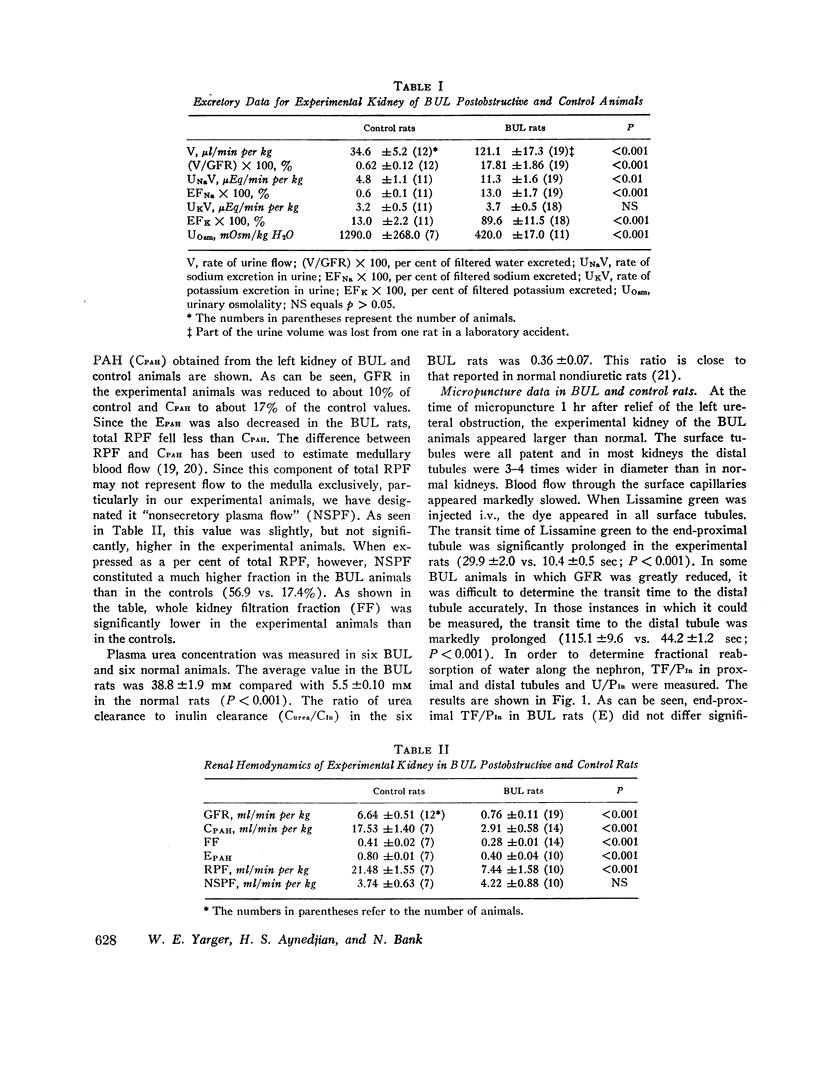
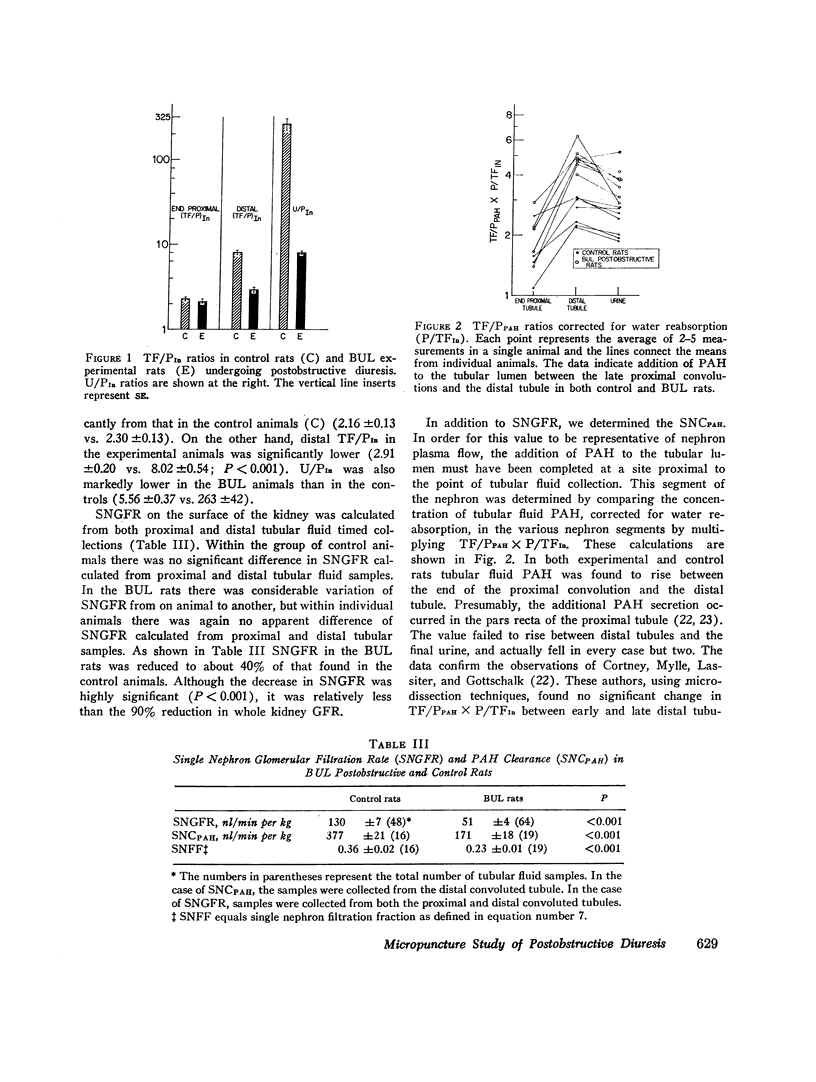
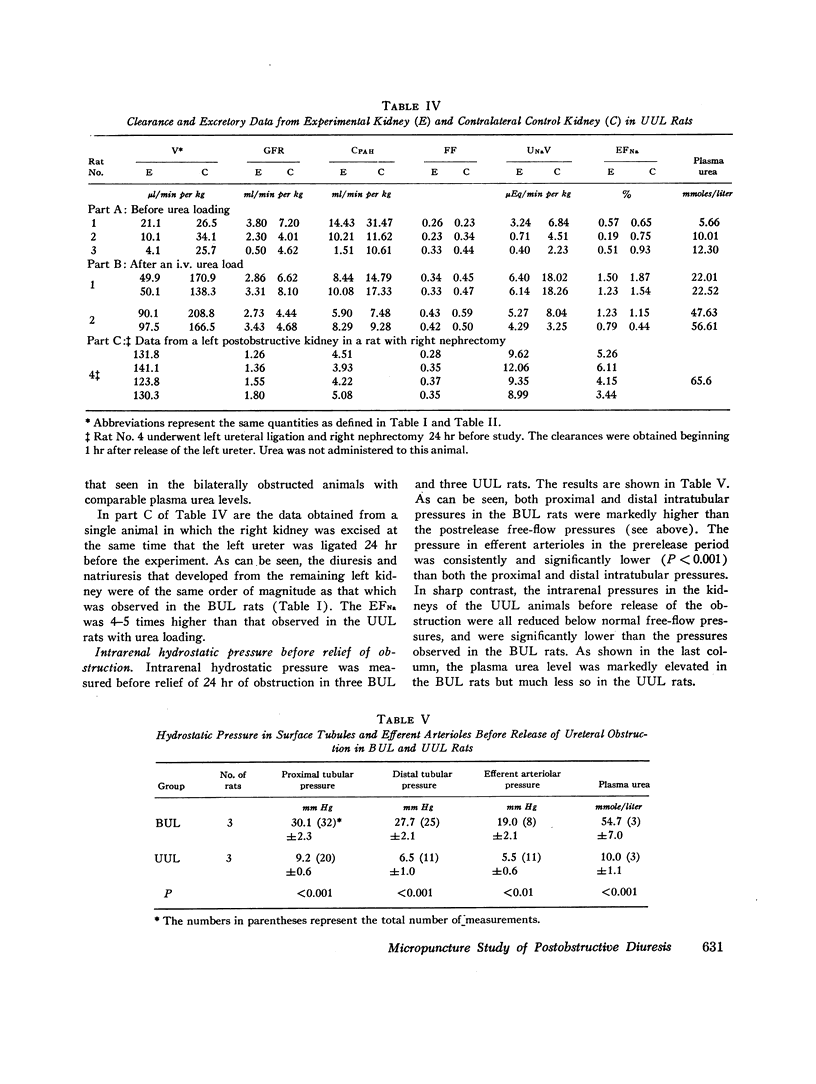
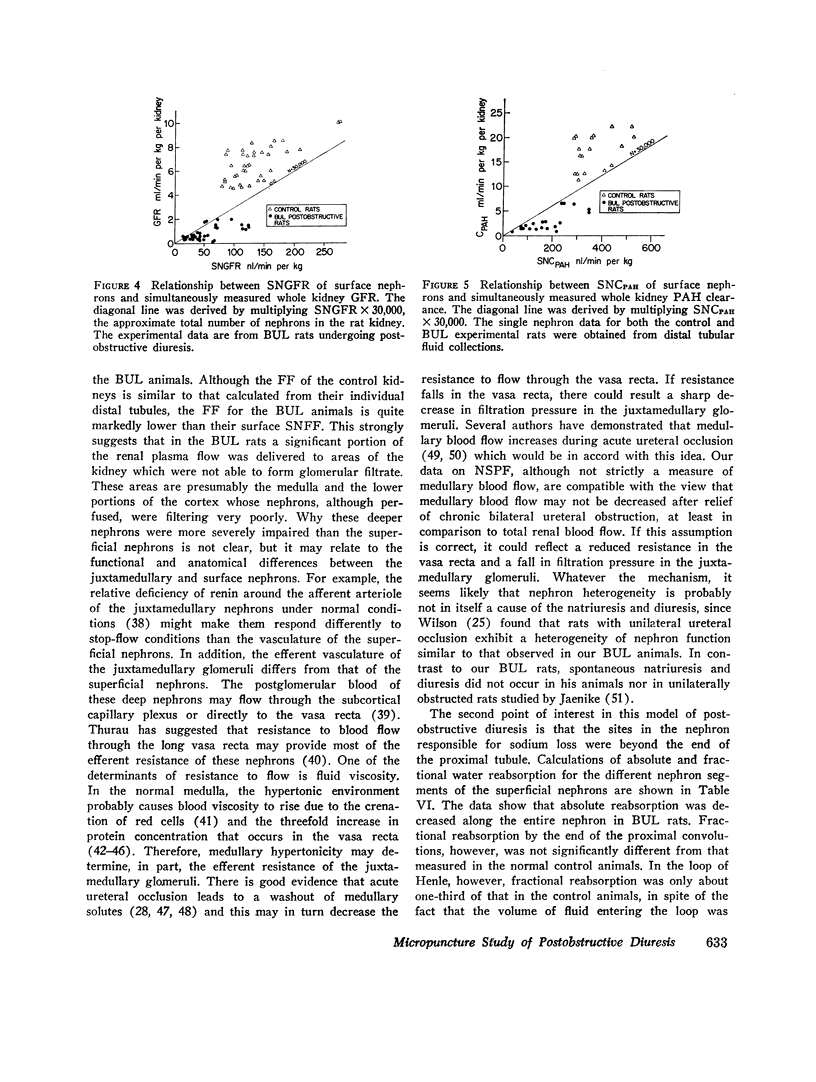
Abstract
In order to investigate the syndrome of postobstructive diuresis, clearance and micropuncture studies were carried out in rats after relief of 24 hr of bilateral (BUL) or unilateral (UUL) ureteral ligation. In rats with BUL, a striking diuresis and natriuresis occurred when the obstruction to one kidney (the experimental kidney) was relieved. The results were not influenced by administration of vasopressin or d-aldosterone. Whole kidney clearances of inulin and p-aminohippuric acid (PAH) in the experimental kidney were reduced to 10% and 20% of normal, respectively. Superficial nephron inulin and PAH clearances were also reduced, but only to 40% and 45%, respectively. These findings suggest a heterogeneity of nephron function in which deep nephrons were functioning poorly or not at all. To investigate the site of impaired tubular reabsorption in the surface nephrons, absolute and fractional water reabsorption was measured. Absolute reabsorption was found to be decreased all along the nephron. Fractional reabsorption in proximal tubules was normal, as indicated by an average endproximal tubular fluid per plasma inulin (TF/PIn) of 2.16 vs. 2.30 in controls. TF/PIn was markedly decreased in distal tubules (2.91 vs. 8.02) and final urine (5.56 vs. 263). These observations indicate that the major sites of impaired sodium reabsorption leading to the diuresis were beyond the proximal tubule.
Rats with 24 hr of UUL did not demonstrate a comparable natriuresis or diuresis either spontaneously when the obstruction was relieved or after i.v. infusion of urea. A major difference between the BUL and UUL rats was that prerelease intrarenal hydrostatic pressure was markedly elevated (30.1 mm Hg) in the former but was below normal free-flow values (9.2 mm Hg) in the latter. Thus, elevation of intrarenal pressure during the period of obstruction may be causally related to the natriuresis and diuresis which occurs after the obstruction is relieved.