Abstract
Knock-in mice were generated that harbored a leucine-to-serine mutation in the α4 nicotinic receptor near the gate in the channel pore. Mice with intact expression of this hypersensitive receptor display dominant neonatal lethality. These mice have a severe deficit of dopaminergic neurons in the substantia nigra, possibly because the hypersensitive receptors are continuously activated by normal extracellular choline concentrations. A strain that retains the neo selection cassette in an intron has reduced expression of the hypersensitive receptor and is viable and fertile. The viable mice display increased anxiety, poor motor learning, excessive ambulation that is eliminated by very low levels of nicotine, and a reduction of nigrostriatal dopaminergic function upon aging. These knock-in mice provide useful insights into the pathophysiology of sustained nicotinic receptor activation and may provide a model for Parkinson's disease.
The mechanism leading from nicotine intake to addiction is not known in detail, but it begins with the activation of neuronal nicotinic acetylcholine receptors (nAChRs). Nicotine elicits dopamine release in several regions of the brain, presumably leading to the reward, motor, and addictive effects (1, 2). The highest-affinity and most abundant nicotine binding in the brain corresponds to the α4β2 nAChR (3). The α4 subunit is the principal partner for the β2 subunit in brain; β2-containing receptors play an important role in nicotine self-administration, in nicotine-stimulated electrophysiological responses in midbrain neurons, and in nicotine-stimulated dopamine release in the ventral striatum (4, 5). The α4 subunit and tyrosine hydroxylase are colocalized in dopaminergic neurons (6). Epidemiological studies show that smokers have a lower incidence of Parkinson's disease (7, 8), suggesting a protective effect of nicotine via modulation of the dopaminergic system.
Both α4 and β2 knockout mice show only subtle alterations in their physiology or behavior until they reach old age (4, 9, 10). We have used a complementary strategy to understand the roles of α4-containing nAChRs. We reasoned that gain of function mutations might generate more noticeable phenotypes, and that these phenotypes would gain relevance from the fact that nicotine and some candidate analgesics (11) are agonists. We have generated lines of knock-in mice by introducing a point mutation into the M2 transmembrane region of the α4 subunit to produce a hypersensitive receptor.
Materials and Methods
Xenopus Oocyte Injections and Electrophysiology.
The α4 and β2 subunits were subcloned into pAMV-PA (12). Capped mRNA transcripts were prepared, and α4/β2 (2 ng, 1:1) or α4L9′S/β2 (0.2–0.5 ng, 1:1) were microinjected into Xenopus oocytes (12). Twenty-four to seventy-two hours later, two-electrode voltage clamp recordings (12) were made in solutions containing zero Ca2+.
Knock-in Mouse Construction.
A 129/SvJ α4 genomic clone containing exon 5 and the L9′S mutation was inserted into pKO Scrambler V907 (Lexicon-Genetics, The Woodlands, TX). A neomycin resistance cassette, with a phosphoglycerate kinase promoter and polyadenylation signal and flanked by loxP sites, was inserted 163 bp downstream from exon 5 for positive selection. The diphtheria toxin A chain gene with the RNA polymerase II promoter was inserted to provide negative selection for random insertion. Embryonic stem (ES) cells were electroporated with the linearized construct and screened by Southern blot; the wild-type (WT) gene contains a 9.7-kb, BamHI–BamHI fragment, and the mutant gene contains a 7.7-kb, BamHI–EcoRI fragment (Fig. 1D). The loxP-flanked neomycin resistance cassette was deleted in some ES cells by transfection with a cytomegalovirus-Cre plasmid; this deletion leaves only the 34 bp of one loxP site in the intron. Two lines of mice were generated by injection of mutated ES cells into C57BL/6 blastocysts, one with the neo cassette still present (neo intact) and another with the neo cassette deleted (neo deleted). The presence of the mutation was confirmed by sequence analysis of PCR-amplified gene segments.
