Summary
Epilepsy is increasingly recognized as a disease that reaches well beyond seizures. Cognitive and psychiatric impairment afflict half of all epilepsy patients, and to date there are no specific treatments for these symptoms. It is unclear which of these comorbidities are directly due to seizures and which are due to separable, parallel mechanisms to those underlying ictal activity. Cellular and molecular mechanisms underlying synaptic modulation are central to both the ictal and non-ictal changes in epilepsy. Current diagnostic methods are rapidly advancing to better delineate the nature and extent of ictal activity, and could soon be critical in identifying patterns unique to the cognitive and psychiatric comorbidities.
Keywords: cognitive comorbidity, connectome, interictal, seizure
The epilepsy spectrum disorder
Epilepsy can be described as a spectrum disorder. Like autism, the disorder is multifactorial, multifaceted, and varies in severity from individual to individual. Over the last several years, our field has become increasingly aware of the complex relationships between epilepsy and a host of cognitive, behavioral, psychiatric, and other neurological disorders, and even sudden death.
Clinical evidence supports the hypothesis that the underlying pathologies responsible for causing epilepsy may directly contribute to the cognitive and other disorders associated with epilepsy. At the same time, increasing evidence points to a direct role for seizures themselves in contributing to the cognitive and behavioral disorders seen in epilepsy. Laboratory models are elucidating both sets of mechanisms.
Epilepsy is defined by the occurrence of seizures, but it is a disorder that is exists within a wide spectrum of other disorders. While seizures often remain the focus of clinical care, the many other cognitive and behavioral disorders associated with epilepsy may be just as troubling, if not more so, to the patient and family. Up to half of all epilepsy patients suffer from some form of cognitive or psychiatric condition(Karouni, et al. 2010; Wiebe and Hesdorffer 2007). A comprehensive and effective approach to treating and managing epilepsy requires a sophisticated appreciation for the intricate relationships between epilepsy and these various other disorders.
Potential for convergence of factors responsible for epileptogenesis and cognitive dysfunction
Data from multiple models of epilepsy suggest that the balance between network excitation and inhibition is disrupted, and that excitatory synaptic composition and efficacy are enhanced, either directly or indirectly (Rakhade and Jensen 2009). Importantly, a key regulator of excitatory synapses is the glutamate receptor, and this receptor is also critical to learning and memory. Hence seizure-induced dysregulation of either glutamate receptor function itself, or that of an upstream mediator or downstream effector may have important effects on learning and cognition. Ictal activity clearly can deliver the enhanced excitability to perturb synaptic homeostasis (for review see Hablitz 2004; Lopantsev et al. 2009), and recent reports have revealed that even brief ictal activity can impair learning (Kleen et al. 2010). At issue is whether brief interictal activity can have long term effects on subsequent activity. Temporal lobe epilepsy is one of the most common forms of adulthood epilepsy, and can be complicated by memory deficit as well as psychiatric disorders. Here both ictal and interictal activity have been associated with disrupted cognition, and animal models have shown numerous cellular and molecular changes resembling those observed in patients in critical hippocampal and limbic structures for memory and emotion. Indeed, epilepsy is seen with greater frequency in patients suffering from depression (LaFrance, et al. 2008) and conversely, depression is a commonly observed comorbidity in chronic epilepsy (see Kanner, this supplement). Serotonin depletion has been suggested to be the predominant neurochemical abnormality in depression, and many antidepressant therapies are directed at restoring serotonin levels to critical neuronal networks thought to be responsible depressed mood. In addition to anti-depressant actions, serotonin agonists also possess anticonvulsant efficacy(Torta and Monaco 2002), suggesting that this monoamine is involved in both depression and epilepsy. Importantly, seizure activity itself can deplete serotonin locally, and could result in further hyperexcitability as well as contribute to depression (see Kanner, this supplement). Local serotonin depletion in brainstem nuclei may also play a role in sudden unexplained death in epilepsy (SUDEP), and raises the question as to whether repeated seizures may impact the raphe nucleus, which is the major source of serotonin in the brain (see Richerson, this supplement).
Perhaps the most sensitive period for seizures affecting cognitive function is during brain development. At this time, synaptic plasticity mechanisms are peaking, and excitatory mechanisms predominate over inhibition (Rakhade and Jensen 2009; see also Brooks-Kayal, this supplement). Given that cascades implicated in learning and memory are largely activity-dependent, there is potential for excessive neuronal activity to have unexpected effects on normal synaptic function. Moreover, in the developing brain, synpatogenesis is occurring at high rates, and appears to share many of the same mechanisms with those of synaptic plasticity (Greer and Greenberg 2008).