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. C., Lee J. B. Effect of osmolality on Na plus-K plus-ATPase in outer renal medulla. Am J Physiol. 1970 Dec;219(6):1742–1745. doi: 10.1152/ajplegacy.1970.219.6.1742. [DOI] [PubMed] [Google Scholar]
- BANK N., AYNEDJIAN H. S. ON THE MECHANISM OF HYPOSTHENURIA IN HYPERCALCEMIA. J Clin Invest. 1965 Apr;44:681–693. doi: 10.1172/JCI105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANK N. Relationship between electrical and hydrogen ion gradients across rat proximal tubule. Am J Physiol. 1962 Sep;203:577–582. doi: 10.1152/ajplegacy.1962.203.3.577. [DOI] [PubMed] [Google Scholar]
- BERLYNE G. M. Distal tubular function in chronic hydronephrosis. Q J Med. 1961 Oct;30:339–355. [PubMed] [Google Scholar]
- BRICKER N. S., SHWAYRI E. I., REARDAN J. B., KELLOG D., MERRILL J. P., HOLMES J. H. An abnormality in renal function resulting from urinary tract obstruction. Am J Med. 1957 Oct;23(4):554–564. doi: 10.1016/0002-9343(57)90226-7. [DOI] [PubMed] [Google Scholar]
- Bercovitch D. D., Kasen L., Blann L., Levitt M. F. The postobstructive kidney. Observations on nephron function after the relief of 24 hr of ureteral ligation in the dog. J Clin Invest. 1971 May;50(5):1154–1165. doi: 10.1172/JCI106588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner R. W., Bennett C. M. Concentration of urine in the mammalian kidney. Am J Med. 1967 May;42(5):777–789. doi: 10.1016/0002-9343(67)90095-2. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L. Postglomerular vascular protein concentration: evidence for a causal role in governing fluid reabsorption and glomerulotublar balance by the renal proximal tubule. J Clin Invest. 