Abstract
Oxidative stress has been suggested to contribute to the pathophysiology of schizophrenia. In particular, oxidative damage to lipids, proteins, and DNA as observed in schizophrenia is known to impair cell viability and function, which may subsequently account for the deteriorating course of the illness. Currently available evidence points towards an alteration in the activities of enzymatic and nonenzymatic antioxidant systems in schizophrenia. In fact, experimental models have demonstrated that oxidative stress induces behavioural and molecular anomalies strikingly similar to those observed in schizophrenia. These findings suggest that oxidative stress is intimately linked to a variety of pathophysiological processes, such as inflammation, oligodendrocyte abnormalities, mitochondrial dysfunction, hypoactive N-methyl-D-aspartate receptors and the impairment of fast-spiking gamma-aminobutyric acid interneurons.[bkyb1] Such self-sustaining mechanisms may progressively worsen producing the functional and structural consequences associated with schizophrenia. Recent clinical studies have shown antioxidant treatment to be effective in ameliorating schizophrenic symptoms. Hence, identifying viable therapeutic strategies to tackle oxidative stress and the resulting physiological disturbances provide an exciting opportunity for the treatment and ultimately prevention of schizophrenia.
Keywords: Schizophrenia, Oxidative Stress, Antioxidant, Immune Response, Parvalbumin, N-methyl-D-aspartate Receptor
1. Introduction
Schizophrenia is a chronic, severe and disabling psychiatric illness that affects about 1% of the population worldwide (Jackobson, 2000; Perala et al., 2007). The symptoms of the disorder can be divided into three main categories: positive symptoms (e.g. delusions and hallucinations), negative symptoms (e.g. flat affect, lack of motivation and deficits in social function) and cognitive deficits (Carpenter, 1994; Tamminga and Holcomb, 2005). Although the symptoms that establish the diagnosis are usually not present until young adulthood, prodromal symptoms and endophenotypic features of cognitive and social deficits can precede psychotic illness and manifest in unaffected relatives.
The prevailing hypothesis for the etiology of schizophrenia is that variations in multiple risk genes, each contributing a subtle effect, interact with each other and with environmental stimuli to impact both early and late brain development (Weinberger, 1987; Lewis and Lieberman, 2000; McDonald & Murray, 2000; Lewis and Levitt, 2002; Sawa and Snyder, 2002; Mueser and McGurk, 2004; Harrison and Weinberger, 2005; Jaaro-Peled et al., 2009). Although a clear mechanism underlying the pathogenesis of schizophrenia remains unknown, oxidative stress as a consequence of aberrant reduction-oxidation (redox) control has become an attractive hypothesis for explaining, at least in part, the pathophysiology of schizophrenia (Cadet and Kahler, 1994; Reddy and Yao, 1996; Fendri et al., 2006; Ng et al., 2008; Behrens and Sejnowski, 2009; Dean et al., 2009a; Do et al., 2009, 2010; Wood et al., 2009a; Yao et al., 2001, 2004, 2006, 2009; Matsuzawa and Hashimoto, 2010; Zhang et al., 2010).
The last four decades have witnessed a great increase in our knowledge of the basic molecular mechanisms underlying oxidative stress. Most remarkably, functional genetic analysis has identified molecular mechanisms that are conserved in yeast, nematodes, flies and mammals. Analysis of these model systems suggests that redox mechanisms are not fixed but are reversible. Similarly, cognitive dysfunction associated with an imbalance in the generation and clearance of reactive oxygen species (ROS) and reactive nitrogen species (RNS) also seems to be variable and possibly open to modification [bkyb2](Kamsler and Segal, 2003; Calabrese et al., 2006). Recent studies have implicated these mechanisms in the control of brain pathology, raising the possibility that altered regulation of fundamental mechanisms of oxidative stress may contribute to the pathogenesis of schizophrenia and related disorders (Floyd, 1999; Chauhan and Chauhan, 2006; Ng et al., 2008; Do et al., 2009; Wood et al., 2009a).
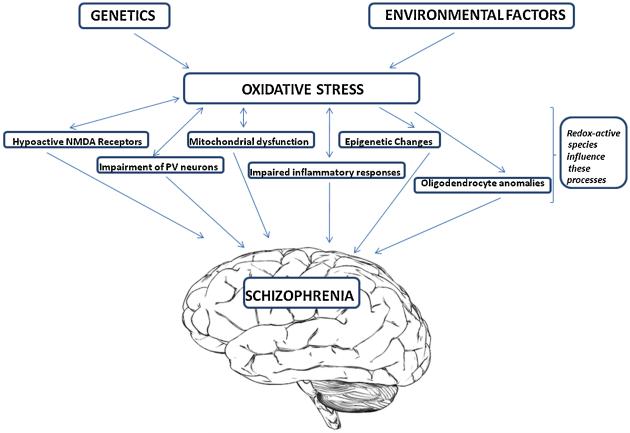
In this review, we explore the basic molecular mechanisms of redox regulation in the brain. We begin with a brief description of oxidative stress and its regulation. Then we turn to a discussion of clinical and pre-clinical findings of redox impairment that induce brain pathology in schizophrenia, through mechanisms that likely involve aberrant inflammatory responses, mitochondrial dysfunction, oligodendrocyte abnormalities, epigenetic changes, hypoactive N-methyl-D-aspartate (NMDA) glutamate receptors and the impairment of fast-spiking gamma-aminobutyric acid (GABA) interneurons (see Figure 1). There is hope that our growing understanding of the molecular basis of oxidative stress mechanisms within the brain will allow us to rise to the challenge of treating and preventing the clinical symptoms and cognitive deficits associated with schizophrenia.
Figure 1.
Schematic illustration of the involvement of oxidative stress in schizophrenia.
2. What is Oxidative Stress?
Oxidative stress occurs when cellular antioxidant defense mechanisms fail to counterbalance and control endogenous ROS and RNS generated from normal oxidative metabolism or from pro-oxidant environmental exposures (Kohen and Nyska, 2002; Berg et al., 2004). The link between oxidative stress and the pathophysiology of disease can be explained by the physiological phenomenon commonly referred to as the ‘oxygen paradox’ (Davies, 1995). This concept states that oxygen plays contradictory roles, one essential for life and the other as a toxic substance (Davies, 1995; Kohen and Nyska, 2002). The deleterious effects of oxygen relate directly to the fact that atomic oxygen is a free radical and molecular oxygen is a biradical (Davies, 1995). The biradical property of oxygen dictates that full reduction of oxygen to water as a terminal event in the electron transport chain requires 4 electrons. The sequential donation of electrons to oxygen during this process can generate ROS as intermediates, and “electron leakage” can also contribute to the formation of ROS [bkyb3](Davies, 1995; Miwa and Brand, 2003; Genova et al., 2003). The most important ROS in humans are hydrogen peroxide (H2O2), superoxide radical (O2•−), and hydroxyl radical (OH•). Reactive nitrogen species include nitric oxide (NO) and peroxinitrite (ONOO•). Fortunately, several cellular antioxidant defense mechanisms exist to counterbalance the production of ROS and RNS, including enzymatic and nonenzymatic pathways (Nordberg and Arner, 2001).
Because the redox status of cells is involved in regulating various transcription factors/activators (e.g., activator protein- and modulating signaling pathways, appropriate ROS/RNS levels are necessary for normal physiological function of living organisms (Sun and Oberley, 1996). Nuclear factor κB, for example, becomes more transcriptionally active in response to the contribution of ROS to the degradation of IκB, the inhibitory partner of nuclear factor κB that sequesters it in the cytosol (Hayden and Ghosh, 2004). Thus ROS can play an important role in modulating inflammation. Excessive ROS may, however, have detrimental effects including modification of macromolecules such as nucleic acids, proteins and lipids (Kohen and Nyska, 2002). Lipid peroxidation is a well-characterized effect of ROS that results in damage to the cell membrane as well as to the membranes of cellular organelles (Rathore et al., 1998). In addition, ROS can contribute to mutagenesis of DNA by inducing strand breaks, purine oxidation, and protein-DNA cross-linking, and other ROS mediated alterations in chromatin structure may significantly affect gene expression (i.e. epigenetic changes) (Konat, 2003). Modification of proteins by ROS/RNS can induce denaturation that renders proteins nonfunctional (Lockwood, 2000; Stadtman and Levine, 2003). Similarly, an overabundance of ROS/RNS can cause inactivation of critical enzymes and induce cell death through activation of kinases and caspase cascades (Cai et al., 1998; Evans et al., 2004; Scherz-Shouval and Elazar, 2007).