There are several major examples where cognition is affected by early life epilepsy. Infantile spasms can occur in an otherwise normally developing child, remit after only weeks, yet result in significant developmental delay and neurocognitive impairment (Goh, et al. 2005). Epilepsy with electrical status in slow wave sleep (ESES) is a particularly problematic variant of refractory epilepsy that exhibits a high incidence of encephalopathy (Nabbout and Dulac 2003). More focal effects can be seen in Landau Kleffner syndrome, where the onset of recurrent temporal lobe ictal and interictal activity coincides with language regression in young children with previously acquired language (Camfield and Camfield 2002). More mild effects of seizures in later childhood may occur in benign rolandic epilepsy, which although spontaneously remitting, is associated with a significantly higher incidence of learning deficits compared to the general population (Camfield and Camfield 2002). An extremely close comorbidity is seen with autism spectrum disorder and epilepsy, where almost 40% of patients with autism have epilepsy, and a greater number have been shown to epileptiform EEGs without clinical epilepsy (Canitano 2006; Deonna and Roulet 2006; Parmeggiani, et al. 2010).
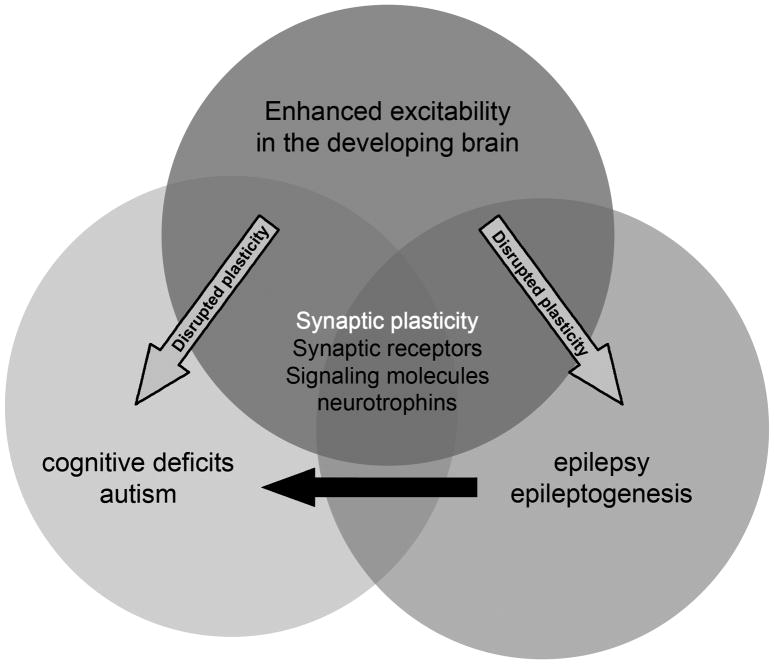
Autism is a particularly interesting case of the interaction between epilepsy and cognitive development. Infancy and early childhood are characterized by some of the highest incidences of epilepsy across the lifespan (Hauser, et al. 1993), and this is also the period when autism becomes manifest. Notably this age window is that of the natural “critical period”, where synaptic plasticity and synpatogenesis are at the highest level of the lifespan (Rakhade and Jensen, 2009). In addition, both epilepsy and autism are thought in part to be due to dysregulated synaptic development (Figure 1). When epilepsy and autism co-occur, it is assumed that they may have both been a result of a primary disruption of synaptic function due to injury or genetic mutation. What is not known is whether epileptic activity, which would further perturb synaptic function, can contribute to secondary symptomatology of autism (Figure 1) (see Berg, this supplement).
Figure 1. Interaction, convergence between brain development, epilepsy and autism.
Brain development is a state of heightened synaptic excitability, to permit the critical period of synaptic plasticity. When disrupted, it can either generate early cognitive disorders such as autism, or if synaptic excitability is excessive, epilepsy. Epilepsy is of high prevalence in the immature brain due to the heighted synaptic tone. Normal brain development, autism, and epilepsy all share in common synaptic activity, and further understanding of how epilepsy and autism may share similar dysregulation of synaptic elements may lead to new therapies. In addition, it is not yet known whether the epileptic seizures themselves, by way of dysregulating synaptic activity at a critical developmental stage, may contribute to autism.
