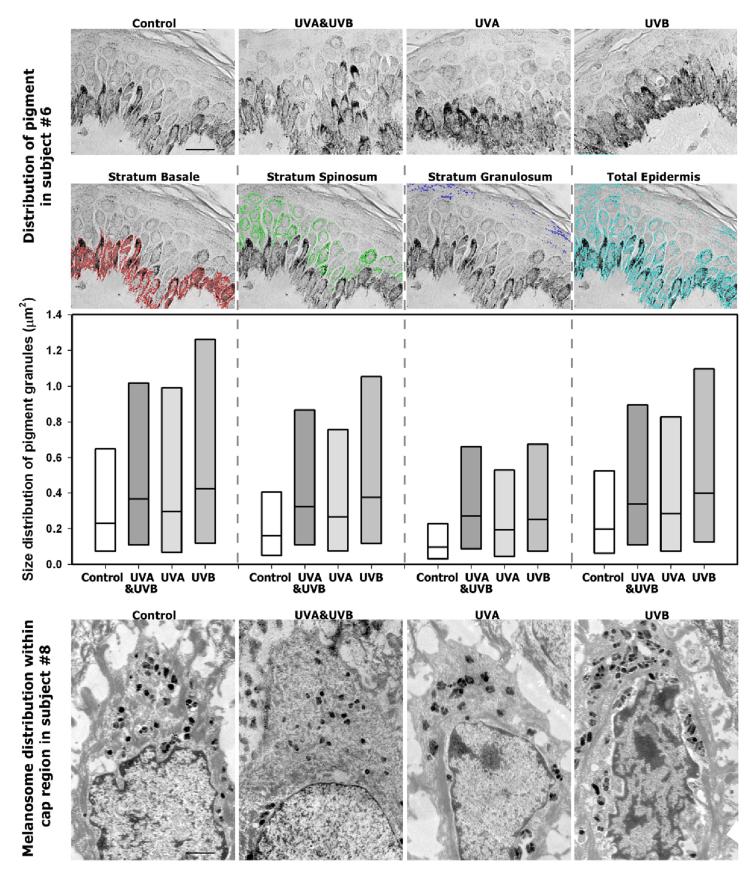
In an effort to resolve the mechanism whereby UVA-tanned skin had visible increases in pigmentation but without any actual increase in melanin content, we analyzed high magnification images of the melanin distribution patterns in the skin (Fig. 5, upper). Image analysis of the pigment granule size in the 3 layers of the epidermis (Fig. 5, middle) revealed that UVB (with or without UVA) increased the size distribution of pigment granules over the control or UVA-exposed areas in all 3 layers. Pigment granule size is defined as a cluster of melanosomes forming a larger pigmented particle visualized by light microscopy with a 100X objective. Although there were no statistical significant differences between the groups, the tendency reflected in the distribution pattern of pigment granule size was for melanin to be dispersed in larger pigment granules after UVB (with or without UVA) exposure compared to UVA only or the unirradiated control in all layers of the skin. UVA elicited a greater level of pigment granule size compared to the unexposed control while maintaining the same melanin positive area as the control. Electron microscopy showed that the supranuclear melanin caps seen in the unirradiated control skin were affected in various ways by UVA and/or UVB (Fig. 5, bottom) although the limited number of specimens (from 1 subject only) that could be used for EM analysis does not allow any significant conclusions to be drawn. UVA did not increase the percentage of melanin positive area and seemed to elicit just as great a local dispersion of melanin granules compared to UVB with or without UVA (although a rearrangement of existing melanin granules seems to occur mostly localized above the nuclei). In contrast, UVB (with or without UVA) increased the overall numbers of melanin granules which were generally dispersed throughout the cytoplasm of the cells, and also increased melanin content in upper layers of the epidermis.
Figure 5.
Relationship of UV tanning to the distribution of melanin. (Top) High magnification light microscopy images of representative areas of skin stained by the Fontana Masson method; example of pigment selection defined as stratum basale layer = red, stratum spinosum layer = green, stratum granulosum layer = blue, total epidermis = cyan to determine pigment granule size in μm2; scale bar = 20 μm. (Middle) Images were captured and analyzed as described in the Methods, and data are reported as box plots of size distribution of pigment granules in the 3 epidermal layers as well as in the total epidermis within 100X images (n=6 subjects, the solid line shows the median value). (Bottom) Electron micrographs taken of specimens from subject 8; scale bar = 1 μm.
