Abstract
We examined model mixed micelles consisting of the nonionic surfactant n-dodecyl-β-D-maltoside, 3-(3-cholamidopropyl)-dimethylammoniopropane sulfonate, and the cholesterol derivative cholesteryl hemisuccinate (CHS) to identify micellar properties that are correlated with the in vitro conformational stability and activity of the human adenosine A2a receptor, a G-protein coupled receptor. Small-angle neutron scattering was used to determine micellar structure and composition as a function of concentration of the various components, and radioligand binding was used as a sensitive probe for receptor activity. Micelles adopted an oblate ellipsoidal morphology and exhibited a reduction in size and change in curvature upon addition of CHS. Our results show a strong correlation between the number of CHS monomers per micelle and the activity of the receptor reconstituted in those micelles. Micelles that yield optimal human adenosine A2a receptor stability closely mimic the cholesterol composition and thickness of mammalian membranes. Thus, successful reconstitution of the receptor is dependent on both specific lipid-protein interactions and the geometry of the micelle environment.
In vitro characterization of membrane protein structure requires solubilization within membrane-mimetic vehicles to facilitate purification and reconstitution outside of the lipid bilayer (1). The simplest strategy for reconstituting membrane proteins is by solubilization within surfactant micelles to form protein-detergent complexes (PDCs) (2). Although surfactant micelles generally promote solubility in polar solvents, they also affect protein folding (2) and activity (3), and can lead to unwanted aggregation (4). Lipid additives, such as sterols that interact with membrane proteins in native bilayers (5), are often added to PDCs to help maintain protein structure and function. However, it is not possible to predict a priori which surfactant-lipid mixtures will effectively stabilize the membrane protein of interest. Therefore, the difficulty of selecting an appropriate environment for stabilizing membrane proteins is a major barrier against characterizing them in vitro. Here, we show how the addition of a cholesterol derivative can affect micelle morphology to benefit the conformational stability (i.e., ligand-binding activity) of a multipass transmembrane protein, the human adenosine A2a receptor (hA2aR).
Our efforts were motivated by an intriguing correlation between the activity of mammalian G-protein coupled receptors (GPCRs) and the presence of cholesterol, which is normally found within mammalian membranes. It is well documented that cholesterol modulates the functional activity of GPCRs in cellular membranes (5). The recently determined crystal structure for the β2-adrenergic receptor, a member of this protein family, revealed specific interactions of cholesterol derivatives with the solved structure. These interactions were required to preserve the structural integrity of the protein, suggesting that a cholesterol-binding motif exists more broadly within Class A GPCRs (6). It is unclear how these critical lipid-protein contacts can be sufficiently emulated within PDCs, as information about how cholesterol and its derivatives affect the self-assembly of commonly used surfactants is lacking.
The most frequently employed surfactants to facilitate membrane protein study are the nonionic alkyl glucosides, specifically n-dodecyl-β-D-maltoside (DDM). It has been observed that altering the composition of DDM micelles by titrating in other surfactants and additives, particularly the cholesterol derivative cholesteryl hemi-succinate (CHS), can drastically impact the activity of GPCRs (7). In previous work, we showed that the activity of purified hA2aR was lost in the presence of single-component DDM micelles, but was retained upon addition of CHS and CHAPS, which was included to promote CHS solubility (8). DDM/CHAPS alone is insufficient to promote activity (see Fig. S1 in the Supporting Material). It is uncertain whether CHS-assisted stabilization is due to changes in micelle morphology, specific protein-CHS interactions, or a combination of both factors.
We examined the self-assembly of DDM micelles in the presence of CHS and CHAPS, and directly measured hA2aR activity in the mixed micelles. To facilitate our approach, we define δ as the combined mass fraction of CHAPS and CHS in the solute (where the mass ratio of CHS/CHAPS in the solute is fixed at 0.2) and the total solute mass fraction of 1 wt%. To systematically study the effects of CHS on micelle architecture, we performed small-angle neutron scattering (SANS) measurements on a series of mixed micelles with δ-values ranging from 0 to 0.85, encompassing a range of CHS concentrations typically used in structural characterization of GPCRs. Concurrently, we examined protein conformational integrity within the same range of δ-values by solubilization and purification of hA2aR, yielding ∼0.004 mM of protein in micellar solution (8). Note that under these conditions, micelles are in large excess for all conditions studied.
Binding of a radiolabeled agonist (3[H]-CGS-21,680) to hA2aR provides a direct measurement of the conformational stability of the protein reconstituted in PDCs (8), where the concentration of active receptors is characterized by the specific amount of radioligand bound (Fig. 1 A). When the radiolabeled receptor was titrated with increasing amounts of unlabeled ligand (N6-cyclohexyl-adenosine (CHA)), a Ki of 59 ± 14 nM was measured (Fig. 1 A, inset), consistent with previous reports (8). This suggests that receptor-ligand affinity is not significantly altered in stabilizing micelles compared with native membranes, further supporting the notion that differences in the amount of radioligand bound are an accurate reflection of the population of active hA2aR.
Figure 1.