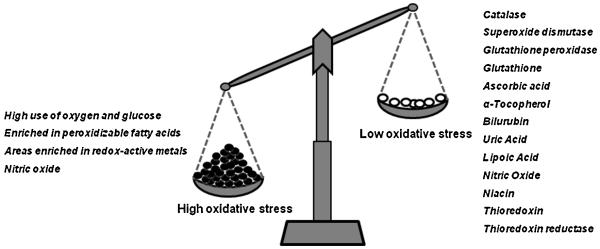
The brain is particularly vulnerable to oxidative damage (Rougemont et al., 2002; McQuillen and Ferriero, 2004), given its relatively low content of anti-oxidant defenses in addition to its high metal content (e.g. iron, zinc, copper and manganese), which can catalyze the formation of ROS/RNS. The brain utilizes more than 20% of oxygen consumed by the body yet comprises only 2% of the total body weight (Dringen, 2000; Berg et al., 2004). The high energy demand from oxidative glucose metabolism plus a high concentration of polyunsaturated fatty acids and relatively low levels of antioxidants are therefore thought to render the brain more vulnerable to oxidative insult than most organs (Bains and Shaw, 1997; Dringen, 2000) (see Figure 2).
Figure 2.
The Brain is susceptible to oxidative damage
3. Antioxidant Systems
The potential toxicity of ROS/RNS in the brain is counteracted by a number of antioxidants that can protect the brain against oxidative damage in several ways, including: (1) removal of ROS/RNS (Ozturk et al., 2005), (2) inhibition of ROS/RNS formation, and (3) binding metal ions needed for catalysis of ROS/RNS generation. Glutathione peroxidase and glutathione reductase are well-known intracellular antioxidant enzymes. Glutathione peroxidase converts peroxides and hydroxyl radicals into nontoxic forms, often with the concomitant oxidation of reduced glutathione (GSH) into the oxidized form glutathione disulfide (GSSG), and glutathione reductase recycles GSSG to GSH. Other enzymes and pathways are also involved in the management of cellular defense against oxidative stress. Notably, catalase and superoxide dismutase, acting in concert with glutathione peroxidase constitute the major defense or primary antioxidant enzymes against superoxide radicals (DeKosky et al., 2004; Dringen et al., 2005). In addition, glutathione-S-transferase and glucose-6-phosphate dehydrogenase help in the detoxification of ROS by decreasing peroxide levels or maintaining a steady supply of metabolic intermediates like GSH and nicotinamide adenine dinucleotide-phosphate (NADPH) necessary for optimum functioning of the primary antioxidant enzymes (Vendemiale et al., 1999). Similarly, thioredoxin and thioredoxin reductase can catalyze the regeneration of many antioxidant molecules, including ubiquinone, lipoic acid, and ascorbic acid (vitamin C), and as such constitute an important antioxidant defense against ROS/RNS. Other notable defense mechanisms against free radical-induced oxidative and nitrosative stress include α-tocopherol (vitamin E), bilurubin, albumin, uric acid, niacin, carotenoids and flavonoids (Nordberg and Arner, 2001; Cho et al., 2009).
Another key antioxidant within biological systems is NO. Nitric oxide is an important messenger molecule involved in many physiological and pathological processes within the mammalian body, both beneficial and detrimental (Mustafa et al., 2009; Shahani and Sawa, 2010). Being a free radical, NO has both pro-and antioxidant properties. Nitric oxide can be protective against oxidative injury, depending on the specific conditions (Kanner et al., 1991). The NO radical can both stimulate lipid oxidation and mediate oxidant-protective reactions in membranes (Radi et al., 1991). Nitric oxide reacts rapidly with peroxyl radicals as a sacrificial chain-terminating antioxidant. However, if left unchecked NO free radical can react with superoxide radical to form highly toxic peroxynitrite. Table 1 shows a list of neutralising antioxidants against ROS/RNS and additional physiological antioxidants.
Table 1.
Redox-active species and their corresponding neutralizing antioxidants. Direct role = direct redox-active species scavenging activity; Indirect Role = prevent the accumulation of toxic species rather than acting directly on ROS/RNS
| ROS | Anti Oxidants (endogenous) | Antioxidants (Exogenous) | |
|---|---|---|---|
| Direct Role | Indirect Role | ||
| Hydroxyl Radical | Glutathione Peroxidase Glucose-6-Phosphate Dehydrogenase Thioredoxin |
- | Ascorbate Flavonoids Lipoic Acid |
| Lipid Peroxide | Glutathione Peroxidase Glutathione-S-Transferase Glucose-6-Phosphate Dehydrogenase Nitric Oxide |
- | α-Tocopherol Carotenoids Flavonoids Ubiquinone Niacin |
| Superoxide Radical | Superoxide Dismutase (cofactor copper/zinc/ manganese) Glutathione Glucose-6-Phosphate Dehydrogenase Thioredoxin |
Albumin | Ascorbate Flavonoids |
| Hydrogen Peroxide | Catalase Glutathione-S-Transferase Glutathione Nitric Oxide Thioredoxin Reductase |
Ferritin Transferrin Hemoglobin |
Ascorbate, Carotenoids Lipoic Acid Glucose-6-Phosphate Dehydrogenase |
| Pro-Oxidant/antioxidant equilibrium |
Thiols (Glutathione, N-acetyl cysteine) NADPH, Thioredoxin |
Bilurubin Uric Acid |
Flavonoids |
4. Alterations in Antioxidant Defense Systems in Schizophrenia
Clinical and preclinical investigations of the actions of antioxidative defense systems in the brain suggest several ways in which ongoing oxidative stress might impact the occurrence and course of schizophrenia. In this section, we describe clinical and preclinical studies that may shed light on the role that oxidative stress plays in schizophrenia.
4.1. Clinical studies
Several studies have documented alterations in antioxidant enzymes in schizophrenia, but this is not always consistent. While reduced levels of the antioxidant enzymes are generally reported in patients with schizophrenia compared with controls (Dadheech et al., 2008; Singh et al., 2008; Raffa et al., 2009), other studies have reported either no change (Srivastava et al., 2001) or a strengthening of antioxidant status in schizophrenia (Kuloglu et al., 2002; Dakhale et al., 2004; Kunz et al., 2008). A recent meta-analysis indicated that there is an increase in the levels of lipid peroxidation products and NO (discussed in further detail below) in schizophrenia, while superoxide dismutase activity was found to be significantly decreased in the disorder (Zhang et al., 2010). This study also showed that the activities of glutathione peroxidase and catalase were not affected in patients with schizophrenia (Zhang et al., 2010). Nonetheless, various reports from different research groups have indicated lower (Reddy et al., 1991), elevated (Herken et al., 2001) or normal (Yao et al.,1998a) catalase levels in schizophrenia patients. The levels of glutathione peroxidase have also been reported to be inconsistent in patients with schizophrenia (Herken et al., 2001; Ranjekar et al., 2003; Gawryluk et al., 2010). The results in the measure of superoxide dismutase are also contradictory, with an increase (Reddy et al.,1991; Zhang et al., 2003), decrease (Mukerjee et al., 1996; Ranjekar et al., 2003) or no change (Yao et al.,1998a) in enzyme activity in patients with schizophrenia. Interestingly, the levels of superoxide dismutase have been found to be high in chronic schizophrenic patients (Reddy et al., 1991; Yao et al., 1998a,b; Zhang et al., 2003) or to be low in neuroleptic-naïve first-episode schizophrenic patients (Raffa et al., 2009), suggesting that the efficacy of neuroleptics may in part be mediated by promoting an endogenous antioxidative mechanism (Padurariu et al., 2010). [bkyb4]
Post-mortem studies have reported a 40% depletion of GSH in the caudate nucleus of schizophrenia patients (Yao et al., 2006). Similarly, Gawryluk and colleagues have recently reported reduced levels of GSH in postmortem prefrontal cortex of patients with schizophrenia (Gawryluk et al., 2010). In addition, magnetic resonance spectroscopy studies have shown that levels of GSH were reduced by 52% in the prefrontal cortex and by 27% in cerebrospinal fluid of drug-naïve schizophrenia patients (Do et al., 2000). However, other spectroscopy studies have failed to detect a decrease in the levels of GSH in the anterior cingulated cortex (Terpstra et al., 2005), posterior medial frontal cortex (Matsuzawa et al., 2008) or the medial temporal lobe (Wood et al., 2009b). In the latter studies, patients were receiving neuroleptic treatment and some of the variability of the results may therefore be related to medication status (drug-naïve versus patients on medication), sample source, ethnicity or the region of the brain investigated.