A case might be made for a causal role for seizures in enhancing cognitive impairment in autism. Epilepsy and autism co-occur at a very high frequency in a number of genetic disorders, most notably Tuberous Sclerosis Complex (TSC). TSC is due to genetic mutation of either the TSC1 or TSC2 gene encoding proteins that inhibit protein synthetic pathway centered on the activity of the mammalian target of rapamycin (mTOR) (Napolioni, et al. 2009). In TSC, absence of normal inhibition of mTOR is thought to cause structural and functional neuronal and glial deficits that can impair normal network development and function. However, mTOR activation can be induced by neuronal activity and calcium-dependent upstream regulators (Tavazoie, et al. 2005). Importantly, a number of recent studies have shown that mTOR can be upregulated in normal, non-mutant brain, by seizure activity itself (Zeng, et al. 2009). This raises the possibility that a feed-forward mechanism might exist in TSC, whereby seizures may exaggerated abnormal neuronal and glial function produced by the cell-autonomous mutations in the TSC pathway. There are other autism-related genes abnormalities that lead to disturbed plasticity that could also be robustly modified by a seizure. In addition to the TSC associated dysregulation of mTOR, we have shown that seizures induce CaMKII activation, which is known to regulate the transcriptional repressor protein methyl-CpG-binding protein 2 (MeCP2) (Zhou, et al. 2006). Loss of function as well as gain of function mutations in MeCP2 are associated with Rett’s syndrome and other X-linked behavioral disorders, suggesting that this pathway may be important in seizure-induced cognitive disorders(Johnston, et al. 2003). Interestingly, MeCP2 activation is CaMKII dependent, and results in altered synaptic function and neurotransmitter expression (Zhou et al. 2006). Postmortem studies of Rett’s syndrome patients show upregulated expression of the AMPA and NMDA glutamate receptor subtypes, suggesting that MeCP2 may be critical for normal synaptic development(Johnston et al. 2003). In the rat, MeCP2 levels peak coincident in the second postnatal week, indicating that this protein may be sensitive to early life seizures and there is robust seizure-induced upregulation of CaMKII activity at this age (Rakhade, et al. 2008). Another synaptic regulator protein associated with autism is reelin, and alterations in reelin affect cortical cytoarchitecture and glutamate receptor expression (Muhle, et al. 2004). Notably, reelin is present in the perinatal period when seizure susceptibility is high and its expression can be upregulated by seizures (Qiu, et al. 2006). Hence, the aforementioned proteins are examples of pathways where synaptic activation, regulation of synapse formation, epilepsy and cognitive impairment may overlap.
Psychiatric disorders in epilepsy
Several psychiatric disorders are of increased prevalence in patients with epilepsy. These include certain personality disorders, as well as disorders of mood and affect, including depression and anxiety (see Kanner this supplement). In addition, amongst epileptic patients, the diagnosis of schizophrenia and paranoid states higher than the general population (Stefansson, et al. 1998). Both epilepsy and schizophrenia can show temporal lobe and hippocampal atrophy (for review see (Cascella, et al. 2009). In addition, auditory hallucinations are common to both TLE patients as well as schizophrenics. A number of genetic studies suggest some common susceptibility genes for epilepsy and schizophrenia, including the LGII gene (Cascella et al. 2009).
Potential interaction between epilepsy and neurodegenerative diseases
Similar to autism and depression, where epilepsy may play both a causal role as well as be a result of a shared primary factor, recent evidence has suggested that a less than coincidental occurrence of epilepsy in dementia. Several mouse models of Alzheimer’s dementia (AD) exhibit recurrent seizures ; (Palop et al. 2007; see also Noebels this supplement). In addition, patients with AD and even Parkinson’s disease have a higher incidence of epilepsy (Palop and Mucke 2009). Indeed, the highest rates of epilepsy across the lifespan are now seen in the elderly(Beghi, et al. 2010). Recently, it was noted that there is a higher rate of epileptiform abnormalities in AD patients, and that these can be highly focal or multifocal rather than generalized (Palop and Mucke 2009). Mechanistically, experimental data from mouse models of AD suggest that glutamate receptors and other ligand and voltage gated ion channels may be dysregulated, increasing the risk for seizure activity (Palop et al. 2007). It is unclear whether seizures can accelerate the progression of neurodegeneration, or whether anticonvulsants may modify neurodegenerative processes.
Future diagnostics for cognitive disorders associated with epilepsy
Currently, epilepsy is a field that is undergoing a revolution with respect to diagnostic technology. In particular, patients with refractory epilepsy that are undergoing consideration for surgical resection are commonly receiving a battery of neurophysiological, neuroimaging, and neuropsychological tests. These include prolonged digital video-EEG recordings, MRI with diffusion tractography, functional MRI, ictal and interictal SPECT and Positron Emission Spectroscopy (PET), Magnetoencephalography (MEG), among others. However, these advanced techniques are directed largely at identifying an ictal focus, whereas they are not routinely used to assess cognitive disorders that my accompany epilepsy in those same patients. Hence, a tremendous opportunity in these patients may be to determine whether there are biomarkers for certain of the cognitive comorbidities associated with their epileptic condition.
