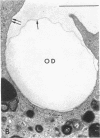
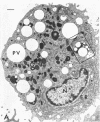
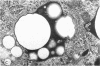
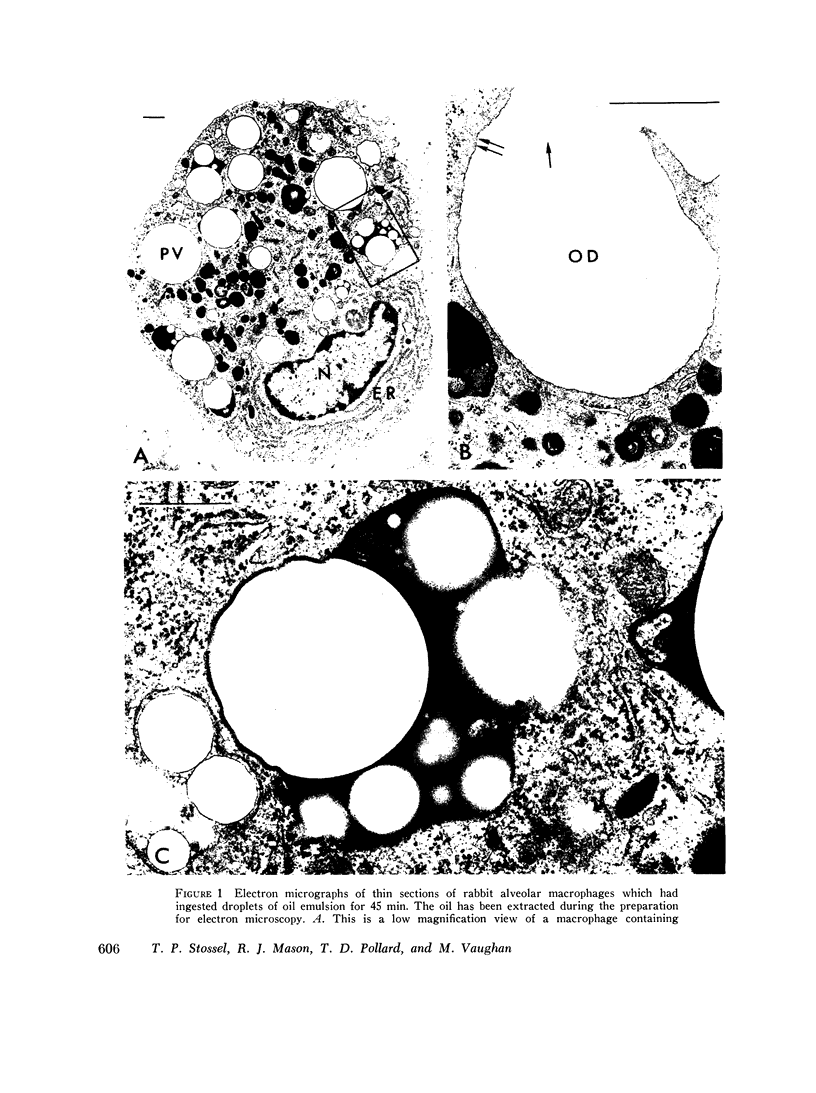
Abstract
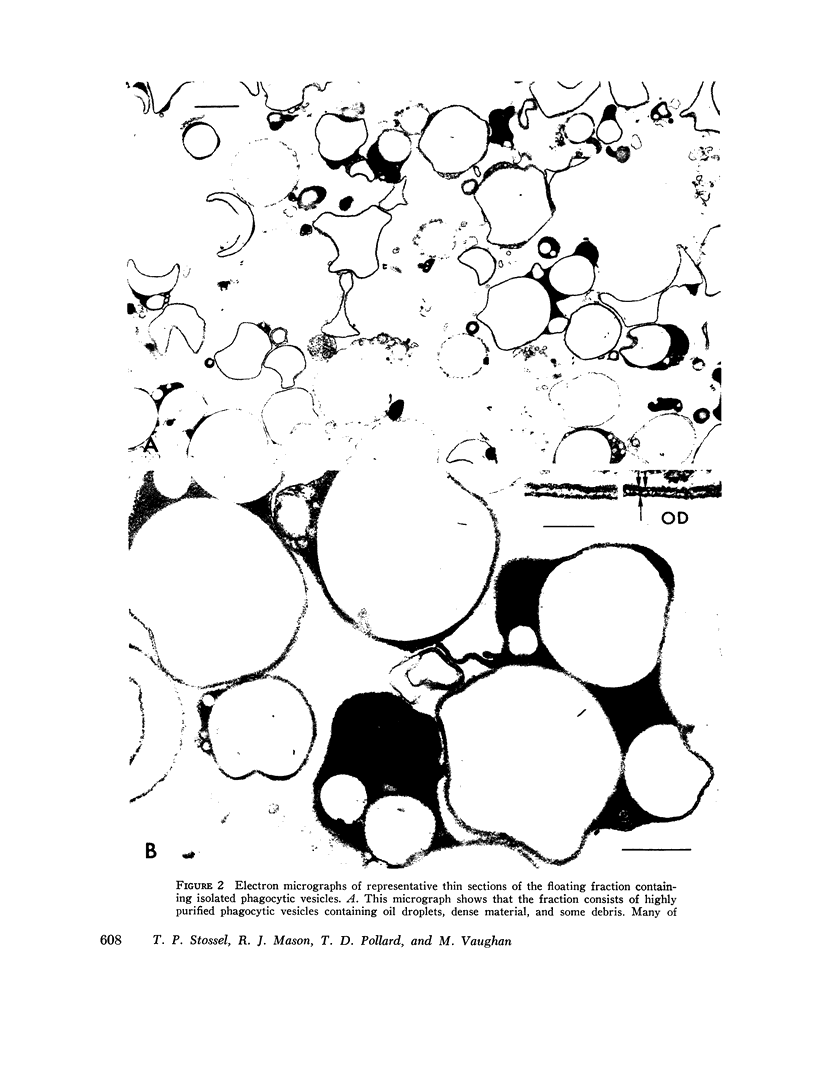
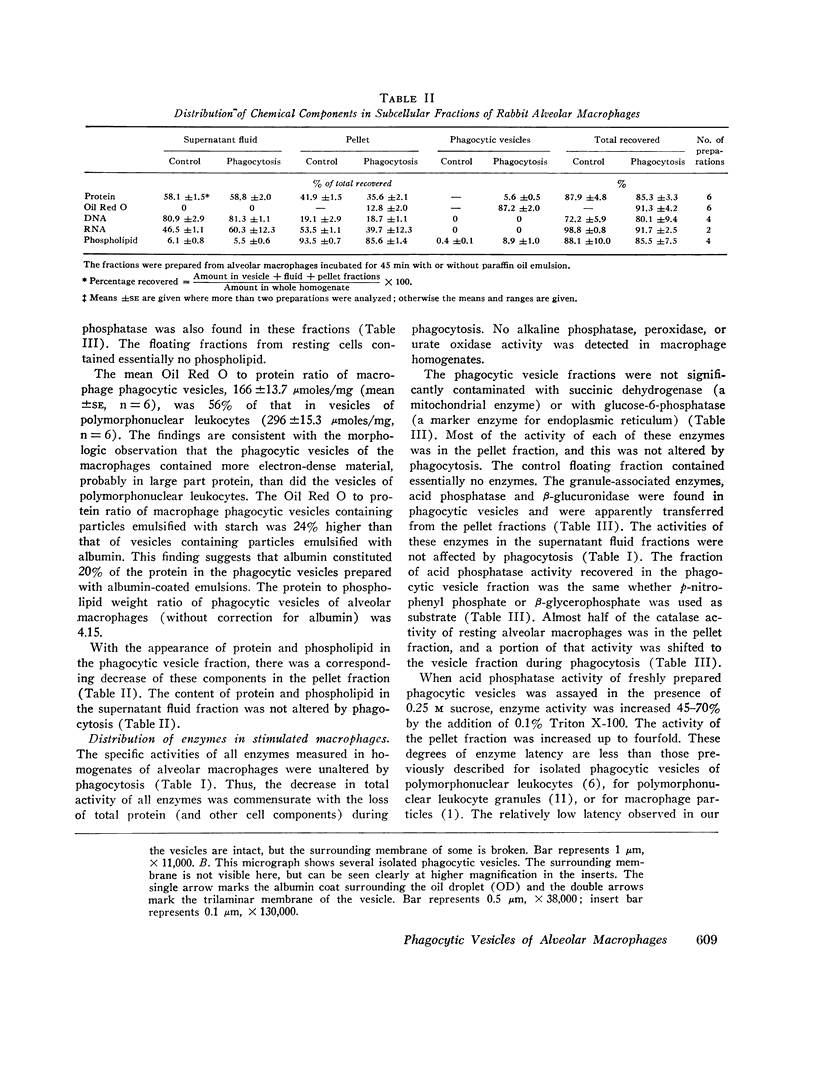
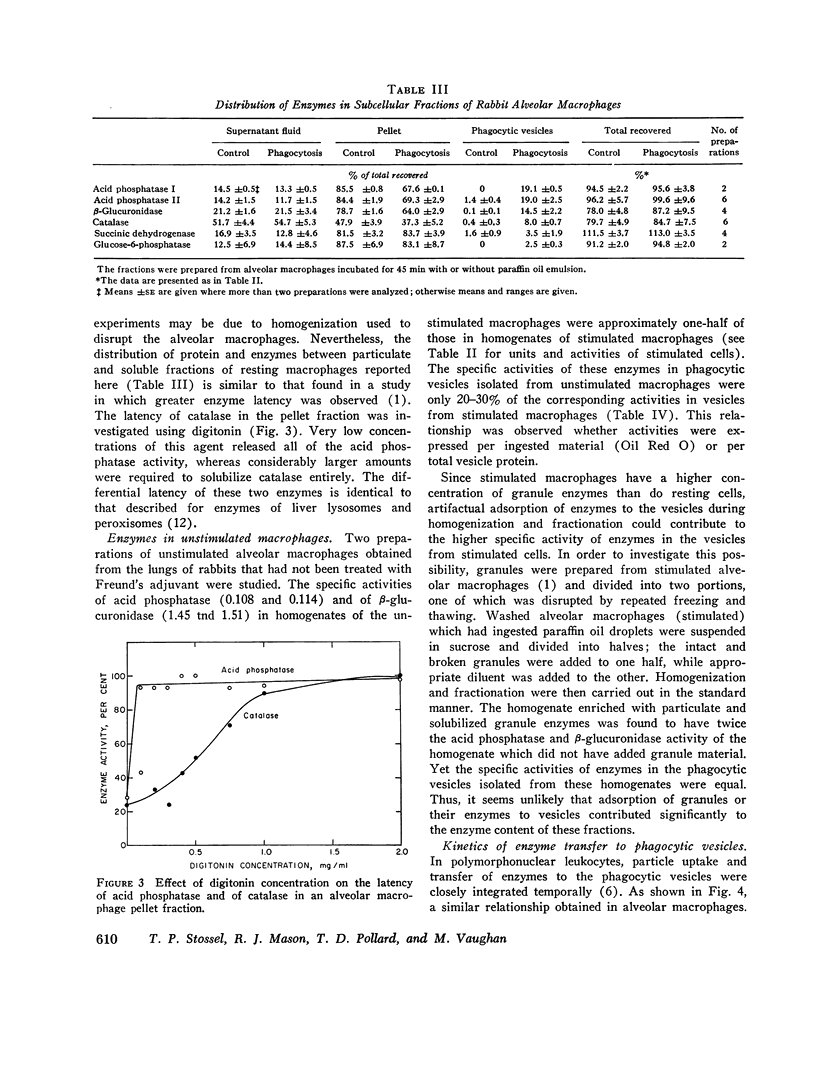
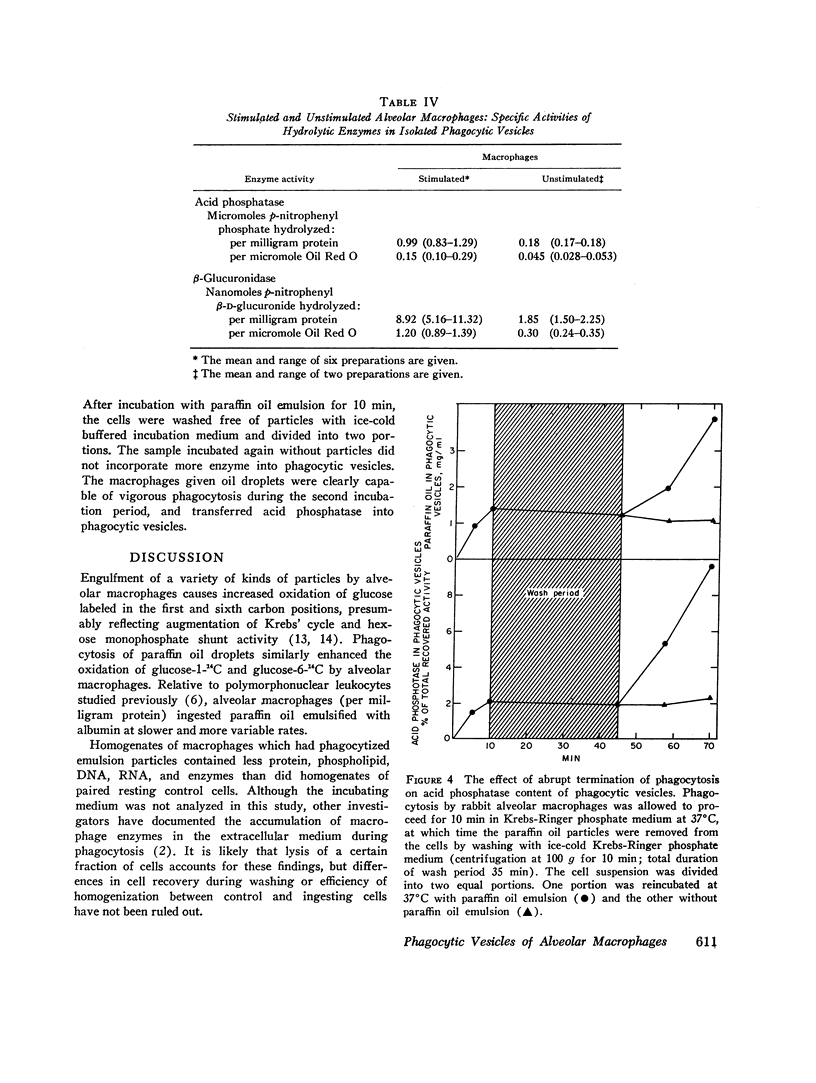
Phagocytic vesicles were obtained by density gradient centrifugation of homogenized rabbit alveolar macrophages that had ingested emulsified paraffin oil contained Oil Red O. The phagocyte vesicles floated and thereby were separated from the soluble fraction and from other cell components which sedimented. The purity of the isolated vesicles was documented by electron microscopy, chemical and enzyme analysis. The vesicles contained 87% of the cell-associated Oil Red O, and were essentially free of DNA, RNA, succinic dehydrogenase, and glucose-6-phosphatase. Acid phosphatase, β-glucuronidase, and catalase were transferred from the sedimenting fraction to the phagocytic vesicle fraction during phagocytosis, whereas enzyme activities of the soluble fraction remained unchanged. Half of the catalase of resting macrophages was in the pellet fraction and, compared with acid phosphatase, greater amounts of digitonin were required to release