To examine a novel cell model based on patient-derived cells from the human olfactory neuroepithelium, an elegant study assessed gene and protein expression and cell function from healthy controls, patients with schizophrenia or Parkinson’s disease (Matigian et al., 2010). The olfactory neuroepithelium is the only neural tissue in the human body that is easily accessible (Féron et al., 1998) and demonstrates disease-dependant alterations in cell biology in schizophrenia (McCurdy et al., 2006), amongst other neurological disorders. In their study, Matigian and colleagues identified several pathways already implicated in schizophrenia including Reelin, Il-8 and Erb signaling in addition to GSH metabolism (Matigian et al., 2010). The findings that molecular profiles from human olfactory neuronal cells are similar to that of post-mortem brain tissue from patients with schizophrenia support the use of this cell model for studying cellular and molecular bases of neurological conditions such as schizophrenia.
The levels of plasma antioxidants (uric acid, albumin and bilirubin) have been reported to be significantly lower in schizophrenia (Yao et al., 1998c; 2000; Reddy et al., 2003). These findings were found to be independent of smoking status (Reddy et al., 2003). Plasma levels of α-tocopherol (McCreadie et al., 1995) and ascorbic acid (Suboticanec et al., 1990) have also been reported to be lower in schizophrenic patients. In contrast, thioredoxin has been shown to be increased during the acute phase of schizophrenia (Zhang et al., 2009), but becomes normalized in chronic schizophrenic patients on long-term antipsychotic pharmacotherapy (Zhang et al., 2009). Serum thioredoxin was also found to be positively correlated with positive symptoms of schizophrenia (Zhang et al., 2009). Other studies have shown the levels of lipid peroxidation products (e.g. malondialdehyde and thiobarbiturate reactive substances) to be increased in plasma, serum (McCreadie et al., 1995; Zhang et al., 2006; Dietrich—Muszalska and Olas, 2009) and red blood cells (Mahadik et al., 1998; Herken et al., 2001) of schizophrenic patients. These observations strengthen the evidence for a defective antioxidant system as an early pathophysiological change associated with the disease, rather than a sequela of drug effects, chronic disease and smoking (Reddy et al., 2003).
4.2. Preclinical studies
Various studies have shown that alterations in antioxidant systems cause cognitive impairment and biochemical changes relevant to schizophrenia. Dean and colleagues have shown that treatment of Sprague-Dawley rats and C57BL/6 mice with 2-cyclohexene-1-one (CHX), a compound that reduces brain GSH levels by conjugating to GSH via glutathione transferase (Masukawa et al., 1989), dose-dependently reduced striatal and frontal cortical GSH levels to levels similar to those observed in patients with schizophrenia (Dean et al., 2009b). In both species, GSH depletion resulted in disruption of short-term spatial recognition memory in a Y-maze test. Because GSH has been shown to modulate glutamatergic activity (Janaky et al., 1994; Oja et al., 2000), it has been suggested that a GSH deficit may contribute to a dysfunction in glutamatergic pathways responsible for long-term potentiation (LTP) (Steullet et al., 2006), a phenomenon critical for learning and required to encode memories (Martin et al., 2000). Treatment with CHX, however, did not affect sensory motor gating as indexed by prepulse inhibition (PPI), a preattentive process that is considered an endophenotype of schizophrenia, in both species following treatment with MK-801 or dizocilipine, an NMDA receptor antagonist, and amphetamine, which increases synaptic dopamine release (Dean et al., 2010). Although in rats treated with CHX, the effect of amphetamine to disrupt PPI was reversed, potentially suggesting a decrease in pre-synaptic dopamine release or dopamine receptor function.Therefore, acutely reduced GSH levels may not be directly involved in the disruption of PPI observed in schizophrenia (Dean et al., 2010).
Experimental evidence from rats exposed to early postnatal (days 5-16) treatment with l-buthionine-(S,R)-sulfoximine (BSO), an accepted rodent model of oxidative stress which induces a transitory deficit in GSH, have shown that GSH deficiency leads to long-lasting behavioral aberrations (Cabungcal et al., 2007). For example, BSO treatment leads to impaired spatial learning and memory (viz. worse performance in the homing hole board task) (Cabungcal et al., 2007). These findings highlight the role of oxidative changes during development in cognitive processes associated with schizophrenia. Specifically, oxidative stress during early development may lead to a dysfunction in integrating sensory information relevant for spatial representation. Such a deficit may arise from a misconnectivity in specific brain regions involved in modulating distinct cognitive processes (Cabungcal et al., 2007).
A recent study demonstrated the importance of altered oxidative stress state in inducing anomalies of brain neural oscillations and neuronal pathology underlying cerebral integration and cognitive functioning (Steullet et al., 2010). The study used a mouse model in which disrupted expression of the modifier (GCLM) subunit of glutamate cysteine ligase (GCL), the rate-limiting enzyme of GSH synthesis, elicits elevated oxidative stress (Yang et al., 2002). These mice exhibited altered behavior during an object recognition task, increased novelty-induced exploration in addition to altered emotion and stress-related behaviors (Steullet et al., 2010). Furthermore, the GSH deficit within these mice resulted in a specific reduction in parvalbumin (PV) containing inhibitory interneurons and a concomitant reduction in β/γ oscillations in the hippocampus of young adult mice (Steullet et al., 2010). The fast-spiking basket and chandelier cells that contain PV are known to play a critical role in regulating synchronous neuronal discharges in multiple frequency bands (e.g., theta, gamma, ripple) via both chemical and electrical synapses (Freund, 2003; Klausberger et al., 2003; Whittington and Traub, 2003; Buzsaki and Draguhn, 2004; Freund and Katona, 2007). These findings therefore highlight the role of oxidative changes in electrophysiological, behavioral and cognitive deficits associated with schizophrenia.
5. Nitric Oxide in Schizophrenia
Evidence is accumulating that NO may be involved in the pathophysiology of schizophrenia given the various roles that NO plays in the brain, such as regulating synaptic plasticity (Holscher and Rose, 1992), neurotransmitter release (Lonart et al., 1992), and neurodevelopment (Truman et al., 1996; Hindley et al., 1997; Downen et al., 1999; Contestabile, 2000; Gibbs, 2003). Nitric oxide is especially important as the second messenger of NMDA receptor activation, which interacts with both dopaminergic and serotonergic pathways (Lorrain and Hull, 1993; Brenman and Bredt, 1997). Abnormal functioning of these pathways has been suggested to be involved in the pathophysiology of schizophrenia. Perhaps relevant to the previous connection are the findings suggesting that nitric oxide synthase (NOS, an enzyme widely expressed throughout the brain and which is responsible for NO production in the central nervous system) inhibitors protect against phencyclidine (PCP) induced schizophrenia-mimicing phenotypes such as PPI deficits and cognitive inflexibility in animals (Johansson et al., 1997; Klamer et al., 2001, 2004; Wass et al., 2008, 2009). Interestingly, postmortem studies have reported elevated levels of NO and NOS in brain tissue of subjects with schizophrenia and have suggested that NOS may be activated in the illness (Baba et al., 2004; Yao et al., 2004; Xu et al., 2005). Similarly, it was shown that NO levels in red blood cells are significantly increased in patients with schizophrenia (Herken et al., 2001). However, the evidence surrounding NO metabolites in schizophrenia has been inconsistent with studies reporting both increased (Zoroglu et al., 2002; Suzuki et al., 2003; Yilmaz et al., 2007) and decreased (Suzuki et al., 2003; Lee and Kim, 2008; Nakano et al., 2010) levels. A negative correlation was observed between NO metabolite levels and positive and negative syndrome scale (PANNS) scores in schizophrenia subjects, indicating that reduced plasma NO metabolites maybe related to the severity of negative symptoms in schizophrenia (Nakano et al., 2010).
