The advent of chemical biology tools for imaging and tracking of biomolecules (proteins, lipids, glycans) in their native environment is providing unique insights into cellular processes that are not achievable with traditional biochemical or molecular biology tools.[1] Bioorthogonal labeling of biomolecules has proven particularly useful for the detection and study of glycans[2] and lipids,[3] based on a highly selective reaction between an abiotic functional tag and a designed chemical probe. With respect to the abiotic tag, azide has been used extensively because of its straightforward chemical introduction, small size, and relative inertness.[4] The finding that azides react rapidly and cleanly with terminal acetylenes in the presence of copper(I), the quintessential “click” reaction, has found tremendous application in life and material sciences.[5] However, because up to 20 mol % of copper(I) species is typically used, such click chemistry is not suitable for labeling of living systems without compromising cell function.[6] Apart from that, the presence of copper may induce oligonucleotide[7] and polysaccharide[8] degradation. To avoid the use of toxic metals, several metal-free bioorthogonal labeling reaction have been developed.[9] In particular, phosphines have been used for covalent ligation to azides, a procedure known as Staudinger ligation.[10] However, owing to the oxygen sensitivity of phosphines, recent focus of chemical ligation is shifting towards strain-promoted cycloaddition reactions with cyclooctynes (Scheme 1 a).[11] Most prominently, azides were find to react with cyclooctynes with high reaction rates in a so-called strain-promoted alkyne–azide cycloaddition (SPAAC).[12] The toolbox of metal-free bioorthogonal reactions was most recently further expanded by our research group[13] and others,[14] by demonstrating that cyclooctynes undergo even more rapid strain-promoted cycloaddition with nitrones (SPANC), a procedure that was found suitable for dual, irreversible, and site-specific N-terminal modification of proteins.[13]
Scheme 1.
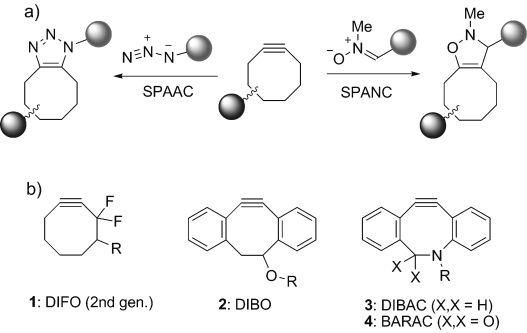
Reactions and structures of cyclooctyne compounds for strain-promoted cycloaddition. a) Cycloaddition with azide (SPAAC) or nitrone (SPANC). b) Structures of the most commonly employed cyclooctynes.
The broad application of metal-free cycloaddition in life sciences is, however, hampered by the limited commercial availability and lengthy synthetic routes for preparation of the most common cyclooctynes (Scheme 1 b). For example, eight synthetic steps are required to generate second-generation DIFO (1),[15] nine steps for DIBAC (3),[16] and seven steps for BARAC (4),[17] while yields are usually low (10 % for 2,[18] 16 % for 4). Additional modifications, such as dibenzoannulation (compounds 2–4), increase lipophilicity and may, therefore lead to non-specific binding to proteins.[17]
Here we report bicyclo[6.1.0]nonyne (BCN) as a novel ring-strained alkyne for metal-free cycloaddition reactions with azides and nitrones. Bicyclononyne derivatives, which were obtained in a highly straightforward process through cyclopropanation of 1,5-cyclooctadiene, are Cs symmetrical and display excellent reaction kinetics in strain-promoted cycloaddition reactions. Functionalized derivatives of BCN were applied in the labeling of proteins and glycans, as well as in the three-dimensional visualization of living melanoma cells.