full activity. Such differential latency has been described for enzymes of peroxisomes vs. those of lysosomes. Compared with polymorphonuclear leukocyte vesicles studied previously, phagocytic vesicles of macrophages had more electron-dense material and lower Oil Red O:protein, phospholipid:protein, and enzyme:protein ratios. It is thus probable that secondary lysosomes become part of the macrophage vesicle. When paraffin oil particles, the stimulus for phagocytic vesicle formation, were washed away from the macrophages, acquisition of hydrolases by preformed vesicles ceased, i.e. transfer of these enzymes into phagocytic vesicles occurred only during or shortly after the formation of new vesicles. As noted previously by others, the content of acid hydrolases of stimulated alveolar macrophages was doubled in comparison to normal cells. The difference between stimulated and normal macrophages was even more marked when isolated phagocytic vesicles were analyzed. Vesicles from stimulated macrophages had 3-5 times more enzyme activity (per milligram of vesicle protein or per amount of paraffin oil ingested) than did vesicles from normal cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE IN VITRO DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. II. THE INFLUENCE OF SERUM ON GRANULE FORMATION, HYDROLASE PRODUCTION, AND PINOCYTOSIS. J Exp Med. 1965 May 1;121:835–848. doi: 10.1084/jem.121.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet A. J. Fine structure of the alveolar macrophage of the cat and modifications of its cytoplasmic components during phagocytosis. Anat Rec. 1970 Jul;167(3):277–289. doi: 10.1002/ar.1091670303. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Dounce A. L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970 Jul;50(3):319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- Gee J. B., Vassallo C. L., Bell P., Kaskin J., Basford R. E., Field J. B. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. J Clin Invest. 1970 Jun;49(6):1280–1287. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M. Pulmonary clearance of infectious agents. Annu Rev Med. 1968;19:315–336. doi: 10.1146/annurev.me.19.020168.001531. [DOI] [PubMed] [Google Scholar]
- HEISE E. R., MYRVIK Q. N., LEAKE E. S. EFFECT OF BACILLUS CALMETTE-GU'ERIN ON THE LEVELS OF ACID PHOSPHATASE, LYSOZYME AND CATHEPSIN IN RABBIT ALVEOLAR MACROPHAGES. J Immunol. 1965 Jul;95:125–130. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial activity of catalase at acid pH. Proc Soc Exp Biol Med. 1969 Nov;132(2):571–574. doi: 10.3181/00379727-132-34263. