Abstract
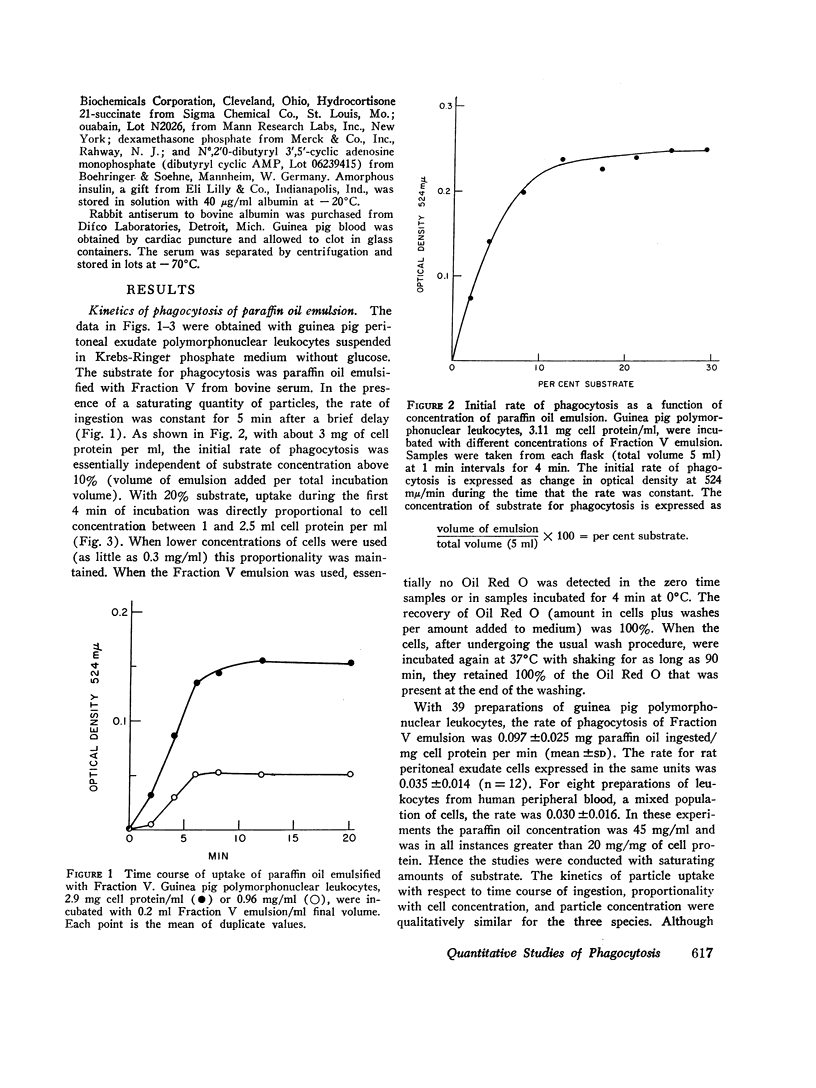
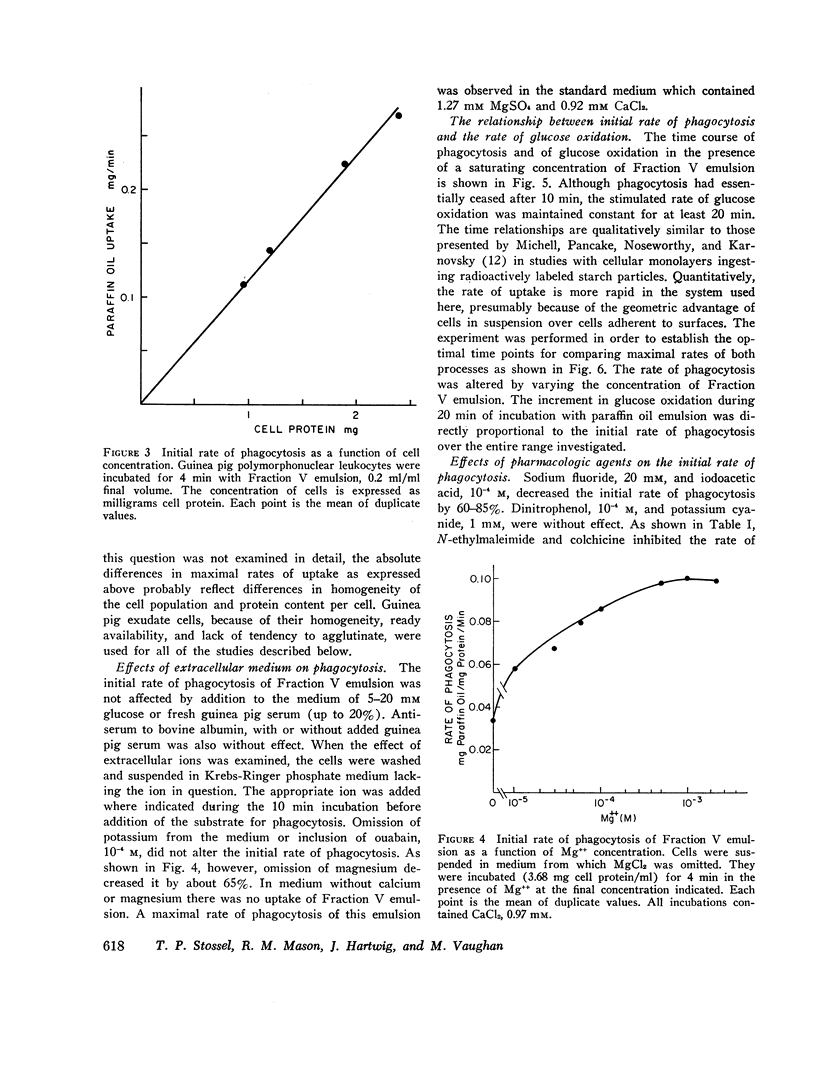
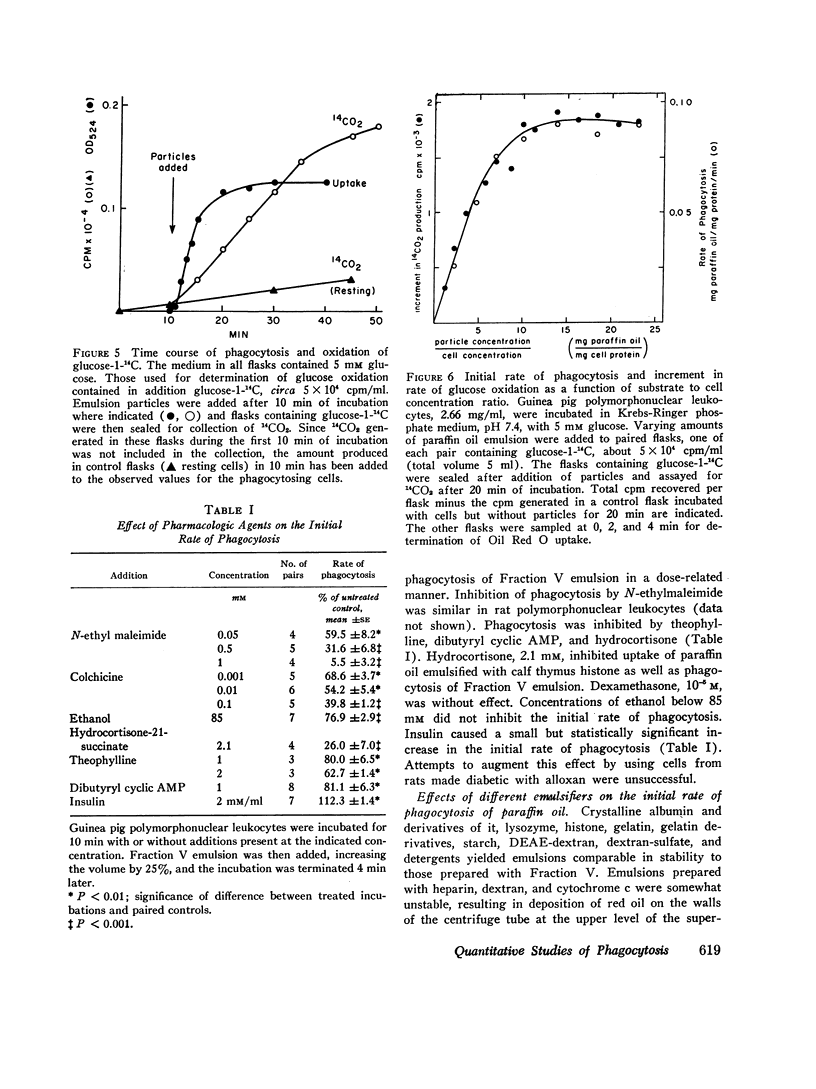
Polymorphonuclear leukocytes suspended in Krebs-Ringer phosphate medium ingest paraffin oil containing Oil Red O emulsified with a variety of substances. Spectrophotometric determination of Oil Red O in the cells after uningested particles have been removed by differential centrifugation provides a quantitative measure of phagocytosis. This system has been used to investigate the effects of several drugs and hormones on the initial rate of phagocytosis and to approach the question of how the surface of a particle influences its acceptability as a substrate for phagocytosis. The rate of uptake of paraffin oil emulsified with bovine albumin was constant for 6 min and was proportional to cell concentration when saturating concentrations of paraffin oil emulsion were used. At lower concentrations of substrate, the initial rate of phagocytosis was directly proportional to paraffin oil concentration. The increment in glucose oxidation associated with phagocytosis varied directly with the initial rate of particle uptake. The rate of ingestion of the albumin emulsion was not altered by serum (2-20%, v/v), glucose (5-20 mM), or omission of potassium from the medium. The rate of phagocytosis was decreased 65% if magnesium was omitted, and was essentially zero in the absence of divalent cations. The initial rate of uptake was inhibited by inhibitors of glycolysis, by N-ethylmaleimide (0.05-1 mM), colchicine (0.001-0.1 mM), theophylline (1 and 2 mM), dibutyryl cyclic AMP (1 mM), hydrocortisone (2.1 mM), and ethanol (85 mM). Inhibitors of oxidative phosphorylation and dexamethasone (0.01 mM) were without effect, while insulin (2 mU/ml) slightly stimulated the phagocytic rate. Paraffin oil emulsified with different agents was used to approach the question of how the surface of a particle influences its acceptability as a substrate for phagocytosis. Emulsions prepared with nonionic detergents, methylated proteins, and