Discussion
As expected, DNA damage caused by repetitive UVA exposure was less than was caused by UVB (with or without UVA), but the results of this study clearly demonstrate that suberythemal doses of UVA do not elicit increased melanin levels and provide little or no photoprotective benefit against a subsequent UV challenge. Although UVB produced higher levels of CPD compared to UVA, the UVB-induced tans did protect to some extent against subsequent CPD formation in the epidermis due to the UV challenge. This protection was particularly evident in basal layers of the epidermis which is the critical area with respect to photocarcinogenesis, since upper layers of the epidermis will soon be lost by desquamation and only cells in the basal layers persist and can pass on their UV-induced mutations to daughter cells. The production of a tan in the skin by UVA in the absence of increased melanin synthesis results from other effects of UVA on existing melanin, e.g. its distribution and/or oxidation. Although we did not measure an increase in the melanin positive area by UVA, the redistribution of melanin granules occurred to some extent that might account for the visual pigmentation differences, particularly if combined with increased oxidation of existing melanin by UVA. It is possible that measuring submicron-sized melanin granules might be beyond the detection capability of light microscopy and/or that melanosome complexes become sufficiently separated from each other and can’t be resolved.
UVA exposure has been shown to produce a brown to black persistent pigmentation that can remain for several weeks due to the oxidation of colorless melanogenic precursors (DHICA, 6H5MI2C and 5H6MI2C) present in the basal and suprabasal layers of the epidermis (Maeda & Hatao, 2004). During our repetitive UV protocol, subjects received daily UVA exposures of 1.76 - 3.40 J/cm2 in the first week and 2.41 - 4.34 J/cm2 in the second week, which would be a sufficient dose to increase DHICA and 6H5MI2C pigment formation according to that previous study (Maeda & Hatao, 2004). Previously, our group calculated concentrations of 6H5MI2C and 5H6MI2C in suction blisters from subjects in this repetitive UV protocol and found a tendency for increased levels of those intermediates following UVA exposure compared to the controls (Wolber et al., 2008). Our working hypothesis is that the UVA-induced tan produced without de novo melanin synthesis is due to the dispersion of larger pigment granules generated either from smaller granules or from the dissociation of aggregated granules compared to the control, although they are not as large as pigment granules in the UVB-induced tans. This, combined with the oxidation of existing melanin due to the action of UVA (Joshi et al., 1987; Novellino et al., 1998) is consistent with the visible UVA-induced tans.
UV exposure causes the dispersion of melanosomes, which is one important tanning response elicited by UV. The deleterious effects of UVA on cytoskeletal components were reported many years ago (Jimbow et al., 1973; Zamansky et al., 1991; Zamansky et al., 1992; Grzanka et al., 2006) and might play a critical role in the redistribution of melanosomes, which are organized intracellularly by the cytoskeleton (Rogers & Gelfand, 2000; Westbroek et al., 2004). Of the proteins previously identified to be associated with cytoskeleton, motor, trafficking and membrane dynamics in melanosomes (Chi et al., 2006; Watabe et al., 2008), Pmel17/SILV and melanophilin were significantly increased after UVB (with or without UVA) while syntenin-1 was significantly decreased after UVB only relative to the control (Storey-adjusted P values of <0.05) (Choi et al., 2010). Nielsen et al. ( 2006) reported a simulation of the effects of melanin dispersion in the epidermis and showed that dispersed melanin (particularly in upper layers of the skin) would have higher UV-protective effects for basal cells compared to the same amount of melanin distributed only in the basal layer. UV protection by melanin in supranuclear melanin caps has also been previously reported (Kobayashi et al., 1998). Unfortunately, in our study, we had only sufficient specimens remaining to allow EM analysis of 1 subject, which does not permit any significant conclusions to be drawn regarding melanosome distribution in the melanin cap region. Nevertheless, those images provide preliminary evidence of differences in melanin dispersion and melanin production elicited by UVA and/or UVB that warrants further investigation. The relationship between melanin content and DNA damage indicates that repetitive UVB exposure shows an increased inverse correlation between melanin content and CPD damage. These results suggest that exposure to UVB may increase protection against subsequent UV damage via increased melanin production supplemented by the redistribution of melanin towards the upper layers of the skin. This potential increase in protection from suberythemal exposures may be better at later times after the last UV exposure when the melanogenic system has been given time to reach its full photoprotective capacity.