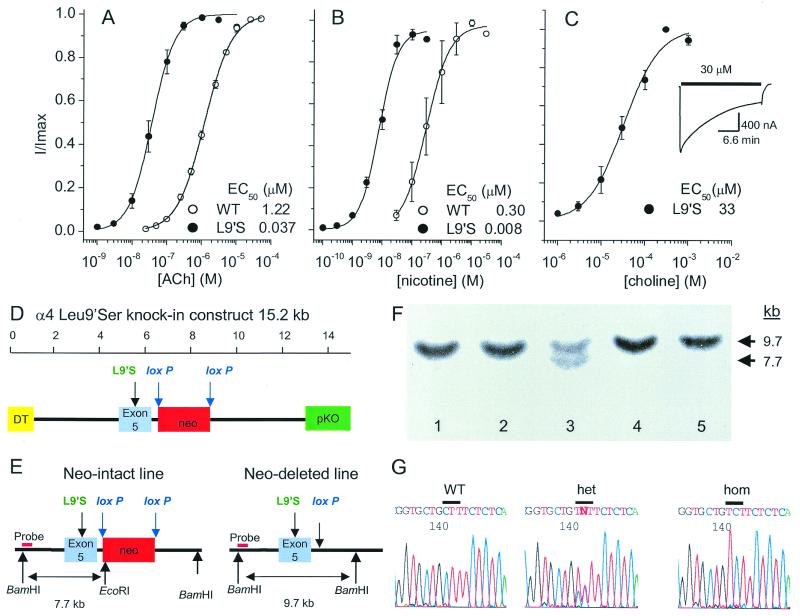
Figure 1.
Physiological design, recombinant construction, and genomic characterization of the α4 knockin mouse strains. Agonist concentration-response relations of WT and mutated (α4L9′S) rat α4β2 receptors expressed in oocytes (five oocytes for each curve). (A) Acetylcholine. (B) Nicotine. (C) Choline. The choline responses of the WT receptor were not studied systematically, because there is no response at choline concentrations up to 1 mM, and higher concentrations of choline block the channel. (Insert) Time course of the response to 30 μM choline, showing partial desensitization. (D) Targeting construct containing exon 5 with the Leu9′Ser mutation, the neomycin resistance gene (neo) flanked by loxP sites, the diphtheria toxin A chain gene (DT), and the pKO V907 vector (pKO). (E) Deletion of the neo cassette by transfecting the neo-intact ES cells with a cytomegalovirus-Cre plasmid generates neo-deleted ES cell lines. (F) Southern blot analysis of genomic DNA from five embryonic stem-cell clones following digestion with BamHI and EcoRI restriction endonucleases and hybridization with a flanking genomic fragment as a probe, indicated in E. Lines analyzed in lanes 1, 2, 4, and 5 contain DNA from two WT genes. Lane 3 shows a line with one WT gene (9.7 kb, BamHI–BamHI as indicated in E) and one mutant gene (7.7 kb, BamHI–EcoRI in E). (G) Sequence analysis of DNA extracted from WT, heterozygous (het), and homozygous (hom) neo-intact mice. The WT sequence at nucleotide position 142, corresponding to the codon at position 9′ in the M2 region, is CTT, encoding leucine; the mutant sequence is TCT, encoding serine.
Histology and Immunocytochemistry.
Embryos were surgically removed from pregnant females. Tails were removed for genotyping. Embryos were fixed by cardiac perfusion (0.1 M PBS and 4% formaldehyde), postfixed for 2 h, dehydrated in 20% sucrose, and cryosectioned (10 μm) in frontal orientation. Staining was performed with antisera for tyrosine hydroxylase (TH) (1:500) (Chemicon) and α4 nAChR (1:500) (Santa Cruz Biotechnology) and fluorescent secondary antibodies (1:500, Alexa 594, Alexa 488; Molecular Probes), respectively. For cell counts, sections were stained with TH-antisera (Pel-Freez; 1:250) for indirect immunohistochemistry according to the Vectastain ABC Elite kit protocol (Vector Laboratories). Diaminobenzidine was used as the chromogen, with nickel enhancement.
Midbrain Progenitor Cells.
Midbrain progenitor cells were harvested, expanded, and differentiated as described (13), but epidermal growth factor (20 ng/ml) and basic fibroblast growth factor (ng/ml) were used as mitogens in media treated with choline oxidase. Cells were studied in whole-cell patch clamp experiments (14).
Locomotion and Other Behaviors.
Ambulation (Fig. 3 A, B, D, and E) was measured in Pasadena. Single events represented disruption of two distinct light beams 10 cm apart in the cage (San Diego Instruments). Nicotine (0.02 mg/kg nicotine equivalent of nicotine hydrogen tartrate salt dissolved in 0.9% saline) or amphetamine (5 mg/kg d-amphetamine sulfate dissolved in 0.9% saline) was injected i.p. in a volume of 100–200 μl (100 μl/20 g body weight).