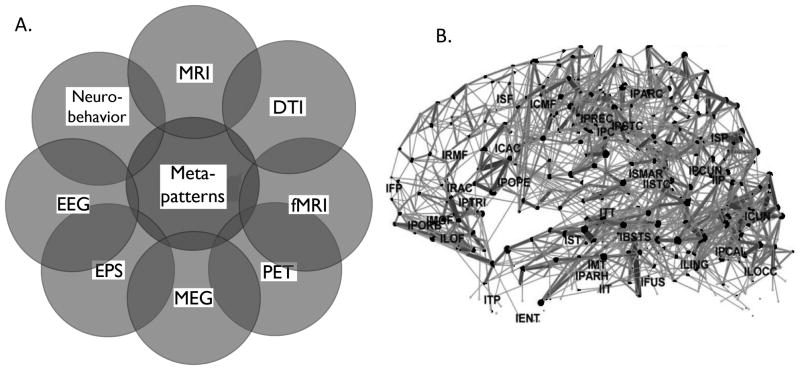
To this end, and important advance in the past decade is the advances in informatics such that data from multiple platforms and techniques can be merged to examine, for the first time, the brain “connectome” (Hagmann, et al. 2010; Sporns, et al. 2005). Connectomics is the multimodal assessment of network connectivity, integrating functional MRI at rest or task-dependent conditions with DTI and structural imaging (Hagmann, et al. 2008) (Figure 2A). Additional data that can be superimposed are neurophysiologic activity as assessed by MEG or EEG, which both have greater temporal resolution than fMRI. Taken together, the computational integration of this multidisciplinary data can reveal unique structure/function relationships, and which networks “wire” and “fire” together. Three dimensional maps can be constructed to display connectivity maps, with different brain areas represented as nodes, and the strength of their connections indicated by thickness of the line connecting one node to another (Hagmann et al. 2008) (Figure 2B).
Figure 2. Connectomics is a method to assess global brain connectivity as well as meta-patterns of brain activity.
A. Connectomics can computationally integrate any combination of multiple modalities, including clinical observation of neurobehavior and genomics, neurophysiological including electroencephalography (EEG), evoked potentials (EP), and magnetoencephalography (MEG), as well as structural including magnetic resonance imaging (MRI), Diffusion tensor imaging (DTI), and functional MRI imaging (fMRI). B. Example of a connectivity map for the left hemisphere, using data obtained from fMRI and DTI. Individual brain regions are indicated by dots, and the strength of connections denoted by thickness of the line connecting these regions. (Figure 2B Reprinted with permission from PLoS Biol(Hagmann et al. 2008).
Modern digital EEG data can examine neurophysiologic activity at frequencies in a much higher range than those in the past. While very high frequency recordings are only available from intracranial recordings, superficial scalp electrodes can detect signals into the gamma range (30–80 Hz). A number of reports link alterations in gamma frequency to a number of neuropsychiatric conditions (Herrmann and Demiralp 2005). Current research is directed at evaluating the potential of even higher frequency oscillations (HFOs) in the range of 80–600 Hz as biomarkers for epilepsy, and regional ictal potential. At present only intracranial electrocorticography (ECoG) performed with the use of electrode grids and strips placed prior to surgical resection of ictal foci can accurately yield recordings in the range exceeding 100Hz, thus confining this measure to those who are being evaluated for epilepsy surgery. HFOs in the range of 80–250Hz are thought to be physiological, but those in the range of 250–600Hz are being shown to be seen primarily in and around epileptic foci (Engel, et al. 2009). To date however, the relationship of HFOs to other neuropsychiatric symptoms in epileptic patients has not been investigated, and may have great potential as a biomarker.
The power of the connectomic approach in assessing brain function is that this combinatorial data can reveal “meta-patterns” of brain function and dysfunction, potentially redefining neurologic disease and showing previously unperceived similarities and differences between disorders. Within epilepsy, interictal connectomic evaluation of default and task dependent networks may yield important new data regarding spatial and temporal relationships of cognitively important network dysfunction to the ictal foci.