proteins with a weak net charge at pH 7.4 were poorly ingested. On the other hand emulsions prepared with agents of strong net positive or negative charge were rapidly taken up. The effect of divalent cations on the rate of phagocytosis varied with the nature of the emulsifier, but was not related in any simple, direct fashion to the net surface charge of the particles. However, it has not been conclusively established that charge was the only variable of the emulsion particles employed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER R. R., STAHMANN M. A. Polypeptide formation by reaction of N-carboxy amino acid anhydrides in buffered aqueous solutions. J Biol Chem. 1953 Oct;204(2):737–744. [PubMed] [Google Scholar]
- Baier R. E., Shafrin E. G., Zisman W. A. Adhesion: mechanisms that assist or impede it. Science. 1968 Dec 20;162(3860):1360–1368. doi: 10.1126/science.162.3860.1360. [DOI] [PubMed] [Google Scholar]
- Bollinger J. N. Metabolic fate of mineral oil adjuvants using 14C-labeled tracers. I. Mineral oil. J Pharm Sci. 1970 Aug;59(8):1084–1088. doi: 10.1002/jps.2600590804. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton R. G., Stokes P. E., Schwartz M. S., Louria D. B. Effect of alcohol and various diseases on leukocyte mobilization, phagocytosis and intracellular bacterial killing. N Engl J Med. 1970 Jan 15;282(3):123–128. doi: 10.1056/NEJM197001152820303. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Goldfinger S. E., Howell R. R., Seegmiller J. E. Suppression of metabolic accompaniments of phagocytosis by colchicine. Arthritis Rheum. 1965 Dec;8(6):1112–1122. doi: 10.1002/art.1780080610. [DOI] [PubMed] [Google Scholar]
- Hénon M., Delaunay A. Action exercée, in vitro, par les ions Ca et Mg sur la sensibilité chimiotactique et le pouvoir phagocytaire de leucocytes polynucléaires. Ann Inst Pasteur (Paris) 1966 Nov;111(5 Suppl):85–93. [PubMed] [Google Scholar]