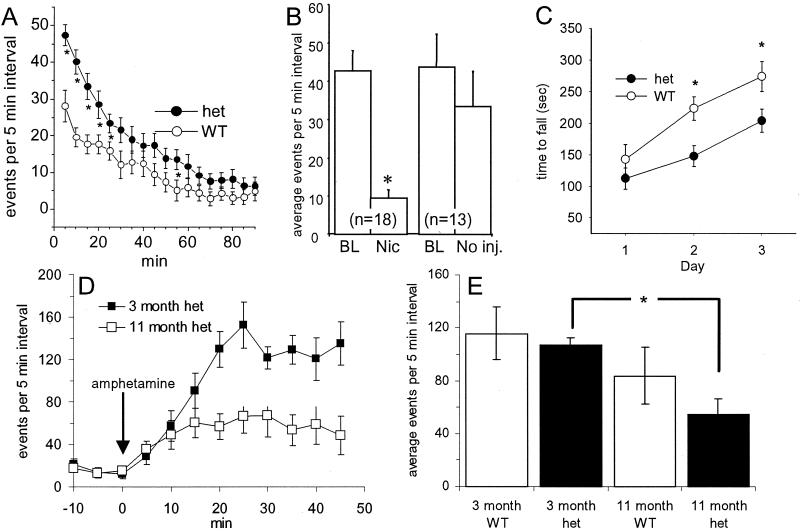
Figure 3.
Spontaneous and drug-modulated locomotion of WT and heterozygous (het) neo-intact mutants. (A) No treatment. Heterozygotes showed significantly higher locomotion than WT mice at the beginning of the experiment (P < 0.001). (B) Nicotine, 0.02 mg/kg, was injected 30 min after the start of behavioral monitoring. The plot shows data averaged over the time periods, 10 min before (BL, baseline) and 5–15 min after injection. Heterozygous mice showed a significant reduction of locomotor activity after nicotine injection (P < 0.05). There was no significant difference in noninjected animals (right-hand bars), nicotine-injected control animals, or saline-injected WT or heterozygous animals (data not shown). (C) Heterozygotes were impaired compared with WTs on the accelerating rotarod (P < 0.012) when tested for three sequential days (n = 20–22 of each genotype). (D and E) The effect of amphetamine on locomotion of WT and heterozygous (het) mice at two ages. Before drug administration, animals were allowed to habituate for 30 min. Ten male WT and 10 male heterozygous mice showing at least a 3-fold increased activity over baseline in response to amphetamine at 3 months of age were selected for longitudinal follow-up studies. (D) Comparison in amphetamine responses for heterozygotes at 3 months vs. 11 months of age. (E) The average activity is plotted for the period between 5 and 45 min after injection. The response at 11 months declines significantly compared with the response at 3 months in heterozygous mice [F(1,9) = 12.72, P < 0.01] but not in WT mice.
Mice were shipped to Boulder and acclimated for 21 days before testing by personnel blind to the genotype at 110–133 days of age (22 each WT mice and mutant littermates). Week 1 involved baseline anxiety, measured in the elevated plus maze (15, 16), followed by mirrored chamber (16, 17) and light/dark box (16, 18). Week 2 involved startle and prepulse inhibition of startle, then 3 days on the accelerating rotarod (Ugo Basile, Varese, Italy), at speeds increasing gradually from 4 to 40 rpm over 500 s. In week 3, contextual and cued fear conditioning was as described (19), except that a 0.7-mA shock was used for fear conditioning. A separate group of mice derived from the first backcross onto C57BL/6J mice also were tested for gross ataxia on a circular mesh (0.5 × 0.5 cm squares) elevated 23 cm from a plastic square arena. All behavioral data were analyzed with ANOVA procedures and spss Version 9 software.
Results
Physiological Design and Recombinant Generation of Mutant Genotypes.