1971 Feb;50(2):336–349. doi: 10.1172/JCI106501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Carone F. A., Everett B. A., Blondeel N. J., Stolarczyk J. Renal localization of albumin and its function in the concentrating mechanism. Am J Physiol. 1967 Feb;212(2):387–393. doi: 10.1152/ajplegacy.1967.212.2.387. [DOI] [PubMed] [Google Scholar]
- Cooke C. R., Brown T. C., Zacherle B. J., Walker W. G. The effect of altered sodium concentration in the distal nephron segments on renin release. J Clin Invest. 1970 Sep;49(9):1630–1638. doi: 10.1172/JCI106380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortney M. A., Mylle M., Lassiter W. E., Gottschalk C. W. Renal tubular transport of water, solute, and PAH in rats loaded with isotonic saline. Am J Physiol. 1965 Dec;209(6):1199–1205. doi: 10.1152/ajplegacy.1965.209.6.1199. [DOI] [PubMed] [Google Scholar]
- EARLEY L. E. Extreme polyuria in obstructive uropathy; report of a case of water-losing nephritis in an infant, with a discussion of polyuria. N Engl J Med. 1956 Sep 27;255(13):600–605. doi: 10.1056/NEJM195609272551305. [DOI] [PubMed] [Google Scholar]
- Eknoyan G., Suki W. N., Martinez-Maldonado M., Anhalt M. A. Chronic hydronephrosis: observations on the mechanism of the defect in urine concentration. Proc Soc Exp Biol Med. 1970 Jul;134(3):634–639. doi: 10.3181/00379727-134-34850. [DOI] [PubMed] [Google Scholar]
- GIEBISCH G., WINDHAGER E. E. RENAL TUBULAR TRANSFER OF SODIUM, CHLORIDE AND POTASSIUM. Am J Med. 1964 May;36:643–669. doi: 10.1016/0002-9343(64)90178-0. [DOI] [PubMed] [Google Scholar]
- GLABMAN S., AYNEDJIAN H. S., BANK N. MICROPUNCTURE STUDY OF THE EFFECT OF ACUTE REDUCTIONS IN GLOMERULAR FILTRATION RATE ON SODIUM AND WATER REABSORPTION BY THE PROXIMAL TUBULES OF THE RAT. J Clin Invest. 