Abstract
Purpose
Oxaliplatin-induced chronic peripheral neurotoxicity (OXCPN) manifests as a loss of sensation and dysesthesia in the distal extremities, which may impair daily activities and increase in incidence with the amount of oxaliplatin delivered. The variation in the reported incidence and severity of OXCPN may be a consequence of differences in the baseline characteristics of patients.
Materials and Methods
This was a prospective study (ClinicalTrials.gov, NCT00977717) in which OXCPN was recorded for all consecutive colon cancer patients treated at Samsung Medical Center (Seoul, Korea) with oxaliplatin-based combination chemotherapy. The primary endpoint was the incidence of severe OXCPN (grade 2 lasting for >7 days, or grade 3). The association of severe OXCPN and pretreatment parameters was evaluated using a multivariate regression model.
Results
Between Jan 2008 and Feb 2010, 100 patients treated with adjuvant folinic acid/fluorouracil plus oxaliplatin (FOLFOX) and 266 patients treated with capecitabine plus oxaliplatin (XELOX) or FOLFOX for advanced disease were registered into our study. The median cumulative dose of oxaliplatin was 796 mg/m2 (range, 85 to 1,583 mg/m2). Severe OXCPN was observed in 126 (34%) patients. Overall, 43 patients discontinued chemotherapy due to toxicity: 23 without severe OXCPN and 20 with severe OXCPN. In univariate analysis, severe OXCPN was frequently observed in patients with age ≥55 years (p<0.01), stage II or III (p<0.01), adjuvant setting (p=0.01), FOLFOX (p<0.01), performance status of 0 (p=0.02), and those with no prior chemotherapy (p<0.01). In a multivariate regression model, the number of chemotherapy cycles and the cumulative oxaliplatin dose were not associated with the development of severe OXCPN.
Conclusion
We failed to find a significant association between patient characteristics at baseline and the development of severe OXCPN after oxaliplatin-based combination chemotherapy. Pharmacogenomic profiling using genome-wide association study in these patients is underway.
Keywords: Colorectal neoplasms, Oxaliplatin, Neurotoxicity
Introduction
Currently, standard therapy for patients with advanced colorectal cancer (CRC) is combination chemotherapy with fluoropyrimidine, sometimes modulated by folinic acid (FA), plus irinotecan or oxaliplatin (1). Oxaliplatin is a platinum analogue that blocks DNA replication and transcription (2). This agent is particularly attractive because of its activity against cisplatin-resistant colon cancer cell lines (3). Oxaliplatin and infusional 5-fluorouracil (5-FU) with or without FA, namely FOLFOX, has been reported to be active and comparatively safe, and is now recommended as the standard first-line chemotherapy for patients with advanced CRC (4,5), as well as in the adjuvant setting (6). The main dose-limiting toxicity of oxaliplatin is peripheral neuropathy, which affects 90% of all treated patients, and which may be reversible after discontinuation of treatment (7). Oxaliplatin neurotoxicity occurs as two distinct syndromes: acute neurosensory toxicity, and chronic, cumulative peripheral neurotoxicity. Oxaliplatin-induced chronic peripheral neurotoxicity (OXCPN) manifests as a loss of sensation and dysesthesia in the distal extremities, which may impair daily activities and increase in incidence with the amount of oxaliplatin delivered. The mechanism of cumulative OXCPN remains unknown; however, it has been suggested that platinum accumulation in the dorsal root ganglion causes apoptosis of terminal sensory neurons (8).
In addition to efforts to identify effective prophylactic or therapeutic agents for OXCPN, there have been studies attempting to define predictive markers for OXCPN. An accurate marker of OXCPN that would provide a prediction of the incidence or severity would prove valuable in controlling this toxicity. In the current study, we evaluated the incidence and severity of OXCPN in order to investigate the association of OXCPN with baseline characteristics in patients with colon cancer.
Materials and Methods
1. Eligibility
This was a prospective study (ClinicalTrials.gov, NCT00977717), in which the incidence and severity of OXCPN was recorded for all consecutive colon cancer patients treated with oxaliplatin-based combination chemotherapy. The study was designed to include 100 consecutive consenting patients who were treated with FA, 5-FU and oxaliplatin (FOLFOX) combination chemotherapy for curatively-resected, stage II to IV, colon cancer. At the same time, colon cancer patients who were treated with oxaliplatin-based first-line chemotherapy for advanced disease were also enrolled. Other eligibility criteria included: age 18 years or older, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or lower, no evidence of neurosensory disease, resolution of all surgery-related complications, and the absence of major organ dysfunctions. All patients were treated and followed at Samsung Medical Center (SMC), Seoul, Korea. The nature and purpose of the study was fully explained and discussed with each patient, and each provided the investigators with written informed consent before registration. This study was approved by the SMC institutional review board.