These results show conclusively that increased melanin production in the skin results from UVB exposure and that UVA has little or no such effect. At this point, one cannot rule out the photoadaptation of the skin that occurs after UV exposure and perhaps the doses used were not sufficient to induce sustainable photoprotection. The capacity to repair DNA is also an important aspect which is not addressed in this study. It is also possible that within the relatively short time period of this study (less than 3 weeks), the melanogenic system has not reached its full photoprotective capacity, but the amount of DNA damage incurred may not warrant any cosmetic benefit perceived from UV tanning. Thus, UVA-tanning contributes very little in the way of photoprotection for the skin phototypes examined, although all types of UV-induced tanning result in DNA and cellular damage which can eventually lead to photocarcinogenesis. Of particular concern is the shift towards UVA-rich sunlamps which seem to afford little or no protection from subsequent UV exposure, despite the visible tan elicited. These results are alarming, especially when considered with recently emerging evidence that UVA exposure due to sunlamps correlates with significantly increased risk of melanomas (Roberts et al., 2009; Coelho & Hearing, 2010; von Thaler et al., 2010; Lazovich et al., 2010).
Materials and Methods
UV Sources
The 3 types of UV radiation used in this study were generated using an Oriel 1600 W Solar UV light simulator (Oriel Instruments, now the Newport Corporation, Stratford, CT, USA) and doses used were measured using a radiometer (IL1700, International Light, Newburyport, MA, USA) prior to each exposure to measure the intensity at the UV exposure test site as previously described (Wolber et al., 2008; Choi et al., 2010). The spectra of the Solar UV light simulator has previously been published (Choi et al., 2010) and the radiometer (IL1700) was calibrated using the measurements made with a Bentham 605 Spectral Radiometer (Bentham Instruments, Berkshire, UK) as previously published (Mahns et al., 2004). For UVA&UVB (290-400 nm), wavelengths below 290 nm were removed with a WG320 optical filter (ITOS GmbH, Mainz, Germany). For UVA (320-400 nm), UVB wavelengths were removed with a UVB/C-blocking filter (Oriel Instruments, now the Newport Corporation). For UVB (290-320 nm), UVA was removed using a combination of a WG320 optical filter (ITOS GmbH), a UG11 optical filter and a 290-320 bandpass filter (Tafelmaier, Rosenheim, Germany). Wavelengths above 400 nm were minimized using a dichroic mirror. Doses were determined using a radiometer (IL1700, International Light) prior to each exposure to measure the intensity at the UV exposure test site.
Pigmented in vitro Reconstituted Human Skin Equivalents and UV Challenge
Human skin equivalents in 12 mm diameter, 3.0 μm sterile culture plate inserts were purchased from MatTek Corp (Ashland, MA, USA). These human skin reconstructs were of White, Asian, and Black origin. They were cultured for 9 days (37°C, 5% CO2) on stands in 6-well plates maintained at the air/liquid interface using 5 ml EPI-100-LLMM medium (which contains αMSH and bFGF) which was changed every 2 days, according to the manufacturer’s instructions. The samples were monitored by macrophotography to evaluate visible pigmentation as described previously (Coelho et al., 2009a). Both day 0 and day 9 human skin equivalents were exposed to challenge doses of UVA/UVB at 50 mJ/cm2 using a TL20W/12RS Lamp (Philips, Eindhoven, Holland) monitored with a UVB detector coupled to a PMA2100 Radiometer (Solar Light Company Inc, Glenside, PA, USA). After the UV exposure, the irradiated and unexposed human skin equivalents were fixed in formalin and embedded in paraffin for further analysis as detailed below.