Extending previous studies with the neuronal homomeric α7 and muscle nAChRs (20–22), we found that the Leu-9′Ser mutation in the α4 subunit (VTLCISVLLSLTVFLLLIT) shifts the dose–response relation for acetylcholine and nicotine about 30-fold to the left for the α4β2 receptor tested in oocytes (Fig. 1 A and B). The mutated receptor also is activated to 20% of maximal values by choline at a concentration (≈10 μM) that is detected in plasma and cerebrospinal fluid (Fig. 1C) (23). We also noted increased 8-(diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride (500 nM, nine oocytes) blockable leakage currents in some oocytes expressing the α4L9′S/β2 receptor (24), and this may arise from constitutive activation of 1–2% of the receptors; that is, channels open even in the absence of agonist (25).
Fig. 1D shows the targeting construct employed in these experiments; Fig. 1E shows the altered region of one α4 allele in 129/SvJ ES cells after homologous recombination and also after Cre recombinase-mediated deletion of the neo selection cassette. Fig. 1F shows a Southern blot indicating presence of the recombinant neo-intact mutant α4 gene in an ES cell line. Similar blots were used then to indicate Cre recombinase-mediated deletion of the neo selection cassette. Chimeric males from the resulting two mouse lines, neo-intact and neo-deleted, were bred to C57BL/6 females to generate animals heterozygous for the mutation, and mice were tested for the mutation by sequencing of genomic DNA (Fig. 1G). Heterozygous animals from the neo-deleted line do not feed and die within 24 hours after birth; a breeding colony bearing this dominant neonatal fatal gene could not be established.
Heterozygous mice from the neo-intact line are viable and fertile, and a breeding colony has been established. (In this paper, further use of “heterozygous” refers to this neo-intact line.) Homozygous mutant animals from the neo-intact line do not feed and die within the first postnatal day. The effect of the Leu-9′Ser mutation is probably reduced in heterozygous neo-intact mice because (i) the extent of hypersensitivity, quantified by the reduction in EC50, is expected to increase with the proportion of mutated to normal α4 subunits in the pentameric α4β2 complex (21, 22); and (ii) in Western blots not shown, we find that 3-month-old heterozygous mice express somewhat lower levels of α4 receptors than do WT littermates, in agreement with previous studies on knock-in animals showing that the neo cassette often results in reduced production of the mutated protein (26).
Deficits of Dopaminergic Neurons.
Embryos from matings of heterozygous neo-intact mice (WT, heterozygous, and homozygous for the mutation) and embryos from matings of neo-deleted chimeras with C57BL/6 females (WT and containing one copy of the mutated gene) were collected at embryonic day (ED) 14, 16, and 18. Double immunolabeling with anti-TH and anti-α4 antibodies revealed numerous TH- and α4-immunoreactive cells in the substantia nigra and the ventral tegmental area of WT and heterozygous animals at age ED 14–18 (Fig. 2A). Brains from mutant homozygous embryos of the neo-intact line and from embryos of the dominant lethal neo-deleted line showed normal numbers of immunoreactive cells at ED 14, but a marked reduction in the number of these cells at ED 16 and 18 (Fig. 2 B and C). The severe deficit of nigral dopaminergic cells was the most remarkable anatomical change we observed in the brain of the knock-in mouse.
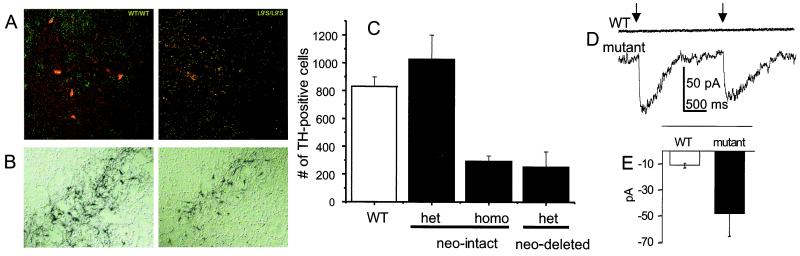
Figure 2.