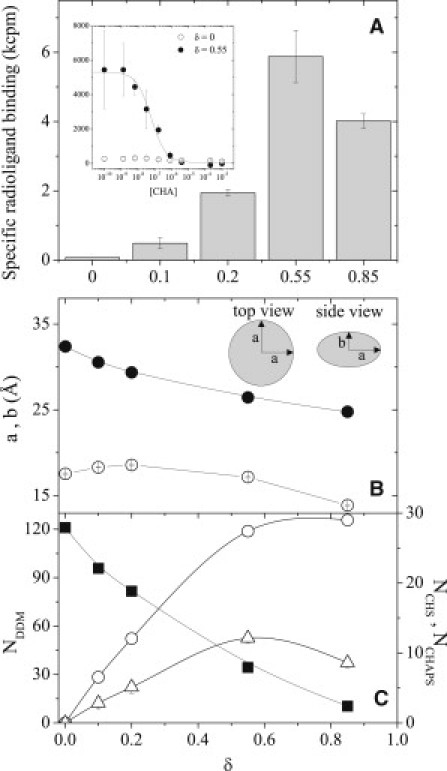
(A) Point competition radioligand binding activity of hA2aR-His10 reconstituted in mixed DDM/CHAPS/CHS micelles versus δ. (Inset) Full competition curves for hA2aR at two values of δ. (B) Major and minor axes a (solid) and b (open) for oblate ellipsoidal micelles (inset) upon addition of CHAPS/CHS. (C) Aggregation numbers for DDM (squares, left axis), CHAPS (circles, right axis), and CHS (triangles, right axis).
In agreement with previous reports, we found that hA2aR was inactive in pure DDM micelles (δ = 0) (8) but displayed detectable activity for all other conditions tested in which CHS was present in PDCs. Surprisingly, DDM-solubilized hA2aR exhibited a nonmonotonic dependence of activity on CHS addition, with a clear maximum at δ = 0.55. This shows that adding an excess amount of sterol to PDCs may actually prove detrimental to protein stability under certain conditions.
To explain this behavior, we performed SANS measurements to quantify the micelle shape and composition in the absence of protein upon addition of CHAPS/CHS. Spectra were fit to a model for uniform oblate ellipsoids (Fig. 1 B) previously used to describe pure DDM micelles (9). This model yields the major and minor elliptical axes, a and b, respectively as a function of CHAPS/CHS composition (Fig. 1 B). The radius of gyration, Rg2 = (2a2 + b2)/5, determined from the model fit is quantitatively consistent with that determined by a model-independent Guinier analysis, confirming that the micelles retain an oblate ellipsoid morphology over the entire range of δ-values. The aggregation number, Ni, of each component i per micelle is calculated assuming uniform distribution of all species within the micelle applied to balance equations for the volume, scattering length density, and absolute scattered intensity at zero angle of individual micelles (Fig. 1 C).
For δ = 0, the determined values of a, b, and NDDM are in quantitative agreement with those reported for pure DDM micelles (9). When the concentration of CHAPS/CHS in solution is increased, a monotonic decrease in the major axis from 32 Å to 25 Å is observed concomitantly with a significant reduction in NDDM. By contrast, a maximum is observed in both the minor axis and the aggregation numbers of CHAPS and CHS with increasing δ, showing that addition of CHAPS/CHS drives changes in curvature of the micelles. Given that the hydrophilic moieties of CHAPS and CHS are small compared with the maltoside headgroup of DDM, these results indicate a decrease in size of the hydrophobic micelle core upon increasing δ. Because the change in minor axis (b) is small compared with that of the major axis (a), we infer that CHS is oriented parallel to the minor axis of the micelle, similar to its orientation in native membranes (5).
Although the exact relationship between the structure of protein-free micelles and PDCs is unknown, in this case a correlation between micellar structure upon CHS addition and hA2aR activity is clearly evident. Specifically, the observed maximum of NCHS = 12 at δ = 0.55 corresponds precisely with the observed maximum in hA2aR ligand binding upon solubilization in DDM/CHAPS/CHS micelles. This demonstrates that a sufficient number of CHS monomers are required within the PDC to ensure conformational stability. To test this correlation quantitatively, we plotted the measured binding activity against NCHS in protein-free micelles. The results show a remarkably strong correlation across the entire range of conditions for which protein activity is observed (Fig. 2). The maximum in NCHS is significantly greater than the estimated number of cholesterol-binding sites based on the hA2aR structure (10,11). Linear extrapolation of the data in Fig. 2 predicts that detectable hA2aR activity occurs only when NCHS exceeds 2, which corresponds to the minimum number of cholesterol-binding sites identified from simulation of two helices of the receptor (11). This suggests that CHS-receptor association is critical for activity. The results presented here demonstrate that addition of CHS influences hA2aR activity both through specific CHS-protein interactions and by altering the PDC structure. It is thought that PDC structure resembles a detergent/sterol belt, with its hydrophobic core equatorial to the transmembrane domains of the protein (12). Our results suggest that the most likely configuration of the amphiphilic belt for DDM/CHAPS/CHS in the presence of hA2aR is an oblate structure, with CHS oriented laterally with respect to receptor helices.
Figure 2.