Altered populations or distribution of NOS-containing neurons have been reported in frontal (Akbarian et al., 1993a) and temporal (Akbarian et al., 1993b) cortices, hypothalamus (Bernstein et al., 1998), and cerebellum (Karson et al., 1996; Bernstein et al., 2001) in schizophrenia. Consistent with a role of NOS in schizophrenia, a number of genetic association studies have reported that single nucleotide polymorphisms in the NOS gene are associated with schizophrenia (Shinkai et al., 2002; Brzustowicz et al., 2004; Fallin et al., 2005; Reif et al., 2006; Tang et al., 2008; Wratten et al., 2009; Cui et al., 2010), although some results are inconsistent with such associations (Okumura et al., 2009). Genetic and functional data for NOS revealed an association between a putative cis-acting polymorphism in the NOS gene and decreased protein NOS expression in the prefrontal cortex of patients with schizophrenia (Cui et al., 2010). The same study also showed that the age of schizophrenia onset was earlier in patients carrying the cis-acting polymorphism in the NOS gene (Cui et al., 2010).
Decreased activity of receptors sensitive to NO has also been reported in schizophrenia. The cholinergic receptors (e.g. α7 nicotinic acetylcholine receptor) known to be sensitive to NO toxicity were decreased in both blood and cortex of patients with schizophrenia (Perl et al., 2003; Matthew et al., 2007). Patients with schizophrenia frequently smoke cigarettes and often smoke heavier than the normal population (Matterson and O’shea, 1984; Goff et al., 1992; Lasser et al., 2000). The high level of smoking has been proposed as a form of self-medication to alleviate symptoms of their illness including depression, anxiety, anhedonia or amotivation (Glassman, 1993; Olincy et al., 1997). In this context, a series of studies in humans has implicated the α7 nicotinic acetylcholine receptor in the physiology of P50 auditory gating (a measure of preattentive auditory processing). Nicotine gum and physostigmine were found to improve gating in the relatives of persons with schizophrenia who also had impaired auditory gating (Adler et al., 1992). Thus, NO appears to influence neurotransmission (e.g. cholinergic transmission) and may play a role in some of the endophenotypes associated with schizophrenia.
6. Imbalance in Homocysteine Metabolism and Epigenetic Changes in Schizophrenia
Hyperhomocysteinaemia (a medical condition characterized by an abnormally elevated level of homocysteine in the blood) can cause oxidative stress via a number of mechanisms such as auto-oxidation of homocysteine to form ROS (Heinecke et al., 1987), increased lipid peroxidation (Jones et al., 1994) and reduced production of glutathione peroxidase (Upchurch et al., 1997). A recent study by Brown and colleagues reported that higher maternal homocysteine levels may be a risk factor for schizophrenia (Brown et al., 2007). Specifically, mothers that have elevated third-trimester homocysteine levels may elevate schizophrenia risk through developmental effects on brain structure and function and/or through subtle damage to the placental vasculature that compromises oxygen delivery to the fetus (Brown et al., 2007). In this context, it has been shown that high levels of homocysteine are negatively correlated with glutathione peroxidase activity (Pasca et al., 2006), suggesting that high levels of homocysteine may also be associated with oxidative stress in schizophrenia.
Because oxidative damage to specific gene promoters results in gene silencing (Lu et al., 2004), it may be that irreplaceable post-mitotic cells, such as neurons, respond to unrepaired DNA damage by silencing expression of the affected genomic region, rather than by undergoing apoptosis. The mechanism of silencing is likely epigenetic; specifically, it may be mediated through dysregulation of DNA methylation. High homocysteine levels have been shown to be accompanied by high S-adenosyl-homocysteine levels with the elevation of S-adenosyl-homocysteine suggested to be associated with DNA hypomethylation and alterations in gene expression (James et al., 2002). Altered gene expression has been shown to be associated with the pathogenesis of schizophrenia (Tsankova et al., 2007; van Vliet et al., 2007; Jiang et al., 2008). S-Adenosyl-homocysteine and its analogs have been reported to be a non-competitive inhibitor of catechol-O-methyltransferase (COMT), an enzyme that catalyses the first step in the degradation of monoamine neurotransmitters such as dopamine, epinephrine and norepinephrine (Coward and Sweet, 1972; Coward et al., 1972, 1973). The more active allele of COMT is reported to be associated with schizophrenia (Egan et al., 2001) and although not immediately intuitive, it might be that elevated levels of homocysteine may play some aggravating role in the pathogenesis of schizophrenia through an indirect effect on COMT (Applebaum et al., 2004).
7. Genetic Susceptibility to Schizophrenia
Genetic factors may also contribute in modulating the threshold for vulnerability to oxidative stress in schizophrenia (for a review see Kodavali et al., 2010). Recent evidence has shown manganese superoxide dismutase (Akyol et al., 2005) and glutathione S-transferase T1 (Saadat et al., 2007) to be associated with schizophrenia. A functional polymorphism in the glutathione S-transferase p1 gene has been reported to be associated with vulnerability to develop psychosis in the setting of methamphetamine abuse (Hashimoto et al., 2005), which may have some bearing on schizophrenia. A mitochondrial DNA sequence variation affecting a subunit of NADPH-ubiquinone reductase (complex 1), a component of the electron transport chain responsible for generating superoxide, has also been associated with schizophrenia patients and with increased superoxide levels in post-mortem brain samples (Marchbanks et al., 2003). In another study, the GCLM subunit of the GCL enzyme has been suggested as a susceptibility gene in schizophrenia (Tosic et al., 2006). Similarly, genetic analysis of the trinucleotide (GAG) repeat polymorphism in the GCL catalytic subunit (GCLC) gene showed a significant association with schizophrenia in two independent case-control studies (Swiss and Danish) (Gysin et al., 2007). The same study revealed an association between disease-associated GCLC GAG trinucleotide repeat genotypes and decreased GCLC protein expression (Gysin et al., 2007). Do and colleagues have recently gone on to demonstrate that patients with the trinucleotide repeat GAG susceptibility polymorphism also show reduced plasma total cysteine levels, and an increase of the oxidized form of cysteine, namely cystine, content (Do et al., 2010). These patients also exhibit increased levels of plasma free serine, glutamine, citrulline, and arginine. These data suggest that schizophrenia patients exhibit alterations of plasma thiols levels in addition to a decreased capacity to synthesize GSH that most likely reflects a dysregulation of redox control and an increased susceptibility to oxidative stress that is most likely of a genetic origin (Gysin et al., 2007, 2009; Do et al., 2010). Taken together, these studies provide additional support for the involvement of oxidative stress in the etiology of schizophrenia.
8. Neurotransmitter Metabolism and Oxidative Stress in Schizophrenia
The biological effects of neurotransmitters are linked to their chemical properties. It has been shown that metabolism of serotonin (Yao et al., 2009), glutamate (Smythies, 1999) and dopamine (Smythies, 1999) play important roles in mediating redox balance within biological systems. These neurotransmitters have generated a great deal of research in a variety of mental disorders, including schizophrenia (Grima et al., 2003; Smythies, 1999; Yao et al., 2009). In this section, we specifically focus on how abnormal metabolism of dopamine and glutamate may play a pathological role in schizophrenia.