Based on the known reactivity enhancement of cyclopropane fusion,[19] we speculated that analogues of bicyclo[6.1.0]nonyne (compound 6, Scheme 2 a) would form a class of versatile cycloalkynes for bioconjugation by combining relative stability with high reactivity. Thus, the synthesis of BCN started by the dropwise addition of ethyl diazoacetate to a large excess (8 equiv) of 1,5-cyclooctadiene in the presence of rhodium acetate.[20] The resulting mixture of diastereomeric compounds exo-5 and endo-5, formed in a 2:1 ratio, was readily separated by chromatography on silica gel (combined yield 76 %). Next, as exemplified for endo-5, the individual stereoisomers were converted into the corresponding hydroxyalkynes by reduction of the ester group, bromination, and elimination—a three-step reaction sequence that was performed within eight hours and required only a single purification step. The desired bicyclo[6.1.0]non-4-yn-9-ol (endo-6) was thus obtained in 61 % overall yield after purification (the diastereomeric exo-isomer of 6 was prepared in 53 % yield form exo-5). Both exo-6 or endo-6 were found sufficiently stable for prolonged storage at −20 °C and did not undergo structural change upon stirring in the presence of 5 mm glutathione for 48 hours in CD3CN/D2O (1:2).[17]
Scheme 2.
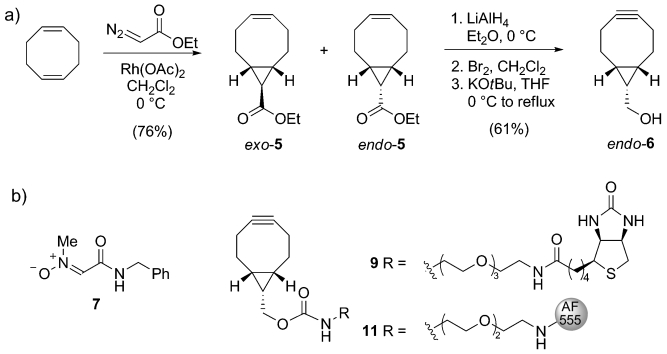
a) Synthesis of 9-hydroxymethylbicyclo[6.1.0]nonyne (endo-6). b) Structures of nitrone 7 and BCN conjugated to biotin (9) or Alexa Fluor 555 (11). THF=tetrahydrofuran.
Next, we investigated the reaction kinetics of 6 in the prototypical SPAAC reaction with benzyl azide. Calculation of second-order reaction kinetics demonstrated that the three-membered ring fusion leads to a near 100-fold rate enhancement over plain cyclooctyne (k≈2×10−3 m−1 s−1), measuring 0.14 m−1 s−1 for endo-6 and 0.11 m−1 s−1 for exo-6. Reaction rates increased, as anticipated,[13] in a more polar mixture (CD3CN/D2O (1:2)), measuring 0.29 and 0.19 m−1 s−1 for endo-6 and exo-6, respectively, values similar to or better than other cyclooctyne systems.[4, 21] Strain-promoted acetylene–nitrone cycloaddition (SPANC)[13] with nitrone 7 (Scheme 2 b) instead of an azide was found to be significantly faster, measuring 1.66 and 1.32 m−1 s−1 for endo-6 and exo-6, respectively.
The usefulness of BCN for bioorthogonal functionalization of biomolecules was next investigated in the one-pot SPANC functionalization of a model peptide with an N-terminal serine. Indeed, we observed a clean conversion of FRATtide, a GSK-1 binding peptide that prevents axin binding,[22] into the expected isoxazoline conjugates upon SPANC labeling with endo-6 or biotinylated BCN-conjugate 9, as judged by mass spectrometric analysis and spot-blot analysis (see the Supporting Information).
We also became interested in whether BCN-conjugates are suitable for labeling of azido-containing proteins. To this end, recombinant virus capsid protein was expressed in auxotrophic E. coli in the presence of azidohomoalanine,[23] thus leading to the introduction of a single azide in nearly 50 % of the isolated protein. Without further separation, the mixture of proteins was subjected to strain-promoted functionalization with BCN-Alexa Fluor 555 conjugate 11 by mixing for three hours in a phosphate buffer (pH 7.5). After washing and dialysis, incorporation of 11 was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; Figure 1 a) and mass spectrometric analysis, which indicated a quantitative mass increase of 1135 Da, that is, the molecular weight of 11 (see the Supporting Information). Next, capsid proteins were assembled into viral capsids by dialysis to a sodium acetate buffer (pH 5.0, 0.01 m CaCl2), and subsequently purified by FPLC. A strongly fluorescent peak appeared around 1.2 mL, which is the common elution volume of virus capsid. Finally, the structure of virus capsids was determined by transmission emission spectroscopy (TEM) indicating the structural integrity of the protein capsids after functionalization with 11 (Figure 1 b).