1965 Aug;44:1410–1416. doi: 10.1172/JCI105246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., MYLLE M. Micropuncture study of pressures in proximal and distal tubules and peritubular capillaries of the rat kidney during osmotic diuresis. Am J Physiol. 1957 May;189(2):323–328. doi: 10.1152/ajplegacy.1957.189.2.323. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., MYLLE M. Micropuncture study of pressures in proximal tubules and peritubular capillaries of the rat kidney and their relation to ureteral and renal venous pressures. Am J Physiol. 1956 May;185(2):430–439. doi: 10.1152/ajplegacy.1956.185.2.430. [DOI] [PubMed] [Google Scholar]
- Hendler E. D., Torretti J., Epstein F. H. The distribution of sodium-potassium--activated adenosine triphosphatase in medulla and cortex of the kidney. J Clin Invest. 1971 Jun;50(6):1329–1337. doi: 10.1172/JCI106612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horster M., Thurau K. Micropuncture studies on the filtration rate of single superficial and juxtamedullary glomeruli in the rat kidney. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):162–181. doi: 10.1007/BF00362733. [DOI] [PubMed] [Google Scholar]
- JAENIKE J. R., BRAY G. A. Effects of acute transitory urinary obstruction in the dog. Am J Physiol. 1960 Dec;199:1219–1222. doi: 10.1152/ajplegacy.1960.199.6.1219. [DOI] [PubMed] [Google Scholar]
- Jaenike J. R. The renal response to ureteral obstruction: a model for the study of factors which influence glomerular filtration pressure. J Lab Clin Med. 1970 Sep;76(3):373–382. [PubMed] [Google Scholar]
- Jamison R. L. Micropuncture study of superficial and juxtamedullary nephrons in the rat. Am J Physiol. 1970 Jan;218(1):46–55. doi: 10.1152/ajplegacy.1970.218.1.46. [DOI] [PubMed] [Google Scholar]
- Johnston H. H., Herzog J. P., Lauler D. P. Effect of prostaglandin E1 on renal hemodynamics, sodium and water excretion. Am J Physiol. 1967 Oct;213(4):939–946. doi: 10.1152/ajplegacy.1967.213.4.939. [DOI] [PubMed] [Google Scholar]