8.1 Dopamine
The classical dopamine hypothesis of schizophrenia postulates a hyperactivity of dopaminergic transmission at the D2 receptor. It is of interest that enzymatic metabolism of dopamine leads to hydrogen peroxide generation, which, via autooxidation of dopamine, leads to the production of ROS such as dopamine quinones and superoxide (Hastings, 1995; Fleckenstein et al., 2007). These ROS may then interact with superoxide dismutase and GSH, leading to a reduction in the available levels of these antioxidants.
Do and colleagues have studied the effect of dopamine in cultured cortical neurons with a low level of GSH. This study suggests that dopamine alone decreased GSH by 40% (Grima et al., 2003). This effect appears to result from the direct conjugation of dopamine semiquinone/quinone with GSH. Furthermore ethacrynic acid, a potent inhibitor of glutathione-S-transferase, decreased GSH in a concentration-dependent manner. When added to ethacrynic acid, dopamine further lowered GSH levels. As this additional decrease is blocked by superoxide dismutase or D1/D2 receptor antagonists, it likely involves the generation of superoxide via activation of dopamine receptors. It also reduces the mitochondrial membrane potential (Grima et al., 2003). Most interestingly, a significant decrease in the number of neuronal processes was induced by a 24-hour application of dopamine with ethacrynic acid, which also reduced the levels of GSH. The underlying mechanism of this effect of reduced GSH level on neuronal morphology may include ROS-evoked lipid peroxidation, leading to membrane alterations, and cytoskeleton modification (Halliwell and Gutteridge, 1998; Valko et al., 2007). These findings are consistent with the reported reduction of neuropil and dendritic spines in regions rich in dopamine innervation (Selemon et al., 1995, 1998; Garey et al., 1998; Glantz and Lewis, 2000; Selemon, 2001) and with the postulated retrograde degeneration of the mediodorsal nucleus of the thalamus projecting to those regions (Pakkenberg, 1990; Popken et al., 2000) in patients with schizophrenia. The degeneration of spines with their synaptic contacts would lead to abnormal cortico-cortical and thalamo-cortical connectivity (Weinberger, 1987; Parnas et al., 1996; Lewis and Lieberman, 2000; Andreasen, 2000). This may in turn be responsible for part of the symptoms of schizophrenia, particularly those involving cognitive and perceptive functions (Goldman-Rakic, 1991; Grima et al., 2003).
8.2 Glutamate
The glutamate hypothesis of schizophrenia posits that the function of the NMDA receptor is compromised in this disease (Coyle, 1996, 2006). This postulate is largely based on the observations that PCP and other NMDA antagonists can induce psychosis that is diagnostically difficult to differentiate from schizophrenia (Javitt and Zukin, 1991; Krystal et al., 1994, 2002; Newcomer and Krystal, 2001). While glutamate serves as the major excitatory neurotransmitter in the central nervous system via ionotropic and metabotropic receptors, it is also known to be excitotoxic at high levels (Platt, 2007). The toxic effects of glutamate on cell viability ensue from two major factors: Firstly, an influx of calcium ions may trigger an osmotic entry of isotonic fluid that renders the cells vulnerable to mechanical stress. Secondly, a cycle of excitation caused by increased calcium entry into cells results in the further release of glutamate. The latter may result in ROS production due to the accumulation of glutamate and calcium related oxidative events (Olney, 1989; Hirose and Chan, 1993).
Whereas the hypofunction of the NMDA receptor results in reduced calcium flow through these channels, the resultant effect is an increased level of free intracellular calcium in large neuronal populations (Olney et al., 1999; Schwartz et al., 1994). This is because deactivation of NMDA receptors on GABAergic interneurons removes the inhibition of major excitatory pathways that innervate primary neurons of cerebrocortical and limbic brain regions (Olney and Farber, 1995; Olney et al., 1999). These pathways are, then, able to induce an abnormal increase in free intracellular calcium in their targets by activating non-NMDA glutamate gated ion channels as well as G protein-coupled receptors, which are capable of mobilizing calcium from internal stores (Sharp et al., 1994) and subsequently triggering oxidative damage (Lidow, 2003).
A recent study has revealed that perinatal PCP administration to rats results in region-specific changes in the levels of GSH and the activities of the enzymes involved in its metabolism (i.e. glutathione peroxidase and glutathione reductase) (Radonjic et al., 2009). Alterations in superoxide dismutase activity and the level of lipid peroxides were also reported (Radonjic et al., 2009). Interestingly, the activity of catalase was not changed in any of the investigated brain structures (Radonjic et al., 2009). Within the prefrontal cortex, GSH, superoxide dismutase and glutathione reductase levels were all reduced whereas an increase in glutathione peroxidase level was observed. In the hippocampus, reduced GSH, glutathione peroxidase and glutathione reductase levels were accompanied with an increased level of lipid peroxides (Radonjic et al., 2009). In addition, GSH content was decreased in the caudate nucleus, while the major findings in the thalamus were, increased levels of lipid peroxides and glutathione reductase activity (Radonjic et al., 2009). These findings suggest that NMDA receptor malfunction might be related to the redox status of patients with schizophrenia.
9. Abnormal Iron Metabolism as a Mechanism for Oxidative Stress
Several studies have implicated imbalances of trace elements, including manganese, zinc, copper, and iron in schizophrenia (Yanik et al., 2004; Rahman et al., 2008). A disruption in the homeostasis of the latter two redox-active metals is particularly significant in light of the increases in oxidative stress parameters such as lipid peroxidation, and the oxidative damage to proteins and nucleic acids. Because free iron has been implicated in undergoing redox transitions in vivo (via superoxide-driven Fenton chemistry: Fe2+ + H2O2 --> Fe3+ + •OH + OH−) with the consequent generation of oxygen free radicals more than any other transition metal (Winterbourn, 1995), the focus of this section will be on abnormalities of brain iron metabolism and its involvement in schizophrenia.
Iron plays an important role in the human body. Almost two thirds of the total amount of iron is located in hemoglobin (Yuan et al., 1995). Most of the remaining iron is located in the liver, spleen, heart and brain. Iron is adept at catalyzing redox reactions within biological systems (Wright and Bacarelli, 2007). Transferrin and ferritin are iron-transporting proteins which also possess antioxidant properties (Loeffler et al., 1995; Nappi and Vass, 2000; Fisher et al., 2007; Madsen and Gitlin, 2007). Both proteins are synthesized in several tissues, including brain (Loeffler et al., 1995; Fisher et al., 2007; Madsen and Gitlin, 2007) and act as antioxidants by reducing the concentration of free ferrous ion (Loeffler et al., 1995). Several authors have reported that the levels of iron, ferritin and transferrin are reduced in the serum of patients with schizophrenia as compared to normal controls (Weiser et al., 1994; Kuloglu et al., 2003; Yanik et al., 2004). Other studies have reported no change in levels of iron in postmortem brains of schizophrenic patients (Casanova et al., 1990; Kornhuber et al., 1994).
A deficiency of maternal iron as a risk factor for schizophrenia spectrum disorders (SSDs) was recently evaluated (Insel et al., 2008). It was postulated that maternal iron deficiency during pregnancy, assessed via maternal hemoglobin concentration, may disrupt essential pathways and iron-dependent processes involving dopaminergic neurotransmission, myelination, and energy metabolism. Disturbances of these pathways during fetal development might heighten the susceptibility to schizophrenia in adulthood. This study showed that reduced maternal hemoglobin concentration was associated with a nearly 4-fold statistically significant increased rate of SSDs (Insel et al., 2008), hence suggesting that maternal iron deficiency may be a risk factor for SSDs among offspring.