2. Chemotherapy
In the adjuvant setting, FOLFOX began no more than 5 weeks after surgery. Patients were treated with a modified FOLFOX regimen which included oxaliplatin 85 mg/m2 iv on day 1, FA 200 mg/m2 iv on day 1, and 5-FU 400 mg/m2 iv bolus on day 1 followed by 2,400 mg/m2 iv over 46 hours. Adjuvant FOLFOX was repeated every 2 weeks until recurrence, unacceptable toxicity, patient withdrawal from the study, or a maximum of 12 cycles. A second group of patients, those with advanced disease, were treated with oxaliplatin 130 mg/m2 iv on day 1 plus capecitabine 2,000 mg/m2/day on days 1 to 14 (XELOX, repeated every 3 weeks), or FOLFOX. Supportive care, including administration of blood transfusions, use of hematopoietic growth factors, antiemetics and analgesics were provided if judged appropriate by the investigators.
3. Evaluation of neurotoxicity
Toxicity was evaluated during treatment according to criteria of the National Cancer Institute (NCI-CTCAE) version 3 (9). FOLFOX patients were seen every 2 weeks. For XELOX patients, clinical adverse events and blood counts were checked on day 1 every 3 weeks. OXCPN was evaluated and graded before starting each chemotherapy cycle. In brief, grade 1 referred to asymptomatic or mild paresthesia, but not interfering with function. Grade 2 OXCPN was defined as moderate paresthesia or dysesthesia interfering with function, but not interfering with activities of daily living (ADL). Patients with painful paresthesia interfering with ADL were regarded as grade III. In addition to subjective assessment, a short, standardized neurologic examination including the testing of exteroceptive sensation in the hands and feet, and testing of proprioceptive sensation was done. In case of grade 2 or 3 OXCPN, chemotherapy was delayed up to a maximum of 2 weeks. Dose modification was done based on hematologic parameters and the degree of non-hematologic toxicity seen during the preceding cycle. In particular, oxaliplatin was reduced in cases of severe OXCPN. In cases of persistent (14 days or longer) grade 2 OXCPN or functional impairment, oxaliplatin was omitted from the regimen until recovery.
4. Statistical considerations
The primary endpoint of the current study was the incidence of severe OXCPN during the treatment period. Severe OXCPN was defined in the protocol as prolonged (7 days or longer) grade 2, or grade 3 of any duration. The time to occurrence of severe OXCPN was the secondary endpoint and was calculated from the date of initiation of therapy to the day on which the toxicity was first noticed. Comparisons were made using Mann-Whitney U tests for non-normally distributed data and paired t-tests for normally distributed data. The Chi-square test was used for comparison of categorical variables. To examine the impact of clinical and treatment variables on the incidence of severe OXCPN, multiple logistic regression models were used. Covariates included age, gender, ECOG performance status (0-1 vs. 2), therapy aim (adjuvant vs. palliative), stage (II or III vs. IV), body surface area, creatinine clearance, and chemotherapy regimens (FOLFOX vs. XELOX). The backward selection procedure with removal criterion p>0.10 based on a chi-square test was used to identify significant parameters. In addition, factors affecting the time to occurrence of severe OXCPN were evaluated using the above covariates plus chemotherapy duration and cumulative dose of oxaliplatin. All p-values were two-sided, with p<0.05 indicating statistical significance.
Results
Between Jan 2008 and Feb 2010, a total of 100 adjuvant patients and 266 palliative patients were enrolled into the study. Detailed characteristics of the patients are listed in Table 1. Two hundred and eighty-seven patients were treated with biweekly FOLFOX, while 79 received 3-weekly XELOX. The median number of chemotherapy cycles administered to patients was 12 (range, 1 to 22) and the median treatment duration was 5.5 months (95% confidence interval [CI], 5.4 to 5.6 months). Patients on adjuvant FOLFOX received a median of 935 mg/m2 of oxaliplatin (range, 510 to 1,020 mg/m2); those in the palliative setting received a median of 899 mg/m2 (range, 500 to 1,583 mg/m2).