Detection and probable pathophysiological basis of dopaminergic neuron deficits in mutant mice. (A) Substantia nigra of WT (Left) and homozygous neo-intact ED 18 embryos, double stained for the α4 subunit (green) and for TH (red). (B) Tyrosine hydroxylase staining of substantia nigra of WT (Left) and heterozygous neo-deleted (Right) ED 18 embryos. (C) Cell counts of TH-positive neurons in substantia nigra of ED 16 to ED 18 embryos from WT, neo-intact, and neo-deleted mice. The heterozygote (het) cell counts do not differ significantly from WT, but both the homozygous (homo) neo-intact (P < 0.01, t test) and the neo-deleted cell counts (P < 0.05, t test) differ significantly from WT. (D) Whole-cell voltage-clamp recording of responses to two consecutive puffs of choline (100 μM, 20 ms) in neuron-like cells differentiated from ED 16 midbrain neuronal progenitor cells. Upper trace, cell from a WT embryo; lower trace, cell derived from a heterozygous neo-intact ED 16 embryo. (E) Mean ± SEM of responses in neuron-like cells derived from heterozygous animals (n = 5 cells) but little or no response in cells from WT animals (n = 7 cells; significant difference, P < 0.05, t test).
Because (α4Leu-9′Ser)β2 mutant receptors expressed in Xenopus oocytes are activated by choline at concentrations that occur in plasma and cerebrospinal fluid (Fig. 1C), it is possible that the deficit of dopaminergic neurons in mutant mouse embryos is caused by constant activation of hypersensitive nAChRs on these neurons. To test whether dopaminergic neurons in heterozygous mutant mice are activated by choline, we isolated midbrain neuronal progenitor cells at ED 14–16, differentiated them, and observed responses to applied choline (100 μM). There were choline-induced inward currents in neuron-like cells derived from heterozygous mice, but much smaller currents in those derived from WT animals (Fig. 2 D and E), ruling out a major contribution from endogenous α7 nicotinic receptors (27). In cells from homozygous neo-intact mice, the responses to choline were so large that recordings were lost after the first application.
Characterization of Heterozygote Motor Activity.
There were no gross physical differences between heterozygotes and WT littermates of both sexes from the neo-intact line. The genotypes did not differ in weight, and all exhibited normal grooming behavior and social interactions with cage mates. There was no significant effect of genotype on any measure of fear conditioning. Nonetheless, detailed characterization revealed a specific pattern of behavioral differences between the heterozygotes and WT mice.
When mice are placed in the test cage, they explore the new surroundings and show a transiently increased level of ambulation for ≈30 min (Fig. 3A). During this period, the knock-in mice show a significantly higher level of ambulation than the WT controls. The genotype X time interaction was characterized by [F(1,17) = 5.2; P < 0.001]. Whereas WT mice are not affected by an administration of 0.02 mg/kg of nicotine, this treatment sharply reduces the level of activity of the knock-in mice [group X time interaction = F(1,14) = 1.89; P < 0.05] (Fig. 3B). Concentrations of nicotine that affect behavior of unhabituated WT mice are 10- to 100-fold higher.
In the accelerating rotarod test, the α4 heterozygotes also exhibited abnormalities in a measure of motor learning (Fig. 3C). Heterozygotes and WTs performed equally on the first day of testing. Both genotypes showed some improvement across days in their ability to remain on the accelerating rotarod [F(2,80) = 30.2; P < 0.001], but heterozygotes performed more poorly overall on later days [F(1,40) = 6.91; P < 0.012]. Because there is little α4 expression in the cerebellum and we detected no gross ataxia in the α4 heterozygotes (all six heterozygous and all 10 WT mice remained on the mesh for the entire 5-min test period), we suggest that cerebellar function is normal in the α4 heterozygotes. Thus the impairment of motor coordination is most likely another correlate of dysfunction of dopaminergic neurons (albeit to a lesser extent than the fatal cell loss in either the homozygous neo-intact animals or in neo-deleted animals carrying even a single copy of the mutant gene). The hypersensitive α4 allele did not produce an overall change in reactivity as measured by acoustic startle response, prepulse inhibition, contextual learning, or auditory cued conditioning.
Locomotor responses to amphetamine were examined as a behavioral measure of nigrostriatal function. Intraperitoneal injection of 5 mg/kg amphetamine stimulated locomotion in young (≈3-month-old) mice, and there was no significant difference between WT mice and their heterozygous littermates. When the same group of animals was tested at 11 months of age, there was reduced amphetamine-induced locomotion in heterozygous but not in WT mice (Fig. 3 D and E). We suggest that the reduction of the amphetamine response in older heterozygous mice is due to accelerated loss of function in their dopaminergic neurons.
Increased Anxiety of Heterozygous Knockin Mice.