In keeping with this notion, a recent study demonstrated the importance of hypoferremia (viz. a cytokine induced reduction of serum non-heme iron) in inducing behavioural and biochemical changes relevant to schizophrenia (Aguilar-Valles et al., 2010). The study used a rat model of localized injury induced by turpentine, which triggers the innate immune response and inflammation, in order to investigate the effects of maternal iron supplementation on the offspring’s dopamine function. Offspring of turpentine-treated mothers exhibited an enhanced behavioral sensitization to amphetamine following repeated exposure to this drug, when compared to control offspring. These behavioral changes were accompanied by increased baseline levels of tyrosine hydroxylase, dopamine and its metabolites, selectively in the nucleus accumbens (Aguilar-Valles et al., 2010). Interestingly, the behavioral and neurochemical changes were prevented by maternal iron supplementation. Given that schizophrenia is associated with increased subcortical dopamine, it is probable that abnormalities in fetal/maternal iron homeostasis may play a role in developmental processes that render the offspring more susceptible to schizophrenia.
Taken together, these results suggest that reduced iron levels may be associated with schizophrenia in a subset of patients. Because oxidative stress can be induced under situations of iron deficiency (Knutson et al., 2000; Casanueva and Viteri, 2003), free radical formation resulting from iron deficiency may lead to functional disturbances and foster genetic alterations that could in turn contribute to the development of schizophrenia.
10. Mitochondrial Dysfunction and Abnormal Energy Metabolism in Schizophrenia
Oxidative phosphorylation in the mitochondria generates superoxide anion. Furthermore, enzymatic oxidation of biogenic amines by monoamine oxidase in the mitochondrial outer membrane produces hydrogen peroxide. Damaged mitochondria not only produce more oxidants, but mitochondria are also vulnerable to oxidative stress (Kowlatowski and Vercesi, 1999). Notably, peroxidation of membrane lipids yields toxic aldehydes (Keller et al., 1997), which impair critical mitochondrial enzymes (Humphries and Szweda, 1998; Ben-Shachar and Laifenfeld, 2004; Martins-de-Souza et al., 2010). Other essential proteins are directly oxidized, yielding carbonyl and nitrated derivatives (Andreazza et al., 2010). Subsequently, increases in membrane permeability to calcium, other ionic imbalances, and impaired glucose metabolism (Hazlett et al., 2004) aggravate the energy imbalance.
Several studies have demonstrated that mitochondrial malfunction can lead to cellular degeneration (Calabrese et al., 2001; Martins-de-Souza et al., 2009, 2010) as a result of the formation of ROS/RNS (Lenaz, 2001). A disturbance of energy metabolism in mitochondria may play a role in the pathophysiology of schizophrenia (Prabakaran et al., 2004; Martins-de-Souza et al., 2009). Notably, a study using a combined transcriptomic, proteomic, metabolomic approach in addition to hierarchial clustering on human prefrontal cortex tissue in order to detect molecular signatures associated with schizophrenia found alterations in proteins associated with mitochondrial function and oxidative stress responses (Prabakaran et al., 2004). Other studies have also suggested increased lactate levels (Prabakaran et al., 2004), and mitochondrial dysfunction with concomitant defects in neuronal oxidative phosphorylation in schizophrenia (Ben-Shachar et al., 1999; Ben-Scachar and Laifenfeld, 2004; Karry et al., 2004; Prabakaran et al., 2004; Martins-de-Souza et al., 2009). Finally, abnormal mitochondrial morphology, size and density have also been reported in the brains of schizophrenic individuals (Ben-Shachar, 2002).
11. Inflammatory Response Induces Oxidative Stress in Schizophrenia
Maternal exposure to infection during pregnancy has been associated with an increased risk of offspring developing schizophrenia (Brown and Susser, 2002; Brown and Derkits, 2010). Although the epidemiological relationship between in utero infections and schizophrenia remain unclear, the maternal cytokine-associated inflammatory response to infection may be a crucial link, as the identity of the pathogen seems irrelevant (Gilmore and Jarskog, 1997; Buka et al., 2001; Pearce, 2001; Brown et al., 2004; Deverman and Patterson, 2009; Meyer et al., 2009; Patterson, 2009; Watanabe et al., 2010). The mechanism linking maternal immune infection to schizophrenia is suspected to occur as follows: maternally infected cells may promote an increased production of inflammatory cytokines that cross the placenta and then increase interleukin (IL)1β, IL-6, (Tumor necrosis factor) TNF-α and (Interferon) IFN-β among others, by fetal cells (Ohyama et al., 2004). DNA fragmentation may then be induced by free radical production associated with the increase in these cytokines, especially interferon-β. The impact of this damage on nuclear and mitochondrial DNA damage in the neuron could be even more severe due to the high neuronal energy consumption rate and the lack of cell turnover. Due to the positive feed-back loops formed in such a mechanism, the disease state could self-sustain and persist resulting in the progressive development of pathological features and clinical symptoms associated with schizophrenia.
In keeping with this notion, inflammatory responses induced by proinflammatory T cells provide a source of free radicals with the capacity to modify proteins, lipids, and nucleic acids that are potentially toxic for neurons. Important work has therefore been directed towards understanding the consequences and the mechanisms linked to T cell dysfunction in patients with schizophrenia (Henneberg et al., 1990; Sperner-Unterweger et al., 1999; Mazzarello et al., 2004; Rudolf et al., 2004; Maxeiner et al., 2009). Craddock and colleagues have recently shown a decreased activation of helper T cells in both unmedicated and minimally medicated schizophrenia patients as compared with controls (Craddock et al., 2007). To follow up on this indication that patients with schizophrenia exhibit physiological differences in T cell responses, Craddock and colleagues undertook a more systematic investigation of T cell proliferative responses by conducting a microarray analysis of differentially expressed genes in isolated T cells from schizophrenia patients and controls. Functional profiling revealed prominent transcript changes in categories pertaining to cell cycle machinery, intracellular signaling, metabolism and oxidative stress (Craddock et al., 2007). These results suggest that altered T cell response may correspond with oxidative stress in some patients with schizophrenia.
The effects of bacterial infections on triggering oxidative stress during fetal brain development have been evaluated by Lanté and colleagues who administered a lipopolysaccharide injection during pregnancy to rats 2 days before delivery (Lanté et al., 2007). This treatment triggered an oxidative stress response in the hippocampus of male fetuses, evidenced by damage to proteins (as indexed by a rapid rise in protein carbonylation) and by decreases in α-tocopherol levels and in the ratio of reduced/oxidized forms of glutathione (GSH/GSSG). In contrast, none of the biochemical changes observed in males were observed in female fetuses. The authors also showed that NMDA synaptic currents and LTP in addition to spatial recognition in the water maze, were impaired in male but not in female offspring exposed to immune activation by lipopolysaccharide in utero. The sex-dependent effects of lipopolysaccharide treatment are consistent with the impression that male schizophrenic patients seem to exhibit greater structural brain abnormalities (Flaum et al., 1990; Nopoulos et al., 1997) in addition to a more severe clinical profile compared to female patients (Flor-Henry, 1990; Aleman et al., 2003), especially in terms of cognitive deficits (Roy et al., 2001).
Interestingly, pretreatment with the antioxidant N-acetyl cysteine (NAC), a precursor of GSH, prevented the lipopolysaccharide induced changes in the biochemical markers of oxidative stress in male fetuses, and the delayed detrimental effects in male offspring, completely restoring both LTP in the hippocampus and spatial recognition performance (Lanté et al., 2007). Together these findings suggest that the antioxidant properties of NAC may provide an efficient supplement for the treatment of symptoms associated with schizophrenia.
12. Oligodendrocyte Dysfunction in Schizophrenia
Schizophrenia has long been considered a disorder consisting of a disconnection between different cortical areas (Friston and Frith, 1995; Stephan et al., 2006). Given that white matter constitutes the anatomical infrastructure for neural connectivity, it has been hypothesized that aberrant connectivity of brain regions may explain altered processing patterns documented by functional neuroimaging and electrophysiology studies in patients with schizophrenia (Bartzokis, 2002; Hulsoff et al., 2004). Consistent with this notion, a reduced density and compromised morphology of oligodendrocytes as well as signs of deviant myelination have been observed in patients suffering from schizophrenia (Uranova et al., 2004, 2007). Oligodendrocytes are the predominant iron-containing cells of the brain (Connor, 1994, 1995). They also contain reduced level of GSH, glutathione peroxidase and mitochondrial manganese superoxide dismutase (Juurlink et al., 1998). In addition, oligodendrocytes are vulnerable to intracellular GSH depletion (Back et al., 1998; Cammer et al., 2002a), which may result in cell death (Oka et al., 1993). This sensitivity to GSH depletion is ameliorated by free radical scavengers such as α-tocopherol (Cammer et al., 2002a,b). Also, cell death can be prevented by NAC (Cammer et al., 2002b). Taken together, oligodendrocytes appear to be highly susceptible to oxidative stress-induced damage, which may lead to myelin deficiency.