- KESSLER R. H. Acute effects of brief ureteral stasis on urinary and renal papillary chloride concentration. Am J Physiol. 1960 Dec;199:1215–1218. doi: 10.1152/ajplegacy.1960.199.6.1215. [DOI] [PubMed] [Google Scholar]
- Kauker M. L., Lassiter W. E., Gottschalk C. W. Micropuncture study of effects of urea infusion in tubular reabsorption in the rat. Am J Physiol. 1970 Jul;219(1):45–50. doi: 10.1152/ajplegacy.1970.219.1.45. [DOI] [PubMed] [Google Scholar]
- Koike T. I., Kellogg R. H. Effect of urea loading on urine osmolality during osmotic diuresis in hydropenic rats. Am J Physiol. 1963 Nov;205(5):1053–1057. doi: 10.1152/ajplegacy.1963.205.5.1053. [DOI] [PubMed] [Google Scholar]
- Kriz W. Der architektonische und funktionelle Aufbau der Rattenniere. Z Zellforsch Mikrosk Anat. 1967;82(4):495–535. [PubMed] [Google Scholar]
- LASSEN N. A., LONGLEY J. B. Countercurrent exchange in vessels of renal medulla. Proc Soc Exp Biol Med. 1961 Apr;106:743–748. doi: 10.3181/00379727-106-26462. [DOI] [PubMed] [Google Scholar]
- LASSITER W. E., MYLLE M., GOTTSCHALK C. W. NET TRANSTUBULAR MOVEMENT OF WATER AND UREA IN SALINE DIURESIS. Am J Physiol. 1964 Apr;206:669–673. doi: 10.1152/ajplegacy.1964.206.4.669. [DOI] [PubMed] [Google Scholar]
- LEVINSKY N. G., DAVIDSON D. G., BERLINER R. W. Effects of reduced glomerular filtration on urine concentration in the presence of antidiuretic hormone. J Clin Invest. 1959 May;38(5):730–740. doi: 10.1172/JCI103853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowitz H. D., Stumpe K. O., Ochwadt B. Micropuncture study of the action of angiotensin-II on tubular sodium and water reabsorption in the rat. Nephron. 1969;6(3):173–187. doi: 10.1159/000179727. [DOI] [PubMed] [Google Scholar]
- MOFFAT D. B., FOURMAN J. THE VASCULAR PATTERN OF THE RAT KIDNEY. J Anat. 1963 Oct;97:543–553. [PMC free article] [PubMed] [Google Scholar]