Fig. 4 shows that heterozygotes were significantly more anxious than WT mice. In the elevated plus maze (Fig. 4A), heterozygotes displayed more anxiety as measured by percentage of entrances into open arm [F(1,44) = 7.35; P < 0.01], percentage of time in open arms [F(1,44) = 10.8; P < 0.002], percentage of time in the closed arms [F(1,44) = 6.64; P < 0.02], and the number of times to the end of the open arm [F(1,44) = 8.6; P < 0.005]. There was no effect of genotype on percentage of time in the center [F(1,44) = 1.82; not significant) or number of total entries into all arms [F(1,44) = 1.29; not significant].
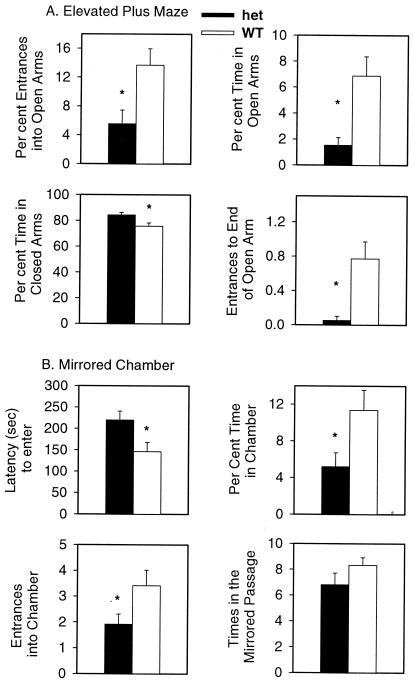
Figure 4.
Increased anxiety in α4 heterozygotes (het) compared with WT mice and in the elevated plus maze (A) and mirrored chamber (B) (n = 22 mice of each genotype) (1, 2). (A) Heterozygotes were significantly more anxious, as measured by percentage of entrances into the open arms (P < 0.01), percentage of time in the open arms (P < 0.002), percentage of time in the closed arms (P < 0.02), and entrances to the end of the open arms (P < 0.005). (B) Heterozygotes were significantly more anxious in the mirrored chamber, as measured by latency to enter the mirrored chamber (P < 0.026), percentage of time in the mirrored chamber (P < 0.03), entrances into the mirrored chamber (P < 0.02), but not by the number of entries into the mirrored passage.
There was no overall difference in activity in this maze, as evidenced by a lack of difference in the total entries in all arms and time spent in the center of the maze. These measures are consistent with overall greater anxiety in the heterozygotes.
In another measure of anxiety, the mirrored chamber test (Fig. 4B), heterozygotes displayed more anxiety as measured by: latency (seconds) to enter mirrored chamber [F(1, 44) = 5.9; P < 0.019] and number of entrances into the mirrored chamber [F(1,44) = 5.9; P < 0.014]. There was no difference in times walking through the mirrored passage [F(1,44) = 2.2; not significant].
In a third test of anxiety, the light/dark box, heterozygotes did not differ from WTs on the following measures: total transitions (15.1 ± 1.7 vs. 17.3 ± 0.9); percentage of time in the light box (34.6 ± 2.8 vs. 31.0 ± 1.3); latency to enter the dark side (17.6 ± 2.7 vs. 12.6 ± 1.9). This lack of difference in the light/dark box compared with increased anxiety measured in other mazes has been observed previously in a corticotropin-releasing hormone receptor-2 null mutant mouse (28). Anxiety is a multidimensional behavior, and each test may evaluate only a subset of these dimensions.
Discussion
We report generation of mice expressing a hypersensitive α4 receptor by mutating a single amino acid in the M2 region. This hypersensitivity results in a severe phenotype: perinatal death of animals that carry either a single copy of the dominant neo-deleted allele or two copies of the neo-intact mutant allele. The phenotype of the α4 nAChR knock-in mice is more severe than that of α4 or β2, or even of β2/β4 subunit null mutants (4, 9, 29), arguing against the possibility that the lethality arises primarily from enhanced desensitization or inactivation of α4-containing receptors or from autonomic dysfunction. The phenotype is also more severe than that of knock-in mice bearing a hypersensitive mutation of the α7 nicotinic receptor at the 9′ position (30); the latter mice display recessive lethality of the neo-deleted line as well as cell death in somatosensory cortex.