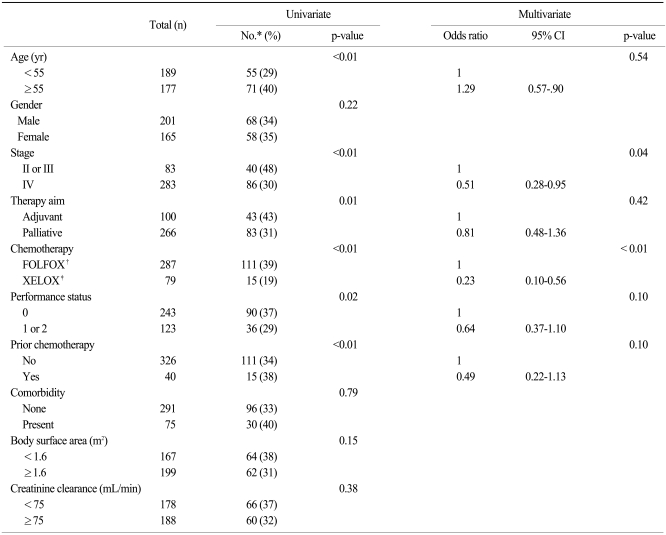
Table 1.
Patient characteristics
*folinic acid, 5-fluorouracil and oxaliplatin, †comorbidities included diabetes (n=52), hypertension and/or history of cardiovascular disease (n=23), viral hepatitis B (n=11) and history of other malignancies (n=2), ‡Eastern Cooperative Oncology Group, §in palliative setting, 23 patients were treated with bevacizumab concomitantly with FOLFOX or XELOX, ∥capecitabine and oxaliplatin.
In 14 patients (3.8%), data for OXCPN were not available because they received only one cycle of therapy or were lost to follow-up. Overall, 299 (85%) patients experienced OXCPN during treatment. The maximum grade of OXCPN was 1 in 163 patients; 2 in 123 patients; and 3 in 13 patients. The time to the development of OXCPN was clearly dependent on the duration of chemotherapy and the cumulative oxaliplatin dose: 42% and 85% of patients experienced OXCPN of any grade after 3 months and 6 months, respectively.
Severe OXCPN was observed in 126 (34%) patients. Overall, 43 patients discontinued chemotherapy due to toxicity: 23 patients without severe OXCPN; and 20 with severe OXCPN (17 prolonged grade 2 OXCPN, 3 grade 3). Management of all patients with severe OXCPN was conservative. Short-acting analgesics and/or oral gabapentin were recommended and, in some patients, oral or transdermal narcotics were prescribed. Oxaliplatin dose reduction and treatment with analgesics resulted in transient improvement, but only one patient showed sustained improvement of severe OXCPN.
Ten baseline parameters were subjected to univariate analysis for their ability to predict severe OXCPN. The following parameters were associated with the development of severe OXCPN (Table 2): age 55 years or older (p<0.01); stage II or III (p<0.01); adjuvant chemotherapy (p=0.01); FOLFOX (p<0.01); performance status of 0 (p=0.02); no prior chemotherapy (p<0.01). Gender, the presence of comorbid illness, body surface area, and creatinine clearance was not a significant predictor of severe OXCPN.
Table 2.
Association of the incidence of severe oxaliplatin-induced chronic peripheral neurotoxicity and baseline characteristics
*number of patients with severe oxaliplatin-induced chronic peripheral neurotoxicity, †folinic acid, 5-fluorouracil and oxaliplatin, ‡capecitabine and oxaliplatin.
These six parameters with univariate significance were entered into a multivariate logistic regression model. These parameters were selected because of their statistical significance in univariate analysis and their perceived clinical relevance. The independent association with severe OXCPN was examined in a backward selection procedure. Two baseline parameters were determined to have independent significance: stage II or III (vs. IV); and FOLFOX (vs. XELOX). Interaction between these two predictive parameters was then considered. A two-way interaction between stage and chemotherapy regimen was found to be highly significant (p<0.01). A model containing an interaction term between stage and regimen no longer showed these two parameters as being significant, and the interaction was eliminated by deleting stage from the model.