Subjects and UV Exposure Schedule
Volunteers with Fitzpatrick skin types II-III enrolled in this study as briefly described above and previously reported (Wolber et al., 2008; Choi et al., 2010). This study was approved by the Research Involving Human Subjects Committee of Beiersdorf AG, and was conducted according to the guidelines of the International Conference on Harmonisation Good Clinical Practice and the ethical principles of the Declaration of Helsinki. Seven subjects enrolled in this study (4 males, 3 females, ages ranging from 26 to 45). The MED for each subject was determined the week before the study as previously reported (Wolber et al., 2008). Three areas on the subjects’ backs (2 × 2 cm each) were exposed daily to the 3 different types of UV. The UVA&UVB area was irradiated 5 times a week at 0.4 MED in week 1 and 5 times a week at 0.5 MED in week 2 (Fig. 1A). Empirically, a dose of 2.3-fold the UVA and 1.1-fold the UVB in the UVA&UVB MED dose was used as the UVA- and UVB-adapted doses that would induce comparable visual tanning responses. An adjacent unexposed area of skin was used as a control for each subject. At the beginning of week 3, a small area within each UV-irradiated area (and the unirradiated control) was then challenged with a 2 MED UV dose. Only the UVA&UVB tan and no tan responses were then assessed by reflection spectroscopy using a SpectroPenTM (Dr. Lange, Berlin, Germany) with the CIE L*a*b* color space system. The CIE L*a*b* parameters L* and b* allow for a vector representation of pigmentation categories by calculating the individual typology angle (ITA° = (atan(L*-50)/b*) × 180/π). All areas were biopsied immediately, after which they were fixed in formalin and embedded in paraffin for further analysis as detailed below.
Immunohistochemistry, Microscopy and Image Analysis
Paraffin-embedded skin biopsies were processed for immunohistochemistry and Fontana-Masson silver stain as detailed previously (Tadokoro et al., 2003; Choi et al., 2010). A mouse monoclonal anti-CPD antibody (clone TDM-2, IgG2a, a gift from Dr. Toshio Mori, Nara Medical University) and a mouse monoclonal anti-DNA antibody (clone AC-30-10, IgM, Progen, Heidelberg, Germany) were used as primary antibodies to detect CPD damage and total genomic DNA, respectively. Magnified images of epidermis in each section were captured using a Leica DMRB/D MLD fluorescence microscope (Leica, Wetzlar, Germany) with a fluorescence filter, a Dage-MTI 3CCD 3-chip color video camera (Dage-MTI, Michigan City, IN, USA), and Scion Image software (Scion, Frederick, MD, USA). The fluorescent signals from CPD were divided by the fluorescent signals from DNA to determine the % CPD positive area and CPD mean fluorescence intensity quantitated from 10 random images for each specimen; care was taken to exclude any potentially saturated images from the analysis.
Using a Leica DMRB/DMLD microscope and Scion Image software, the melanin content was analyzed by quantifying the mean density minus background level of 10 random images per specimen as previously described (Tadokoro et al., 2003) and also to determine the % positive area for melanin within the epidermis. In addition, images of stained samples were captured using the Zeiss Axiovert S100 inverted microscope (Carl Zeiss, Inc., Oberkochen, Germany) with a Hamamatsu ORCA-ER B/W CCD digital camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan). Axiovision v 4.6 (Carl Zeiss MicroImaging GmbH, Jena, Germany) digital image processing software was used to quantify the melanin pigment granule size, and stratum corneum thickness. Using the Measurement Program Wizard within the software, we created a routine that allows segmentation of pigmentation based on an empirically determined threshold value. To determine melanin pigment granule size, the program measures the area of segmented pigment in all 3 epidermal layers (not including the stratum corneum) within a 100X image. These values were exported to a spreadsheet and Student’s t-test was used for statistical analysis (5 random images were quantitated per specimen).