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- LEAKE E. S., GONZALEZ-OJEDA D., MYRVIK Q. N. ENZYMATIC DIFFERENCES BETWEEN NORMAL ALVEOLAR MACROPHAGES AND OIL-INDUCED PERITONEAL MACROPHAGES OBTAINED FROM RABBITS. Exp Cell Res. 1964 Feb;33:553–561. doi: 10.1016/0014-4827(64)90020-5. [DOI] [PubMed] [Google Scholar]
- Leake E. S., Evans D. G., Myrvik Q. N. Ultrastructural patterns of bacterial breakdown in normal and granulomatous rabbit alveolar macrophages. J Reticuloendothel Soc. 1971 Feb;9(2):174–199. [PubMed] [Google Scholar]
- Leake E. S., Myrvik Q. N. Digestive vacuole formation in alveolar macrophages after phagocytosis of Mycobacterium smegmatis in vivo. J Reticuloendothel Soc. 1966 May;3(1):83–100. [PubMed] [Google Scholar]
- Leake E. S., Myrvik Q. N. Interaction of lysosome-like structures and phagosomes in normal and granulomatous alveolar macrophages. J Reticuloendothel Soc. 1970 Nov;8(5):407–420. [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Michell R. H., Karnovsky M. J., Karnovsky M. L. The distributions of some granule-associated enzymes in guinea-pig polymorphonuclear leucocytes. Biochem J. 1970 Jan;116(2):207–216. doi: 10.1042/bj1160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R. L., Ferris B., Hirsch J. G. Macrophage plasma membranes. I. Isolation and studies on protein components. J Exp Med. 1971 Apr 1;133(4):785–806. doi: 10.1084/jem.133.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi E., Selvaraj R. J., Sbarra A. J. The biochemical activities of rabbit alveolar macrophages during phagocytosis. Exp Cell Res. 1965 Dec;40(3):456–468. doi: 10.1016/0014-4827(65)90226-0. [DOI] [PubMed] [Google Scholar]
- Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Function of h(2)o(2), myeloperoxidase, and hexose monophosphate shunt enzymes in phagocytizing cells from different species. Infect Immun. 1970 Apr;1(4):338–344. doi: 10.1128/iai.1.4.338-344.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER W. C., HOGEBOOM G. H. Intracellular distribution of enzymes. IX. Certain purine-metabolizing enzymes. J Biol Chem. 1952 Mar;195(1):161–166. [PubMed] [Google Scholar]
- SWANSON M. A. Phosphatases of liver. I. Glucose-6-phosphatase. J Biol Chem. 1950 Jun;184(2):647–659. [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel M. G., Korn E. D. Phagocytosis of latex beads by Acahamoeba castellanii (Neff). 3. Isolation of the phagocytic vesicles and their membranes. J Cell Biol. 1969 Oct;43(1):90–104. doi: 10.1083/jcb.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]