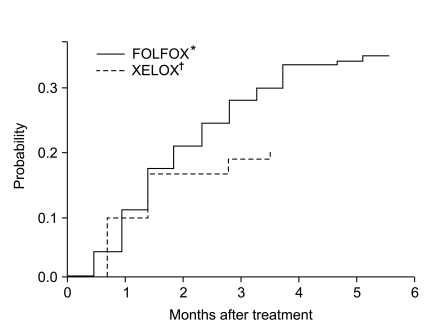
Fig. 1 shows time to occurrence of severe OXCPN by chemotherapy regimen. When, for exploratory purposes, we compared the cumulative hazard rates between patients who received FOLFOX versus XELOX, the chance of developing severe OXCPN was significantly higher in those receiving FOLFOX (p=0.03). The cumulative hazard of developing severe OXCPN increased above 10% after 1.5 months, and increased up to 30% at 4 months. However, a multivariate model containing chemotherapy regimen and the cumulative oxaliplatin dose revealed that neither biweekly FOLFOX (odds ratio, 1.60; 95% CI, 0.88 to 4.93; p=0.09) nor cumulative oxaliplatin dose (odds ratio, 1.27; 95% CI, 0.59 to 2.88; p=0.51) had independent adverse significance.
Fig. 1.
Cumulative hazard of developing severe oxaliplatin-induced chronic peripheral neurotoxicity according to chemotherapy regimens. *folinic acid, 5-fluorouracil and oxaliplatin, †capecitabine and oxaliplatin.
Discussion
Oxaliplatin is the standard of treatment in conjunction with 5-FU/FA for locally-advanced and metastatic colon cancer. OXCPN is initially mild in severity and merely a bothersome side effect of oxaliplatin. However, it can substantially reduce quality of life. Importantly, OXCPN, and not tumor progression, is often the cause of chemotherapy discontinuation. Numerous potential neuroprotective agents have been tested against OXCPN, such as carbamazepine, gabapentin, as have supplements such as intravenous calcium or magnesium (10). However, to date, efforts to establish a neuroprotective agent against OXCPN have been unsuccessful. Once OXCPN has developed, treatment of severe OXCPN includes only conservative measures, such as oxaliplatin dose reduction, the avoidance of cold, or analgesics.
The risk of developing OXCPN may be related to treatment schedule, dose of oxaliplatin, cumulative dose, time of infusion and pre-existing peripheral neuropathy (11). Although reports to date show that an association of prolonged use of oxaliplatin and development of OXCPN is likely, the true incidence and risk factors of severe OXCPN are unknown. Therefore, we attempted to evaluate severe OXCPN prospectively in consecutive patients with colon cancer treated with oxaliplatin-based chemotherapy.
The incidence of OXCPN in patients treated with FOLFOX or XELOX was 82%, and severe (prolonged grade 2 or grade 3) OXCPN was observed in 34% of patients. Only chemotherapy regimen (biweekly FOLFOX) was an independent risk factor for severe OXCPN. Our analyses indicated that more frequent exposure to oxaliplatin, regardless of the dose, is the most significant risk factor for the development of severe OXCPN.
It is of note that the difference in the hazard of developing severe OXCPN was not related to the cumulative oxaliplatin dose or treatment duration. The finding is rather unexpected because previous reports suggested that OXCPN develops progressively as the cumulative oxaliplatin dose increases (8). One possible explanation is that the grading of OXCPN was based on subjective and clinical examinations. Despite a number of comprehensive toxicity scales, accurate grading of OXCPN still represents a matter of controversy, mainly due to the fact that there are variations in interpreting clinical aspects, which leads to poor reliability. Physician-based assessments, such as the NCI-CTCAE neuropathy score that was used in the current study, require patient cooperation and physician training. Although quantitative tests of neuropathy, including electrophysiological measures, are available (12), they are not routinely used to make treatment decisions for OXCPN (13). Currently, a widely accepted, and validated, grading system of OXCPN is lacking. Furthermore, one may argue that this study represents only a small group of patients with colon cancer. However, the strength of the current study lies in a uniform patient population and the prospective design. All patients were treated with standardized FOLFOX or XELOX regimens for their colon cancer, and chemotherapy-related symptoms were absent in most of the patients before study entry.