The brain of mutant embryos showed no gross abnormalities. We chose to specifically investigate substantia nigra dopaminergic neurons, both because these cells express high densities of α4-containing receptors (6) and because NURR1 knock-out mice, which specifically lack these neurons, also die shortly after birth (31). Some mutant nAChRs that cause human slow-channel congenital myasthenic syndrome are activated by serum levels of choline, and this continuous channel activity is thought to contribute to the pathophysiology of the disease (32). We present evidence that continuous activation by choline is a likely mechanism underlying the deficit of dopaminergic neurons. Previous data also establish that chronic stimulation of nicotinic receptors in the brain produces developmental anomalies, including extensive cytotoxicity and premature withdrawal from the cell cycle (33). Detailed studies of other brain regions that express α4 receptors have yet to be completed. Knock-in animals with gain of function mutations in nicotinic receptors may become useful models for neurodegenerative diseases.
Our results with the viable neo-intact heterozygous α4 knock-in mice support a role for α4-containing receptors in the control of baseline anxiety. Nicotine is known to reduce anxiety or to produce a bimodal effect on anxiety (34). Nicotinic receptors modulate the release of neurotransmitters that have critical roles in the regulation of anxiety (γ-aminobutyric acid, dopamine, and serotonin) (35), and it will be important to investigate the hypothesis that these mice have reduced γ-aminobutyric acid release. Functional changes due to the hypersensitive nicotinic receptors on dopaminergic neurons in the α4 heterozygotes could also contribute at least in part to increased anxiety. (Increased anxiety is often associated with Parkinson's disease.) Consistent with this possibility, treatment with D2 but not D1 dopamine receptor antagonists increases anxiety in rodents (36). Finally, locomotion of the heterozygous knock-in mice is extremely sensitive to nicotine, suggesting that these mice may also become a model for effects of nicotine on mammalian brain and behavior.
Acknowledgments
We thank Shirley Pease and her colleagues for generating and maintaining the mice. This research was supported by grants from the National Institutes of Health (NS-11756, MH-49176, DA-10156, and DA11836) and from the California Tobacco-Related Disease Research Program and by fellowships from the Alexander von Humboldt Foundation, the German Academic Exchange Foundation, and the Wellcome Trust.
Abbreviations
- nAChRs
nicotinic acetylcholine receptors
- ES
embryonic stem
- WT
wild type
- TH
tyrosine hydroxylase
- ED
embryonic day
References
- 1.Nisell M, Nomikos G G, Hertel P, Panagis G, Svensson T H. Synapse. 1996;22:369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Marshall D L, Redfern P H, Wonnacott S. J Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- 3.Wonnacott S. In: Nicotine Psychopharmacology: Molecular, Cellular, and Behavioural Aspects. Wonnacott S, Russell M A H, Stolerman I P, editors. Oxford: Oxford Univ. Press; 1990. pp. 226–277. [Google Scholar]
- 4.Picciotto M R, Zoli M, Zachariou V, Changeux J-P. Biochem Soc Trans. 1997;25:824–829. doi: 10.1042/bst0250824. [DOI] [PubMed] [Google Scholar]
- 5.Picciotto M R, Zoli M, Rimondini R, Lena C, Marubio L M, Pich E M, Fuxe K, Changeux J P. Nature (London) 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo-Jimenez M D, Bourgeois J P, Marubio L M, Le Sourd A M, Ottersen O P, Rinvik E, Fairen A, Changeux J P. J Neurosci. 1999;19:6475–6487. doi: 10.1523/JNEUROSCI.19-15-06475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorell J, Rybicki B, Johnson C, Peterson E. Neurology. 1999;52:115–119. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- 8.Hellenbrand W, Seidler A, Robra B, Vieregge P, Oertel W, Joerg J, Nischan P, Schneider E, Ulm G. Int J Epidemiol. 1997;26:328–339. doi: 10.1093/ije/26.2.328. [DOI] [PubMed] [Google Scholar]