Figure 1.
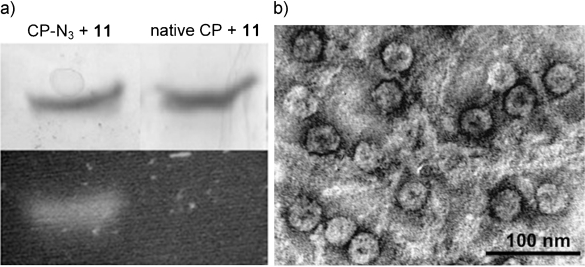
BCN modification of azido-containing virus capsid protein and assembly into virus capsids. a) SDS-PAGE analysis of reaction of BCN-AF555 conjugate (11) with capsid protein containing azide (left) or without azide (right). Top: Coomassie Brilliant Blue staining, bottom: fluorescence image. b) TEM pictures of fluorescent capsids (images recorded on a JEOL 1010 TEM, the sample was deposited on a hydrophilized Formvar carbon-coated TEM grid and consequently negatively stained with 0.2 % uranyl acetate).
The bioavailability and tolerability of labeling surface glycans on living human melanoma MV3 cells was addressed using the chemical reporter strategy.[1b] MV3 melanoma cells are highly invasive and metastatic, and their abundant production of surface glycans was previously implicated in invasion processes.[24] Thus, MV3 cells were incubated with peracetylated N-azidoacetyl-D-mannosamine (Ac4ManNAz), labeled with BCN-biotin conjugate 9, and stained with streptavidin-Alexa Fluor 488 (Figure 2). To be able to compare the efficiency of labeling of 9 to that of dibenzocycylooctyne (DIBO, 2), one of the most reactive cyclooctyne systems known to date,[18] cells were also labeled with a DIBO-biotin conjugate. In all cases, cells retained morphological integrity and cell surface fluorescence, with consistently higher labeling for BCN than for DIBO, as detected by confocal microscopy (Figure 2 a) and flow cytometry (Figure 2 b). By using flow cytometry, high monophasic intensities and excellent signal-to-noise ratio (SNR) were found for both DIBO-biotin (SNR=116) and BCN-biotin (SNR=221). No signs of label-induced cytotoxicity were detected after propidium iodide staining (see the Supporting Information).
Figure 2.
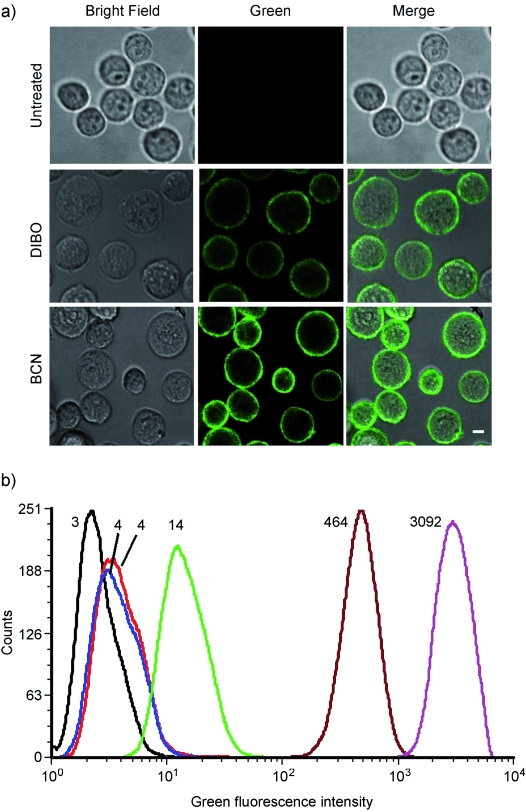
Surface and total fluorescence intensity of MV3 melanoma, cultured in the absence or presence of Ac4ManNAz (50 μm), followed by labeling with a cyclooctyne-biotin conjugate and secondary labeling with streptavidin-Alexa Fluor 488. a) Representative confocal images of unlabeled cells (top), cells labeled with DIBO-biotin (middle) or BCN-biotin 9 (bottom). Bar: 10 μm. b) Label intensity assessed by flow cytometry, indicated as mean fluorescence intensity (MFI). Numbers denote the average of green fluorescent cells for that particular experiment. Black trace: untreated; red trace: Ac4ManNAz + SA-AF488; blue trace: w/o Ac4ManNAz + DIBO + SA-AF488; dark red trace: Ac4ManNAz + DIBO + SA-AF488; green trace: w/o Ac4ManNAz + BCN + SA-AF488; magenta trace: Ac4ManNAz + BCN + SA-AF488.