Conclusion
Epilepsy is being redefined in the 21st century, with the advent of new understanding related to its cellular and molecular underpinnings, and the technological advances that allow more comprehensive assessment of the structural and functional effects of epileptogenesis. More emphasis needs to be placed on the non-ictal symptoms of epilepsy, both from a clinical care standpoint but also with respect to clinical and basic research. The challenge will be to determine to what extent the causes and treatment of comorbid neuropsychiatric disorders are the same or different. These neuropsychiatric disorders need to be focused on as a primary research focus, using many of the techniques previously used for assessment of the ictal aspects of epilepsy, including the technological advances represented by the emerging field of connectomics. Finally, we need to understand how epilepsy is embedded in other neurological and psychiatric disease states, and to what degree seizures or subclinical ictal activity is contributing to the progression of the other disease entity. Epilepsy, when redefined, may be far more prevalent than we currently estimate.
Acknowledgments
Dr. Jensen is funded by NIH NS03718 and DP1 OD003347.
Footnotes
Disclosure: Dr. Jensen receives funds from Lundbeck Pharma for an investigator initiated research project.
References
- Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, Tomson T, Hauser WA. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–675. doi: 10.1111/j.1528-1167.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia. 2002;43(Suppl 3):27–32. doi: 10.1046/j.1528-1157.43.s.3.3.x. [DOI] [PubMed] [Google Scholar]
- Canitano R. Epilepsy in autism spectrum disorders. Eur Child Adolesc Psychiatry. 2006 doi: 10.1007/s00787-006-0563-2. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Schretlen DJ, Sawa A. Schizophrenia and epilepsy: is there a shared susceptibility? Neurosci Res. 2009;63:227–235. doi: 10.1016/j.neures.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonna T, Roulet E. Autistic spectrum disorder: evaluating a possible contributing or causal role of epilepsy. Epilepsia. 2006;47(Suppl 2):79–82. doi: 10.1111/j.1528-1167.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Goh S, Kwiatkowski DJ, Dorer DJ, Thiele EA. Infantile spasms and intellectual outcomes in children with tuberous sclerosis complex. Neurology. 2005;65:235–238. doi: 10.1212/01.wnl.0000168908.78118.99. [DOI] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Hablitz JJ. Regulation of circuits and excitability: implications for epileptogenesis. Epilepsy Curr. 2004;4:151–153. doi: 10.1111/j.1535-7597.2004.44011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Gerhard S, Ellen Grant P, Wedeen V, Meuli R, Thiran JP, Honey CJ, Sporns O. MR connectomics: Principles and challenges. J Neurosci Methods. 2010 doi: 10.1016/j.jneumeth.2010.01.014. in press. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota:1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Mullaney B, Blue ME. Neurobiology of Rett syndrome. J Child Neurol. 2003;18:688–692. doi: 10.1177/08830738030180100501. [DOI] [PubMed] [Google Scholar]
- Karouni M, Arulthas S, Larsson PG, Rytter E, Johannessen SI, Johannessen Landmark C. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010 doi: 10.1007/s00228-010-0861-y. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol. 2010;67:250–257. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFrance WC, Jr, Kanner AM, Hermann B. Psychiatric comorbidities in epilepsy. Int Rev Neurobiol. 2008;83:347–383. doi: 10.1016/S0074-7742(08)00020-2. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Both M, Draguhn A. Rapid plasticity at inhibitory and excitatory synapses in the hippocampus induced by ictal epileptiform discharges. Eur J Neurosci. 2009;29:1153–1164. doi: 10.1111/j.1460-9568.2009.06663.x. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Nabbout R, Dulac O. Epileptic encephalopathies: a brief overview. J Clin Neurophysiol. 2003;20:393–397. doi: 10.1097/00004691-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Napolioni V, Moavero R, Curatolo P. Recent advances in neurobiology of Tuberous Sclerosis Complex. Brain and Development. 2009;31:104–113. doi: 10.1016/j.braindev.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmeggiani A, Barcia G, Posar A, Raimondi E, Santucci M, Scaduto MC. Epilepsy and EEG paroxysmal abnormalities in autism spectrum disorders. Brain Dev. 2010;32:783–789. doi: 10.1016/j.braindev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26:12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ, Jensen FE. Early Alterations of AMPA Receptors Mediate Synaptic Potentiation Induced by Neonatal Seizures. J Neurosci. 2008;28:7979–7990. doi: 10.1523/JNEUROSCI.1734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kotter R. The human connectome: A structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson SB, Olafsson E, Hauser WA. Psychiatric morbidity in epilepsy: a case controlled study of adults receiving disability benefits. J Neurol Neurosurg Psychiatry. 1998;64:238–241. doi: 10.1136/jnnp.64.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(Suppl 2):8–13. doi: 10.1046/j.1528-1157.2002.043s2008.x. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Hesdorffer DC. Epilepsy: being ill in more ways than one. Epilepsy Curr. 2007;7:145–148. doi: 10.1111/j.1535-7511.2007.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]