Instead of compromising oligodendrocyte functions, oxidative stress may also directly damage myelin. For instance, peroxide and hydroxyl radical can react with the polyunsaturated fatty acids that are present in myelin sheaths, directly triggering demyelination (Halliwell, 1992). It has recently been reported that free radical-related molecules such as NO and peroxynitrite generated by activated microglia (Merrill et al., 1993; Li et al., 2005) in addition to inflammatory cytokines such as TNF-α and IFN-γ (Buntinx et al., 2004) result in cytotoxicity toward oligodendroctyes. Furthermore, TNF-α has been shown to compromise the growth of oligodendrocytes and the expression of mRNA for myelin basic protein in cultures (Cammer and Zhang., 1999). The same study showed that TNF-α also inhibited the survival and proliferation of the oligodendrocyte progenitors and their subsequent differentiation into mature myelinating phenotypes. In summary, impaired redox function and inflammatory induction, in combination with previously described deficits in the expression of oligodendrocyte-related genes (Hakak et al., 2001; McCullumsmith et al., 2007; Iwamoto et al., 2008), suggest a multifactorial pathway linking oxidative stress to the abnormalities of myelination observed in schizophrenia.
13. Redox Dysregulation of NMDA-Receptor Mediated Transmission in Parvalbumin-Containing Interneurons
Although the evidence from experimental studies and from postmortem investigation shows that NMDA receptor dysfunction has relevance to schizophrenia, it is still debatable as to which specific NMDA receptor subunits are involved in the cascade of molecular events leading to the neuronal deficits and dysfunction associated with schizophrenia.
Postmortem evidence from human brain has shown that the expression of the NR2A subunit is reduced in subjects with schizophrenia (Beneyto and Meador-Woodruff, 2008). In fact, NR2A expression levels have been shown to be reduced in glutamic acid decarboxylase (GAD) 67 positive neurons in subjects with schizophrenia (Woo et al., 2004, 2008). The reduced NR2A expression is possibly due to a reduced NMDA receptor activity at the affected interneurons because it has been shown in other studies that NR2A expression seems to be down-regulated by reduced glutamatergic input and vice versa (Kinney et al., 2006; Behrens et al., 2007; Xi et al., 2009).
Receptors that contain the NR2A subunit are tightly regulated by redox-active agents including GSH (Kohr et al., 1994; Choi and Lipton, 2000; Lipton et al., 2002) and play a pivotal role in the maintenance of the function of PV interneurons (Kinney et al., 2006). In line with these observations, treatment of intermediate duration with the NMDA receptor antagonist MK-801 has been shown to almost completely down-regulate the expression of NMDA receptor subunits and PV in microdissected PV-positive interneurons in the rat frontal cortex (Xi et al., 2009). In this context, we have recently found that, in schizophrenia, the expression of the mRNA for the NR2A subunit of the NMDA receptor in PV neurons also appears to be decreased (Bitanihirwe et al., 2009). This latter finding supports the notion that reduced glutamatergic inputs to PV neurons via the NMDA receptor contributes to the down-regulation of PV and GAD67 messenger RNA transcripts (Kinney et al., 2006; Behrens et al., 2007) and hence plays a central role in the functional disturbances of PV neurons in schizophrenia (Olney and Farber 1995; Lisman et al., 2008; Gonzalez-Burgos et al., 2010; Woo et al., 2010).
Behrens and colleagues have recently shown that prolonged exposure to ketamine, an NMDA receptor antagonist, induces the release of the pro-inflammatory cytokine IL-6, which results in a subsequent induction and activation of the electron transporter and ROS-generating NADPH oxidase (Nox) enzyme (Behrens et al., 2007). Superoxide overproduction as a result of NOX activation could result in the chain of events that initiates processes resulting in reduced expression of GABAergic markers and the consequent loss of inhibitory capacity in PV interneurons (Behrens et al., 2007, 2008; Dugan et al., 2009). Given that ketamine augmented NOX expression in the mouse brain, and that both apocynin (an inhibitor of NOX activity) pretreatment and NOX deficiency prevented ROS generation and the decrease of PV expressing interneurons, NOX activation was indicated as a major contributor to the pathogenesis of ketamine-induced psychosis and possibly also to schizophrenia (Behrens et al., 2007, 2008; Sorce et al., 2010). Together these findings provide evidence for a potential pathological link between NMDA hypofunction, enhanced neuronal production of IL-6 and oxidative stress, which may in turn be associated with the GABAergic dysfunction often observed in the brain of patients with schizophrenia (Behrens et al., 2007, 2008; Behrens and Sejnowski, 2009; Dugan et al., 2009).
14. Current Therapeutic Modalities
Therapy using antioxidants has the potential to prevent, delay, or ameliorate many neurologic disorders including schizophrenia (Delanty and Dichter, 2000, Moosmann and Behl, 2002, Ng et al., 2008, Dodd et al., 2008; Seybolt, 2010). For example, supplementation of omega-3 poly unsaturated fatty acids in combination with ascorbic acid and α-tocopherol is effective in improving psychopathology (viz. increased scores on the Brief Psychiatric Rating and the PANNS) in chronic-medicated schizophrenic patients (Arvindakshan et al., 2003). Similarly, it has been reported that treatment with Ginkgo biloba extract (a powerful flavonoid antioxidant) and haloperidol results in better PANNS scores (Zhang et al., 2001a), enhances the effectiveness of the antipsychotic and reduces some extrapyramidal side effects (Zhang et al., 2001b). Atypical antipsychotic medication with ascorbic acid (Michael et al., 2002; Dakhale et al., 2005), α-tocopherol (Michael et al., 2002), and lipoic acid (Kim et al., 2008) have also been shown to improve the clinical outcome of patients with schizophrenia.
Moreover, treatment with NAC the rate limiting factor in the synthesis of GSH (Dodd et al., 2008), has been shown to improve core symptoms of schizophrenia (Berk et al., 2008; Lavoie et al., 2008; Bulut et al., 2009). Specifically, administration of NAC has been shown to improve cognitive functioning as indexed by mismatch negativity (MMN) (Lavoie et al., 2008). Mismatch negativity is an auditory event related potential component that is elicited when a sequence of repetitive standard sounds is interrupted infrequently by physically deviant (e.g. pitch, intensity, location, duration), “oddball” stimuli. The MMN occurs rapidly following deviant stimuli; the response begins 50 ms following the onset of the deviation and peaks after an additional 100–150 ms. Physiologically, the MMN is the first measurable brain response component that differentiates between usual and unusual auditory stimuli and shares many of the properties of an automatic, memory-based comparison process (Naatanen et al., 1989). Using this task, a differential response to deviant stimuli compared to distracters (i.e. infrequent tones of a higher pitch) presented in an auditory oddball paradigm, has been shown to be impaired in schizophrenia (Shelley et al., 1991; Javitt et al.,1993; Catts et al., 1995; Shutara et al., 1996; et al., 1998). This impairment may in part be mediated by NMDA receptor hypofunction (Javitt and Zukin, 1991; Umbricht et al., 2000; Coyle, 2006), which, in turn, may result from GSH deficit (Light and Braff, 2005; Steullet et al., 2006). In this context, it has been suggested that NAC treatment could increase GSH levels in schizophrenia patients (Lavoie et al., 2008), restoring normal GSH levels and thus improving NMDA reception functioning, which is thought to be reflected by the amplitude of the MMN (Javitt et al, 1998). Treatment with antioxidants may therefore serve as an effective therapeutic strategy in schizophrenia.