Functional cell integrity was confirmed after incorporating MV3 cells into three-dimensional collagen lattices yielding spontaneous and vigorous invasion. Owing to its high signal-to-noise ratio, labeling with BCN revealed fine, subcellular details, which can discriminate surface glycan distribution states on individual living cells as shown by densitometry experiments (see the Supporting Information). Whereas cells in suspension retain a near-homogeneous distribution of azidosialic acids on the cell surface (as in Figure 2), invading cells show the redistribution and accumulation of sialic acid at actin-rich contact sites with collagen fibers, consistent with their role in cell adhesion and migration (Figure 3).[25, 26, 27] Thereby, submicron resolution reveals fine surface distribution of sialic acids at leading edge filopodia, focal clusters at actin-rich contact sites to collagen fibers, and substantial glycan-rich deposits into the tissue matrix from the trailing edge (see the Supporting Information).
Figure 3.
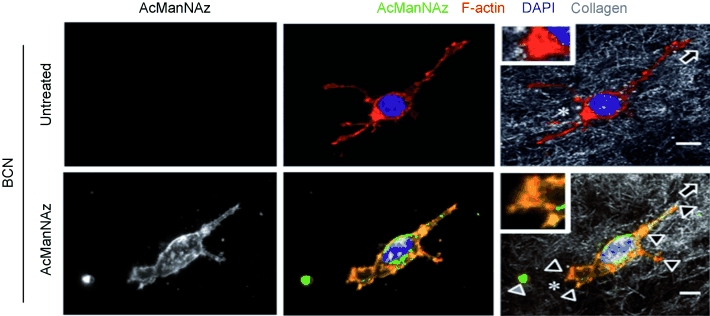
Live-cell staining and redistribution of glycans during invasive cell migration through a three-dimensional collagen matrix. Focal accumulation of sialic acid on migrating MV3 melanoma cell at interaction sites to collagen fibers and partial colocalization with F-actin. Insets, trailing edge. Direction of migration was determined from retraction fibers (asterisks) and deposited sialic acid-rich material lacking F-actin from the cell rear (gray arrowhead).[28] Bar: 5 μm. Focalized glycan distribution at cell matrix interactions (black arrowheads).
In conclusion, in view of the non-toxic labeling procedure, the tunable fluorescent properties by choice of dye and the high signal-to-noise ratio, BCN will be useful for addressing molecular glycan function studies in live-cell and other systems. A key advantage of BCN over earlier cyclooctynes lies in the combination of its exceptionally easy preparation with high reactivity. Furthermore, it should be noted that the lack of conformational isomerism in bicyclo[6.1.0]non-4-ynes[29] leads to sharp peaks in the 1H NMR spectrum, which is further simplified by the Cs symmetry of BCN. An additional advantage of a symmetrical cyclooctyne is the formation of a single regioisomer upon cycloaddition, an aspect of particular advantage in areas where the formation of homogeneous adducts is mandatory.[11] Thus, BCN will allow a broad range of applications that require the highly efficient and metal-free conjugation of two separate molecular entities, for example in life sciences, material science, surface modification, and molecular diagnostics.