- McGiff J. C., Crowshaw K., Terragno N. A., Lonigro A. J. Release of a prostaglandin-like substance into renal venous blood in response to angiotensin II. Circ Res. 1970 Jul;27(1 Suppl 1):121–130. [PubMed] [Google Scholar]
- Navar L. G., Baer P. G. Renal autoregulatory and glomerular filtration responses to gradated ureteral obstruction. Nephron. 1970 Jul;7(4):301–316. doi: 10.1159/000179831. [DOI] [PubMed] [Google Scholar]
- PILKINGTON L. A., BINDER R., DEHAAS J. C., PITTS R. F. INTRARENAL DISTRIBUTION OF BLOOD FLOW. Am J Physiol. 1965 Jun;208:1107–1113. doi: 10.1152/ajplegacy.1965.208.6.1107. [DOI] [PubMed] [Google Scholar]
- REUBI F. Objections à la théorie de la séparation intrarénale des hématies et du plasma (Pappenheimer). Helv Med Acta. 1958 Oct;25(4):516–523. [PubMed] [Google Scholar]
- ROUSSAK N. J., OLEESKY S. Waterlosing nephritis, a syndrome simulating diabetes insipidus. Q J Med. 1954 Apr;23(90):147–164. [PubMed] [Google Scholar]
- Roch-Ramel F., Diézi J., Chométy F., Michoud P., Peters G. Disposal of large urea overloads by the rat kidney: a micropuncture study. Am J Physiol. 1970 Jun;218(6):1524–1532. doi: 10.1152/ajplegacy.1970.218.6.1524. [DOI] [PubMed] [Google Scholar]
- SELKURT E. E. EFFECT OF URETERAL BLOCKADE ON RENAL BLOOD FLOW AND URINARY CONCENTRATING ABILITY. Am J Physiol. 1963 Aug;205:286–292. doi: 10.1152/ajplegacy.1963.205.2.286. [DOI] [PubMed] [Google Scholar]
- Seldin D. W., Eknoyan G., Suki W. N., Rector F. C., Jr Localization of diuretic action from the pattern of water and electrolyte excretion. Ann N Y Acad Sci. 1966 Nov 22;139(2):328–343. doi: 10.1111/j.1749-6632.1966.tb41207.x. [DOI] [PubMed] [Google Scholar]
- Stumpe K. O., Lowitz H. D., Ochwadt B. Function of juxtamedullary nephrons in normotensive and chronically hypertensive rats. Pflugers Arch. 1969;313(1):43–52. doi: 10.1007/BF00586327. [DOI] [PubMed] [Google Scholar]