For preparation of paraffin-embedded skin tissues for electron microscopy, the surrounding wax of the paraffin-embedded tissues was carefully removed and the remaining tissues were placed in xylene for 30 min. After a few changes of xylene the tissues were immersed in a mixture of xylene and ethanol for a few minutes and then in pure ethanol. The samples were quickly rehydrated by placing them in a gradient of ethanol and water and briefly rinsed in 0.1 M sodium cacodylate buffer (pH= 7.4). The samples were postfixed in 1% osmium tetroxide in the above buffer for 1 hr. After several rinses in the buffer, the samples were dehydrated in a series of ethanol (20%, 40%, 60%, 75%, 95% for 10 min and 100% for 30 min with 3 changes) and infiltrated with Epon-Aradite (Ted Pella, Redding, CA) for several days (25% of Epon-Aradite and ethanol for 2 hr, 50% for overnight, 75% for 8 hr and 100% for 1 day with 2 changes). Samples were polymerized at 60°C for 2 days. Ultrathin sections (about 90 nm) were cut on the Leica EM UC6 Ultra-microtome and collected on 1 mm wide slot grids. Sections were counter-stained with uranyl acetate and lead citrate, and examined under a FEI CM120 (FEI Co., Hillsboro, OR, USA) transmission electron microscope (equipped with a Gatan GIF100 image filter) operating at a beam energy of 120keV. Images were acquired by using a Gatan 1k × 1k cooled CCD camera at a magnification of 2800X.
Statistical evaluation was completed using Microsoft Office Excel 2003 Analysis ToolPak. Statistical differences between irradiated specimens and controls with or without the 2 MED challenge were determined by Student’s t-test with a two-tailed distribution.
Significance.
The UVA component in suberythemal repetitive exposures mimicking incident sunlight, received in doses relative to everyday life situations, does not increase melanin production and affords little or no photoprotection. This raises serious concerns for individuals, particularly young adolescents, who might have the misconception that tans induced by UVA-rich sunlamps are protective against subsequent UV exposures. The modest photoprotective effects afforded by repetitive exposure to suberythemal UVB are not without risks, and are due to increased production, coverage and distribution of melanin. This study clarifies important protective differences between the UVA and/or UVB components of suberythemal repetitive UV exposures.
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Cancer Institute at NIH.
Abbreviations Used
- 64PP
6,4-photoproducts
- CPD
cyclobutane pyrimidine dimers
- MED
minimal erythema dose
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- UV
ultraviolet
References
- Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23:171–186. doi: 10.1111/j.1755-148X.2010.00679.x. [DOI] [PubMed] [Google Scholar]
- Agar N, Young AR. Melanogenesis: a photoprotective response to DNA damage? Mutat. Res. 2005;571:121–132. doi: 10.1016/j.mrfmmm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DC. Ultraviolet wavebands and melanoma initiation. Pigment Cell Melanoma Res. 2008;21:520–524. doi: 10.1111/j.1755-148X.2008.00500.x. [DOI] [PubMed] [Google Scholar]
- Berneburg M, Grether-Beck S, Kurten V, Ruzicka T, Briviba K, Sies H, Krutmann J. Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion. J. Biol. Chem. 1999;274:15345–15349. doi: 10.1074/jbc.274.22.15345. [DOI] [PubMed] [Google Scholar]
- Berwick M. Are tanning beds “safe”? Human studies of melanoma. Pigment Cell Melanoma Res. 2008;21:517–519. doi: 10.1111/j.1755-148X.2008.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besaratinia A, Synold TW, Chen HH, Chang C, Xi B, Riggs AD, Pfeifer GP. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10058–10063. doi: 10.1073/pnas.0502311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black HS, de Gruijl FR, Forbes PD, Cleaver JE, Ananthaswamy HN, De Fabo EC, Ullrich SE, Tyrrell RM. Photocarcinogenesis: an overview. Photochem. Photobiol. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J. Invest. Dermatol. Symp. Proc. 1996;1:136–142. [PubMed] [Google Scholar]
- Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruls WA, Slaper H, van der Leun JC, Berrens L. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem. Photobiol. 1984;40:485–494. doi: 10.1111/j.1751-1097.1984.tb04622.x. [DOI] [PubMed] [Google Scholar]
- Caron-Schreinemachers AL, Kingswijk MM, Bos JD, Westerhof W. UVB 311 nm tolerance of vitiligo skin increases with skin photo type. Acta Derm. Venereol. 2005;85:24–26. doi: 10.1080/00015550410022203. [DOI] [PubMed] [Google Scholar]
- Chi A, Valencia JC, Hu ZZ, Watabe H, Yamaguchi H, Mangini N, Huang H, Canfield VA, Cheng KC, Yang F, Abe R, Yamagishi S, Shabanowitz J, Hearing VJ, Wu CH, Appella E, Hunt DF. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J. Proteome Res. 2006;5:3135–3144. doi: 10.1021/pr060363j. [DOI] [PubMed] [Google Scholar]
- Choi W, Miyamura Y, Wolber R, Smuda C, Reinhold W, Liu H, Kolbe L, Hearing VJ. Regulation of human skin pigmentation in situ by repetitive UV exposure - Molecular characterization of responses to UVA and/or UVB. J. Invest. Dermatol. 2010;130:1685–1696. doi: 10.1038/jid.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIE Technical Committee 2-17 . CIE Technical Report N° CIE 85, Solar Spectral Irradiance. Commision Internationale de l’Eclairage (CIE) Central Bureau; Vienna, Austria: 1989. [Google Scholar]
- Coelho SG, Hearing VJ. Hypothesis: UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57–63. doi: 10.1111/j.1755-148X.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho SG, Koo E, Hearing VJ. Standardization of in vitro macrophotography for assessment of cutaneous responses. Photochem. Photobiol. 2009a;85:1032–1037. doi: 10.1111/j.1751-1097.2009.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho SG, Zhou Y-C, Bushar HF, Miller SA, Zmudzka BZ, Hearing VJ, Beer JZ. Long-lasting pigmentation of human skin, a new look at an overlooked response to UV. Pigment Cell Melanoma Res. 2009b;22:238–241. doi: 10.1111/j.1755-148X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fabo EC, Noonan FP, Fears TR, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64:6372–6376. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- de Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol. Appl. Skin Physiol. 2002;15:316–320. doi: 10.1159/000064535. [DOI] [PubMed] [Google Scholar]
- Gambichler T, Sauermann K, Altintas MA, Paech V, Kreuter A, Altmeyer P, Hoffmann K. Effects of repeated sunbed exposures on the human skin. In vivo measurements with confocal microscopy. Photodermatol. Photoimmunol. Photomed. 2004;20:27–32. doi: 10.1111/j.1600-0781.2004.00079.x. [DOI] [PubMed] [Google Scholar]
- Gange RW, Blackett AD, Matzinger BA, Sutherland BM, Kochevar IE. Comparative protection efficiency of UVA- and UVB-induced tans against erythema and formation of endonuclease-sensitive sites in DNA by UVB in human skin. J. Invest. Dermatol. 1985;85:361–364. doi: 10.1111/1523-1747.ep12276983. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. New Eng. J. Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- Grzanka D, Domaniewski J, Grzanka A, Zuryn A. Ultraviolet radiation (UV) induces reorganization of actin cytoskeleton in CHOAA8 cells. Neoplasma. 2006;53:328–332. [PubMed] [Google Scholar]
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol. Clin. 2003;21:725–732. doi: 10.1016/s0733-8635(03)00085-8. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Pathak MA, Fitzpatrick TB. Effect of ultraviolet on the distribution pattern of microfilaments and microtubules and on the nucleus in human melanocytes. Yale J. Biol. Med. 1973;46:411–426. [PMC free article] [PubMed] [Google Scholar]
- Johnson BE. The influence of radiation on the skin and the basis of protection. Int. J. Cosmet. Sci. 1983;5:131–139. doi: 10.1111/j.1467-2494.1983.tb00334.x. [DOI] [PubMed] [Google Scholar]