- 9.Zoli M, Picciotto M R, Ferrari R, Cocchi D, Changeux J-P. EMBO J. 1999;18:1235–1244. doi: 10.1093/emboj/18.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross S A, Wong J Y, Clifford J J, Kinsella A, Massalas J S, Horne M K, Scheffer I E, Kola I, Waddington J L, Berkovic S F, et al. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannon A W, Decker M W, Holladay M W, Curzon P, Donnelly-Roberts D, Puttfarcken P S, Bitner R S, Diaz A, Dickenson A H, Porsolt R D, et al. Science. 1998;279:77–81. doi: 10.1126/science.279.5347.77. [DOI] [PubMed] [Google Scholar]
- 12.Nowak M W, Gallivan J P, Silverman S K, Labarca C G, Dougherty D A, Lester H A. Methods Enzymol. 1998;293:504–529. doi: 10.1016/s0076-6879(98)93031-2. [DOI] [PubMed] [Google Scholar]
- 13.Potter E D, Ling Z D, Carvey P M. Cell Tissue Res. 1999;296:235–246. doi: 10.1007/s004410051285. [DOI] [PubMed] [Google Scholar]
- 14.Li Y X, Zhang Y, Lester H A, Schuman E M, Davidson N. J Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lister R G. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 16.Bowers B, Collins A, Tritto T, Wehner J. Behav Genet. 2000;30:111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- 17.Toubas P L, Abla K A, Cao W, Logan L G, Seale T W. Pharmacol Biochem Behav. 1990;35:121–126. doi: 10.1016/0091-3057(90)90215-4. [DOI] [PubMed] [Google Scholar]
- 18.Crawley J, Goodwin F K. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 19.Young E A, Owen E H, Meiri K F, Wehner J M. Brain Res. 2000;860:95–103. doi: 10.1016/s0006-8993(00)02021-7. [DOI] [PubMed] [Google Scholar]
- 20.Revah F, Bertrand D, Galzi J L, Devillers-Theiry A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux J-P. Nature (London) 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- 21.Labarca C, Nowak M W, Zhang H, Tang L, Deshpande P, Lester H A. Nature (London) 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- 22.Filatov G N, White M M. Mol Pharmacol. 1995;48:379–384. [PubMed] [Google Scholar]
- 23.Klein J, Gonzalez R, Koppen A, Loffelholz K. Neurochem Int. 1993;22:293–300. doi: 10.1016/0197-0186(93)90058-d. [DOI] [PubMed] [Google Scholar]
- 24.Zhong W, Gallivan J, Zhang Y, Li L, Lester H, Dougherty D. Proc Natl Acad Sci USA. 1998;95:12088–12093. doi: 10.1073/pnas.95.21.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertrand S, Devillers-Thiery A, Palma E, Buisson B, Edelstein S J, Corringer P J, Changeux J P, Bertrand D. NeuroReport. 1997;8:3591–3596. doi: 10.1097/00001756-199711100-00034. [DOI] [PubMed] [Google Scholar]
- 26.Single F N, Rozov A, Burnashev N, Zimmermann F, Hanley D F, Forrest D, Curran T, Jensen V, Hvalby O, Sprengel R, et al. J Neurosci. 2000;20:2558–2566. doi: 10.1523/JNEUROSCI.20-07-02558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F. NeuroReport. 1998;9:3097–3101. doi: 10.1097/00001756-199809140-00033. [DOI] [PubMed] [Google Scholar]
- 28.Bale T L, Contarino A, Smith G W, Chan R, Gold L H, Sawchenko P E, Koob G F, Vale W W, Lee K F. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe C B, Armstrong D, Patrick J W, Role L W, Beaudet A L, De Biasi M. J Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orr-Urtreger A, Broide R S, Kasten M R, Dang H, Dani J A, Beaudet A L, Patrick J W. J Neurochem. 2000;74:2154–2166. doi: 10.1046/j.1471-4159.2000.0742154.x. [DOI] [PubMed] [Google Scholar]
- 31.Zetterstrom R, Solomin L, Jansson L, Hoffer B, Olson L, Perlmann T. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 32.Zhou M, Engel A G, Auerbach A. Proc Natl Acad Sci USA. 1999;96:10466–10471. doi: 10.1073/pnas.96.18.10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger F, Gage F H, Vijayaraghavan S. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.File S E, Kenny P J, Ouagazzal A M. Behav Neurosci. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill A B, Brioni J D. Pharmacol Biochem Behav. 1994;49:755–757. doi: 10.1016/0091-3057(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 36.Timothy C, Costall B, Smythe J W. Pharmacol Biochem Behav. 1999;62:323–327. doi: 10.1016/s0091-3057(98)00157-9. [DOI] [PubMed] [Google Scholar]