- Suki W. N., Guthrie A. G., Martinez-Maldonado M., Eknoyan G. Effects of ureteral pressure elevation on renal hemodynamics and urine concentration. Am J Physiol. 1971 Jan;220(1):38–43. doi: 10.1152/ajplegacy.1971.220.1.38. [DOI] [PubMed] [Google Scholar]
- Suki W., Eknoyan G., Rector F. C., Jr, Seldin D. W. Patterns of nephron perfusion in acute and chronic hydronephrosis. J Clin Invest. 1966 Jan;45(1):122–131. doi: 10.1172/JCI105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THURAU K. RENAL HEMODYNAMICS. Am J Med. 1964 May;36:698–719. doi: 10.1016/0002-9343(64)90181-0. [DOI] [PubMed] [Google Scholar]
- Tune B. M., Burg M. B., Patlak C. S. Characteristics of p-aminohippurate transport in proximal renal tubules. Am J Physiol. 1969 Oct;217(4):1057–1063. doi: 10.1152/ajplegacy.1969.217.4.1057. [DOI] [PubMed] [Google Scholar]
- WINBERG J. Renal function in water-losing syndrome due to lower urinary tract obstruction before and after treatment. Acta Paediatr. 1959 Mar;48(2):149–163. doi: 10.1111/j.1651-2227.1959.tb16030.x. [DOI] [PubMed] [Google Scholar]
- WINDHAGER E. E., GIEBISCH G. Micropuncture study of renal tubular transfer of sodium chloride in the rat. Am J Physiol. 1961 Mar;200:581–590. doi: 10.1152/ajplegacy.1961.200.3.581. [DOI] [PubMed] [Google Scholar]
- WITTE M. H., SHORT F. A., HOLLANDER W., Jr MASSIVE POLYURIA AND NATRURESIS FOLLOWING RELIEF OF URINARY TRACT OBSTRUCTION. Am J Med. 1964 Aug;37:320–326. doi: 10.1016/0002-9343(64)90015-4. [DOI] [PubMed] [Google Scholar]
- Wells R. Syndromes of hyperviscosity. N Engl J Med. 1970 Jul 23;283(4):183–186. doi: 10.1056/NEJM197007232830406. [DOI] [PubMed] [Google Scholar]
- Wilde W. S., Vorburger C. Albumin multiplier in kidney vasa recta analyzed by microspectrophotometry of T-1824. Am J Physiol. 1967 Nov;213(5):1233–1243. doi: 10.1152/ajplegacy.1967.213.5.1233. [DOI] [PubMed] [Google Scholar]