- Johnson BE, Mandell G, Daniels G. Melanin and cellular reactions to ultraviolet radiation. Nature. 1972;235:147–149. doi: 10.1038/newbio235147a0. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Carraro C, Pathak MA. Involvement of reactive oxygen species in the oxidation of tyrosine and DOPA to melanin and in skin tanning. Biochem. Biophys. Res. Commun. 1987;142:265–274. doi: 10.1016/0006-291x(87)90480-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Nakagawa A, Muramatsu T, Yamashina Y, Shirai T, Hashimoto MW, Ishigaki Y, Ohnishi T, Mori T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J. Invest. Dermatol. 1998;110:806–810. doi: 10.1046/j.1523-1747.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. J. Dermatol. Sci. 2000;23(Suppl 1):S22–S26. doi: 10.1016/s0923-1811(99)00077-8. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Gerberick GF, Veres D, Irwin CJ, Kaidbey KH. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J. Am. Acad. Dermatol. 1995;32:53–62. doi: 10.1016/0190-9622(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol. Biomark. Prevent. 2010;19:1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Hatao M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J. Invest. Dermatol. 2004;122:503–509. doi: 10.1046/j.0022-202X.2004.22223.x. [DOI] [PubMed] [Google Scholar]
- Mahns A, Wolber R, Stab F, Klotz LO, Sies H. Contribution of UVB and UVA to UV-dependent stimulation of cyclooxygenase-2 expression in artificial epidermis. Photochem. Photobiol. Sci. 2004;3:257–262. doi: 10.1039/b309067a. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Ananthaswamy HN. Molecular mechanisms of photocarcinogenesis. Front. Biosci. 2002;7:765–783. doi: 10.2741/matsumur. [DOI] [PubMed] [Google Scholar]
- Meinhardt M, Krebs R, Anders A, Heinrich U, Tronnier H. Effect of ultraviolet adaptation on the ultraviolet absorption spectra of human skin in vivo. Photodermatol. Photoimmunol. Photomed. 2008;24:76–82. doi: 10.1111/j.1600-0781.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- Miescher G. Das Problem des Lichtschutzes und der Lichtgewöhnung. Strahlentherapie. 1930;35:403–423. [Google Scholar]
- Miller SA, Coelho SG, Zmudzka BZ, Bushar HF, Yamaguchi Y, Hearing VJ, Beer JZ. Dynamics of pigmentation induction by repeated ultraviolet exposures: dose, dose interval and UV spectrum dependence. Brit. J. Dermatol. 2008;159:921–930. doi: 10.1111/j.1365-2133.2008.08708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DL, Fernandez AA, Nairn RS, Garcia R, Paniker L, Trono D, Thames HD, Gimenez-Conti I. Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9329–9334. doi: 10.1073/pnas.1000324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura Y, Coelho SG, Wolber R, Miller SA, Wakamatsu K, Zmudzka BZ, Ito S, Smuda C, Passeron T, Choi W, Batzer J, Yamaguchi Y, Beer JZ, Hearing VJ. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20:2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA. 2006;103:13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KP, Zhao L, Stamnes JJ, Stamnes K, Moan J. The importance of the depth distribution of melanin in skin for DNA protection and other photobiological processes. J. Photochem. Photobiol. 2006;82:194–198. doi: 10.1016/j.jphotobiol.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Novellino L, d’Ischia M, Prota G. Nitric oxide-induced oxidation of 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carboxylic acid under aerobic conditions: non-enzymatic route to melanin pigments of potential relevance to skin (photo)protection. Biochim. Biophys. Acta. 1998;1425:27–35. doi: 10.1016/s0304-4165(98)00060-9. [DOI] [PubMed] [Google Scholar]
- Ortonne JP. The effects of ultraviolet exposure on skin melanin pigmentation. J. Intl. Med. Res. 1990;18:8C–17C. [PubMed] [Google Scholar]
- Patton EE, Mitchell DL, Nairn RS. Genetic and environmental melanoma models in fish. Pigment Cell Melanoma Res. 2010;23:314–337. doi: 10.1111/j.1755-148X.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse AD, Gaskell SA, Marks R. Epidermal changes in human skin following irradiation with either UVB or UVA. J. Invest Dermatol. 1987;88:83–87. doi: 10.1111/1523-1747.ep12465094. [DOI] [PubMed] [Google Scholar]