Another explanation for the current results is that the clinical parameters, although clinically meaningful for predicting the incidence of OXCPN overall, might not have a defined role as a predictive marker of severe OXCPN. Patients treated with anticancer chemotherapy exhibit variation, both in terms of tumor response and in the incidence or severity of toxicity. The etiology of this variation is multifactorial with genetic factors likely contributing to a significant extent. Pharmacogenetic and genomic studies can be used to identify the genetic variants that contribute to the variation in susceptibility to chemotherapy-induced activity and toxicity (14). The traditional approach of pharmacogenomic studies has been the candidate gene approach, in which a gene or pathway is identified as potentially important based on literature evidence and then subjected to further study. Oxaliplatin is metabolized via the conjugation of a platinum-diaminocyclohexane metabolite to glutathione. This reaction is catalyzed by the enzyme glutathione-S-transferase (GST), of which the P1 (GSTP1) subclass of this enzyme is highly expressed in intestine (7). Ruzzo et al. (15) reported that the incidence of oxaliplatin neuropathy was higher in Val/Val genotype patients, whereas another study reported the opposite (16). Gamelin et al. (17) did a study to find genetic variations involving oxalate metabolism and the glutathione cycle, but failed to prove an association between GSTP1 and oxaliplatin neuropathy. While studies are beginning to point to some potential pharmacogenomic markers of oxaliplatin neuropathy, they have not yielded conclusive findings that have allowed for the identification of patients at risk of the toxicity.
Conclusion
We failed to find baseline clinical parameters that were significant predictors of the development of severe OXCPN in colon cancer patients treated with oxaliplatin-based combination chemotherapy. A genome-wide polymorphism study is underway to describe variations between patients and to investigate a possible association with the development of severe OXCPN (18).
Footnotes
This study was supported by research funds from the In-Sung Foundation for Medical Research, Seoul, Korea (CA98691), and a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (0412-CR01-0704-0001).
References
- 1.Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005;23:4553–4560. doi: 10.1200/JCO.2005.17.749. [DOI] [PubMed] [Google Scholar]
- 2.Woynarowski JM, Chapman WG, Napier C, Herzig MC, Juniewicz P. Sequence- and region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol Pharmacol. 1998;54:770–777. doi: 10.1124/mol.54.5.770. [DOI] [PubMed] [Google Scholar]
- 3.Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998;9:1053–1071. doi: 10.1023/a:1008213732429. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 7.Kweekel DM, Gelderblom H, Guchelaar HJ. Pharmacology of oxaliplatin and the use of pharmacogenomics to individualize therapy. Cancer Treat Rev. 2005;31:90–105. doi: 10.1016/j.ctrv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev. 2008;34:368–377. doi: 10.1016/j.ctrv.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Braun AH, Dirsch O, Hilger RA, Schleucher N, Tewes M, Rustum YM, et al. Preclinical evaluation of the combination of epidermal growth factor inhibitor ZD1839 (Iressa) and irinotecan (SN-38) in human colon cancer cells. Proc Am Soc Clin Oncol. 2002;21:abstr 329. [Google Scholar]
- 10.Grothey A. Oxaliplatin-safety profile: neurotoxicity. Semin Oncol. 2003;30(4 Suppl 15):5–13. doi: 10.1016/s0093-7754(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 11.Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer. 2005;5(Suppl 1):S38–S46. doi: 10.3816/ccc.2005.s.006. [DOI] [PubMed] [Google Scholar]
- 12.Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology. 2003;61:1297–1300. doi: 10.1212/01.wnl.0000092015.03923.19. [DOI] [PubMed] [Google Scholar]
- 13.Cleeland CS, Farrar JT, Hausheer FH. Assessment of cancer-related neuropathy and neuropathic pain. Oncologist. 2010;15(Suppl 2):13–18. doi: 10.1634/theoncologist.2009-S501. [DOI] [PubMed] [Google Scholar]
- 14.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruzzo A, Graziano F, Loupakis F, Rulli E, Canestrari E, Santini D, et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol. 2007;25:1247–1254. doi: 10.1200/JCO.2006.08.1844. [DOI] [PubMed] [Google Scholar]
- 16.Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006;12:3050–3056. doi: 10.1158/1078-0432.CCR-05-2076. [DOI] [PubMed] [Google Scholar]
- 17.Gamelin L, Capitain O, Morel A, Dumont A, Traore S, Anne le B, et al. Predictive factors of oxaliplatin neurotoxicity: the involvement of the oxalate outcome pathway. Clin Cancer Res. 2007;13:6359–6368. doi: 10.1158/1078-0432.CCR-07-0660. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Won H, Son E, Lee J, Park S, Park J, et al. Genetic polymorphism associated with chronic neurotoxicity and recurrence in curatively-resected colon cancer patients receiving oxaliplatin-based adjuvant chemotherapy. J Clin Oncol. 2010;28:abstr 3583. [Google Scholar]