References
- 1a.Best MD. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 1b.Sletten EM, Bertozzi CR. Angew. Chem. 2009;121:7108–7133. doi: 10.1002/anie.200900942. Angew. Chem. Int. Ed.2009, 48, 6974–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prescher JA, Bertozzi CR. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 3.Charron G, Wiljon J, Hang HC. Curr. Opin. Chem. Biol. 2009;13:382–391. doi: 10.1016/j.cbpa.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Debets MF, van der Doelen CWJ, Rutjes FPJT, van Delft FL. ChemBioChem. 2010;11:1168–1184. doi: 10.1002/cbic.201000064. [DOI] [PubMed] [Google Scholar]
- 5.Meldal M, Tornøe CW. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 6.Link AJ, Vink MKS, Tirrell DA. J. Am. Chem. Soc. 2004;126:10598–10602. doi: 10.1021/ja047629c. [DOI] [PubMed] [Google Scholar]
- 7.Gierlich J, Burley GA, Gramlich PME, Hammond DM, Carell T. Org. Lett. 2006;8:3639–3642. doi: 10.1021/ol0610946. [DOI] [PubMed] [Google Scholar]
- 8.Lallana E, Fernandez-Megia E, Riguera R. J. Am. Chem. Soc. 2009;131:5748–5750. doi: 10.1021/ja8100243. [DOI] [PubMed] [Google Scholar]
- 9.Becer CR, Hoogenboom R, Schubert U. Angew. Chem. 2009;121:4998–5006. doi: 10.1002/anie.200900755. Angew. Chem. Int. Ed.2009, 48, 4900–4908. [DOI] [PubMed] [Google Scholar]
- 10.Köhn M, Breinbauer R. Angew. Chem. 2004;116:3168–3178. doi: 10.1002/anie.200401744. Angew. Chem. Int. Ed.2004, 43, 3106–3116. [DOI] [PubMed] [Google Scholar]
- 11.Lutz J-F. Angew. Chem. 2008;120:2212–2214. Angew. Chem. Int. Ed.2008, 47, 2182–2184. [Google Scholar]
- 12a.Blomquist AT, Liu LH. J. Am. Chem. Soc. 1953;75:2153–2154. [Google Scholar]
- 12b.Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 13.Ning X, Temming RP, Dommerholt J, Guo J, Ania DB, Debets MF, Wolfert MA, Boons GJ, van Delft FL. Angew. Chem. 2010;122:3129–3132. doi: 10.1002/anie.201000408. Angew. Chem. Int. Ed.2010, 49, 3065–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKay CS, Moran J, Pezacki JP. Chem. Commun. 2010;46:931–933. doi: 10.1039/b921630h. [DOI] [PubMed] [Google Scholar]
- 15.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J. Am. Chem. Soc. 2008;130:11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debets MF, van Berkel SS, Schoffelen S, Rutjes FPJT, van Hest JCM, van Delft FL. Chem. Commun. 2010;46:97–99. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- 17.Jewett JC, Sletten EM, Bertozzi CR. J. Am. Chem. Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ning X, Guo J, Wolfert MA, Boons G-J. Angew. Chem. 2008;120:2285–2287. doi: 10.1002/anie.200705456. Angew. Chem. Int. Ed.2008, 47, 2253–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier H, Schuh-Popitz C, Peiersen H. Angew. Chem. 1981;93:286–287. Angew. Chem. Int. Ed. Engl.1981, 20, 270–271. [Google Scholar]
- 20.Evans PA, editor. Modern Rhodium-Catalyzed Organic Reactions. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 21.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem. Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GM, Frame S, Goedert M, Nathke I, Polakis P, Cohen P. FEBS Lett. 1999;458:247–251. doi: 10.1016/s0014-5793(99)01161-8. [DOI] [PubMed] [Google Scholar]
- 23.Link AJ, Tirrell DA. Methods. 2005;36:291–298. doi: 10.1016/j.ymeth.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Goebeler M, Kaufmann D, Brocker EB, Klein CE. J. Cell Sci. 1996;109:1957–1964. doi: 10.1242/jcs.109.7.1957. [DOI] [PubMed] [Google Scholar]
- 25.Chiang CH, Wang CH, Chang HC, More SV, Li WS, Hung WC. J. Cell. Physiol. 2010;223:492–499. doi: 10.1002/jcp.22068. [DOI] [PubMed] [Google Scholar]
- 26.Christie DR, Shaikh FM, Lucas JA, IV, Lucas JA, III, Bellis SL. J. Ovarian Res. 2008;1:3. doi: 10.1186/1757-2215-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Beilis SL. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 28.Mayer C, Maaser K, Daryab N, Zanker KS, Brocker EB, Friedl P. Eur. J. Cell Biol. 2004;83:709–715. doi: 10.1078/0171-9335-00394. [DOI] [PubMed] [Google Scholar]
- 29.Antony-Mayer C, Meier H. Chem. Ber. 1988;121:2013–2018. [Google Scholar]