- Preston DS, Stern RS. Nonmelanoma cancers of the skin. New Eng. J. Med. 1992;327:1649–1662. doi: 10.1056/NEJM199212033272307. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Hornung CA, Polk HC., Jr. Another duel in the sun: weighing the balances between sun protection, tanning beds, and malignant melanoma. Clin. Pediatr. (Phila) 2009;48:614–622. doi: 10.1177/0009922809332589. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Gelfand VI. Membrane trafficking, organelle transport, and the cytoskeleton. Curr. Opin. Cell Biol. 2000;12:57–62. doi: 10.1016/s0955-0674(99)00057-5. [DOI] [PubMed] [Google Scholar]
- Roser-Maass E, Holzle E, Plewig G. Protection against UV-B by UV-A-induced tan. Arch. Dermatol. 1982;118:483–486. [PubMed] [Google Scholar]
- Ruegemer J, Schuetz B, Hermann K, Hein R, Ring J, Abeck D. UV-induced skin changes due to regular use of commercial sunbeds. Photodermatol. Photoimmunol. Photomed. 2002;18:223–227. doi: 10.1034/j.1600-0781.2002.180501.x. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc. Natl. Acad. Sci. USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J. Am. Acad. Dermatol. 1998;38:89–98. doi: 10.1016/s0190-9622(98)70544-4. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin and photosensitivity. FASEB J. 2003;17:1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, Hearing VJ. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Invest. Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- Tran TT, Schulman J, Fisher DE. UV and pigmentation: molecular mechanisms and social controversies. Pigment Cell Melanoma Res. 2008;21:509–516. doi: 10.1111/j.1755-148X.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Thaler AK, Kamenisch Y, Berneburg M. The role of ultraviolet radiation in melanomagenesis. Exp. Dermatol. 2010;19:81–88. doi: 10.1111/j.1600-0625.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- Wagner JK, Parra EJ, Lin H, Jovel C, Shriver MD. Skin responses to ultraviolet radiation: effects of constitutive pigmentation, sex, and ancestry. Pigment Cell Res. 2002;15:385–390. doi: 10.1034/j.1600-0749.2002.02046.x. [DOI] [PubMed] [Google Scholar]
- Watabe H, Valencia JC, Le Pape E, Yamaguchi Y, Nakamura M, Rouzaud F, Hoashi T, Kawa Y, Mizoguchi M, Hearing VJ. Involvement of dynein and spectrin with early melanosome transport and melanosomal protein trafficking. J. Invest. Dermatol. 2008;128:162–174. doi: 10.1038/sj.jid.5701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbroek W, Lambert J, De Schepper S, Kleta R, Van Den BK, Seabra MC, Huizing M, Mommaas M, Naeyaert JM. Rab27b is up-regulated in human Griscelli syndrome type II melanocytes and linked to the actin cytoskeleton via exon F-Myosin Va transcripts. Pigment Cell Res. 2004;17:498–505. doi: 10.1111/j.1600-0749.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- Wikonkal NM, Brash DE. Ultraviolet radiation signature mutations in photocarcinogenesis. J. Investig. Dermatol. Symp. Proc. 1999;4:6–10. doi: 10.1038/sj.jidsp.5640173. [DOI] [PubMed] [Google Scholar]
- Wolber R, Schlenz K, Wakamatsu K, Smuda C, Nakanishi Y, Hearing VJ, Ito S. Pigmentation effects of solar simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21:487–491. doi: 10.1111/j.1755-148X.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc. Natl. Acad. Sci. USA. 2006;103:4111–4115. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- Zamansky GB, Nguyen U, Chou IN. An immunofluorescence study of the effects of ultraviolet radiation on the organization of microfilaments, keratin intermediate filaments, and microtubules in human keratinocytes. Cell Motil. Cytoskeleton. 1992;22:296–306. doi: 10.1002/cm.970220409. [DOI] [PubMed] [Google Scholar]
- Zamansky GB, Perrino BA, Chou IN. Disruption of cytoplasmic microtubules by ultraviolet radiation. Exp. Cell Res. 1991;195:269–273. doi: 10.1016/0014-4827(91)90527-2. [DOI] [PubMed] [Google Scholar]