- Leirer R., Meyer H., Steinbach G. Einfluss der Magnesiumkonzentration des Blutserums auf die Phagozytoseaktivität der Blutphaagozyten. Arch Exp Veterinarmed. 1969 Nov;23(5):1021–1025. [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Rubin W., Hook E. W. The effect of an NADH oxidase inhibitor (hydrocortisone) on polymorphonuclear leukocyte bactericidal activity. J Clin Invest. 1970 Jul;49(7):1381–1388. doi: 10.1172/JCI106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Pancake S. J., Noseworthy J., Karnovsky M. L. Measurement of rates of phagocytosis: the use of cellular monolayers. J Cell Biol. 1969 Jan;40(1):216–224. doi: 10.1083/jcb.40.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickenberg I. D., Root R. K., Wolff S. M. Leukocytic function in hypogammaglobulinemia. J Clin Invest. 1970 Aug;49(8):1528–1538. doi: 10.1172/JCI106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. PARTICLE UPTAKE BY POLYMORPHONUCLEAR LEUCOCYTES AND EHRLICH ASCITES-CARCINOMA CELLS. Biochem J. 1963 Oct;89:150–156. doi: 10.1042/bj0890150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SELA M., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 1. Antigenicity of polypeptidyl gelatins. Biochem J. 1960 Apr;75:91–102. doi: 10.1042/bj0750091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer H. L., Sachs G., Clark L. C., Jr Fluorocarbon effects on tissue metabolism. Fed Proc. 1970 Sep-Oct;29(5):1746–1750. [PubMed] [Google Scholar]
- Stossel T. P., Murad F., Mason R. J., Vaughan M. Regulation of glycogen metabolism in polymorphonuclear leukocytes. J Biol Chem. 1970 Nov 25;245(22):6228–6234. [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F. Phagocytosis of E. coli by enzyme-treated alveolar macrophages. Am J Physiol. 1971 Apr;220(4):958–966. doi: 10.1152/ajplegacy.1971.220.4.958. [DOI] [PubMed] [Google Scholar]
- WILKINS D. J., BANGHAM A. D. THE EFFECT OF SOME METAL IONS ON IN VITRO PHAGOCYTOSIS. J Reticuloendothel Soc. 1964 Jul;1:233–242. [PubMed] [Google Scholar]
- Weisman R. A., Korn E. D. Phagocytosis of latex beads by Acanthamoeba. I. Biochemical properties. Biochemistry. 1967 Feb;6(2):485–497. doi: 10.1021/bi00854a017. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]