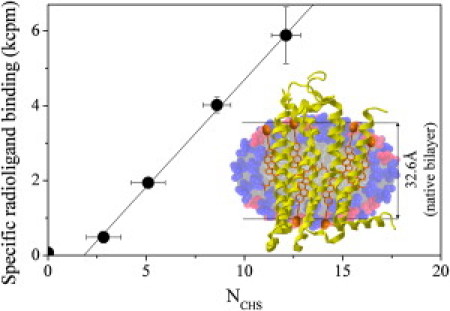
Linear correlation of activity with NCHS in DDM/CHAPS/CHS micelles. (Inset) Cross-section of a micelle with δ = 0.55, showing the number and hypothesized orientation of DDM (blue), CHAPS (red), and CHS (orange). The structure for hA2aR is superimposed (yellow), reproduced to scale.
Within the framework of this hypothesis, in Fig. 2 we compare the crystal structure of a truncated form of hA2aR (10) with the structure of protein-free DDM/CHAPS/CHS micelles determined by SANS for δ = 0.55 (NDDM = 34, NCHAPS = 27, NCHS = 12), where the number and hypothesized orientation of CHS within the hydrophobic interior are in accordance with the protein's width. We note that the structure of micelles that best promote hA2aR activity exhibits several similarities to the native plasma membrane. First, the minor axis of the micelles is nearly identical to the hydrophobic thickness of the lipid bilayer (Fig. 2) in which hA2aR normally resides (10). Furthermore, the mole fraction of CHS is ∼20%, which is close to that usually found for cholesterol within membranes (13), suggesting that the structural rigidity of the PDC also plays a role in maintaining protein conformational stability. Although our observations are empirical, they provide a rational explanation for specific conditions under which in vitro activity is optimized.
In summary, we observe a quantitative correlation between hA2aR activity and DDM/CHAPS/CHS micelle morphology and composition. Specifically, the optimum in conformational stability corresponds to micelles that contain a maximum number of CHS monomers and whose dimensions most closely mimic the mammalian membrane. Although this result is intuitive, the optimal conditions are nonobvious, as the maximum aggregation number of CHS does not trivially correspond to the maximum amount of CHS in solution. These results can serve as detailed guidelines for the rational engineering of mixed micelles for structural characterization of membrane proteins.
Acknowledgments
This work was supported by the National Institutes of Health (RR15588), the Delaware Center for Neutron Science, and fellowships from the Integrative Graduate Education and Research Traineeship Program of the National Science Foundation, and the NASA Harriett G. Jenkins Predoctoral Fellowship Program to M.A.O. The manuscript was prepared under cooperative agreement 70NANB7H6178, National Institute of Standards and Technology, U.S. Department of Commerce.
Supporting Material
References and Footnotes
- 1.Sanders C.R., Kuhn Hoffmann A., Ellis C.D. French swimwear for membrane proteins. ChemBioChem. 2004;5:423–426. doi: 10.1002/cbic.200300830. [DOI] [PubMed] [Google Scholar]
- 2.Seddon A.M., Curnow P., Booth P.J. Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Chiu M.L., Tsang C., MacWilliams M.P. Over-expression, solubilization, and purification of GPCRs for structural biology. Comb. Chem. High Throughput Screen. 2008;11:439–462. doi: 10.2174/138620708784911456. [DOI] [PubMed] [Google Scholar]
- 4.Garavito R.M., Ferguson-Miller S. Detergents as tools in membrane biochemistry. J. Biol. Chem. 2001;276:32403–32406. doi: 10.1074/jbc.R100031200. [DOI] [PubMed] [Google Scholar]
- 5.Pucadyil T.J., Chattopadhyay A. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog. Lipid Res. 2006;45:295–333. doi: 10.1016/j.plipres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Hanson M.A., Cherezov V., Stevens R.C. A specific cholesterol binding site is established by the 2.8 A structure of the human β2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss H.M., Grisshammer R. Purification and characterization of the human adenosine A(2a) receptor functionally expressed in Escherichia coli. Eur. J. Biochem. 2002;269:82–92. doi: 10.1046/j.0014-2956.2002.02618.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Malley M.A., Lazarova T., Robinson A.S. High-level expression in Saccharomyces cerevisiae enables isolation and spectroscopic characterization of functional human adenosine A(2)a receptor. J. Struct. Biol. 2007;159:166–178. doi: 10.1016/j.jsb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmins P.A., Leonhard M., Welte W. A physical characterization of some detergents of potential use for membrane protein crystallization. FEBS Lett. 1988;238:361–368. [Google Scholar]
- 10.Jaakola V.P., Griffith M.T., Stevens R.C. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyman E., Higgs C., Voth G.A. A role for a specific cholesterol interaction in stabilizing the Apo configuration of the human A(2A) adenosine receptor. Structure. 2009;17:1660–1668. doi: 10.1016/j.str.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.le Maire M., Champeil P., Moller J.V. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta. 2000;1508:86–111. doi: 10.1016/s0304-4157(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 13.Mouritsen O.G., Zuckermann M.J. What's so special about cholesterol? Lipids. 2004;39:1101–1113. doi: 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