15. Conclusion
There is growing evidence supporting increased oxidative stress in schizophrenia with likely contributions from environment, genetic and immunological factors. However, the exact molecular mechanisms are yet to be determined. Indeed, the maintenance of redox balance within cells is a primary component of homeostasis underlying neuronal survival. It may not be too surprising therefore that any process that leads to a disruption of the redox balance can drastically interfere with a range of other biochemical processes and result in neuronal deficits and dysfunction.
Compared to other organs in the body, brain tissue is more vulnerable to oxidative stress due to its high oxygen consumption, high content of polyunsaturated fatty acids and low levels of antioxidant enzymes. Even so, neuronal cells are endowed with a range of protective mechanisms. The difficulty is that these may be overwhelmed by additional oxidative load and that a failure of protective mechanisms may allow endogenous oxidative processes to damage cells and result in the pathophysiology of neurological disease. Pathways and mechanisms that control oxidative stress such as GSH metabolism, can modulate pathology and cognitive deficits in rodent models of schizophrenia (Cabuncgal et al., 2007; Dean et al., 2000b, Steullet et al., 2010). However, the role of these conserved pathways in the onset and progression of schizophrenia in humans is still unclear. The resolution of this basic issue will depend on future clinical interventions that target these pathways to ascertain their role in cognitive dysfunction and neuropathogenesis. Tackling of the oxidative stress involvement offers a novel therapeutic target for schizophrenia. However, only when the mechanisms and involvement of oxidative stress in the pathogenesis of schizophrenia are understood, will approaches to antioxidant therapy be designed effectively and targeted. It is thus noteworthy that preliminary results of some clinical trials have suggested improved cognitive functioning in individuals with schizophrenia who receive antioxidant therapy, particularly NAC (Berk et al., 2008; Lavoie et al., 2008; Bulut et al., 2009). Hence, identifying viable therapeutic strategies to restore redox balance and the physiological disturbances that result from oxidative stress provide an exciting opportunity for the treatment and ultimately prevention of schizophrenia.
Research highlights.
Evidence of oxidative stress in schizophrenia.
Redox dysregulation during neurodevelopment may play a role in schizophrenia.
Antioxidants may prove to be a useful adjunctive treatment for schizophrenia.
Acknowledgements
We thank Dr. Jean-Charles Paterna and Dr. Helen Pothuizen for carefully reading through the manuscript and providing helpful comments. We are also grateful to two anonymous reviewers, whose comments have greatly improved the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adler LE, Hoffer LJ, Griffith J, Waldo M,C, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32:607–16. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Aguilar-Valles A, Flores C, Luheshi GN. Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS One. 2010;5:e10967. doi: 10.1371/journal.pone.0010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Bunney WE, Jr., Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry. 1993a;50:169–77. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Viñuela A, Kim JJ, Potkin SG, Bunney WE, Jr., Jones EG. Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry. 1993b;50:178–87. doi: 10.1001/archpsyc.1993.01820150016002. [DOI] [PubMed] [Google Scholar]
- Akyol O, Yanik M, Elyas H, Namli M, Canatan H, Akin H, Yuce H, Yilmaz HR, Tutkun H, Sogut S, Herken H, Ozyurt H, Savas HA, Zoroglu SS. Association between Ala-9Val polymorphism of Mn-SOD gene and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:123–31. doi: 10.1016/j.pnpbp.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–71. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Brain Res Rev. 2000;31:106–112. doi: 10.1016/s0165-0173(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Andreazza A,C, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67(4):360–8. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- Applebaum J, Shimon H, Sela BA, Belmaker RH, Levine J. Homocysteine levels in newly admitted schizophrenic patients. J Psychiatr Res. 2004;38:413–6. doi: 10.1016/j.jpsychires.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr. Res. 2003;62:195–204. doi: 10.1016/s0920-9964(02)00284-0. [DOI] [PubMed] [Google Scholar]
- Baba H, Suzuki T, Arai H, Emson PC. Expression of nNOS and soluble guanylate cyclase in schizophrenic brain. Neuroreport. 2004;15:677–80. doi: 10.1097/00001756-200403220-00020. [DOI] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Brain Res. Rev. 1997;25:335–58. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27:672–83. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J. Neurosci. 2008;28:13957–66. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–86. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int. Rev. Neurobiol. 2004;59:273–96. doi: 10.1016/S0074-7742(04)59011-6. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D. Mitochondrial dysfunction in schizophrenia: a possible linkage to dopamine. J. Neurochem. 2002;83:1241–51. doi: 10.1046/j.1471-4159.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Zuk R, Gazawi H, Reshef A, Sheinkman A, Klein E. Increased mitochondrial complex I activity in platelets of schizophrenic patients. Int. J. Neuropsychopharmacol. 1999;2:245–253. doi: 10.1017/S1461145799001649. [DOI] [PubMed] [Google Scholar]
- Berg D, Youdim MB, Riederer P. Redox imbalance. Cell Tissue Res. 2004;318(1):201–13. doi: 10.1007/s00441-004-0976-5. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol. Psychiatry. 2008;64:361–8. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Krell D, Braunewell KH, Baumann B, Gundelfinger ED, Diekmann S, Danos P, Bogerts B. Increased number of nitric oxide synthase immunoreactive Purkinje cells and dentate nucleus neurons in schizophrenia. J. Neurocytol. 2001;30:661–70. doi: 10.1023/a:1016520932139. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, Falkai P, Bogerts B. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83:867–75. doi: 10.1016/s0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Lim MP, Kelley JF, Kaneko T, Woo TU. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MB., Jr. 5-Hydroxyindoleacetic acid (5HIAA) and homovanillic acid (HVA) following probenecid in acute psychotic patients treated with phenothiazines. Psychopharmacologia. 1973;28:309–18. doi: 10.1007/BF00422751. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Bredt DS. Synaptic signaling by nitric oxide. Curr. Opin. Neurobiol. 1997;7:374–8. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- Brown AS, Bottiglieri T, Schaefer CA, Quesenberry CP, Jr., Liu L, Bresnahan M, Susser ES. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch. Gen. Psychiatry. 2007;64:31–9. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr. Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- Brzustowicz LM, Simone J, Mohseni P, Hayter JE, Hodgkinson KA, Chow EW, Bassett AS. Linkage disequilibrium mapping of schizophrenia susceptibility to the CAPON region of chromosome 1q22. Am J Hum Genet. 2004;74(5):1057–63. doi: 10.1086/420774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav. Immun. 2001;15:411–20. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, Stinissen P, Ameloot M. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J. Neurosci. Res. 2004;76:834–845. doi: 10.1002/jnr.20118. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–9. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Preissmann D, Delseth C, Cuénod M, Do KQ, Schenk F. Transitory glutathione deficit during brain development induces cognitive impairment in juvenile and adult rats: relevance to schizophrenia. Neurobiol. Dis. 2007;26:634–45. doi: 10.1016/j.nbd.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Kahler LA. Free radical mechanisms in schizophrenia and tardive dyskinesia. Neurosci. Biobehav. Rev. 1994;18:457–67. doi: 10.1016/0149-7634(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998;273:11401–4. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Guagliano E, Sapienza M, Mancuso C, Butterfield DA, Stella AM. Redox regulation of cellular stress response in neurodegenerative disorders. Ital. J. Biochem. 2006;55:263–82. [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB. Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem. Res. 2001;26:739–64. doi: 10.1023/a:1010955807739. [DOI] [PubMed] [Google Scholar]
- Cammer W. Apoptosis of oligodendrocytes in secondary cultures from neonatal rat brains. Neurosci. Lett. 2002a;327:123–7. doi: 10.1016/s0304-3940(02)00392-0. [DOI] [PubMed] [Google Scholar]
- Cammer W. Protection of cultured oligodendrocytes against tumor necrosis factor-alpha by the antioxidants coenzyme Q(10) and N-acetyl cysteine. Brain Res. Bull. 2002b;58:587–92. doi: 10.1016/s0361-9230(02)00830-4. [DOI] [PubMed] [Google Scholar]
- Cammer W, Zhang H. Maturation of oligodendrocytes is more sensitive to TNF alpha than is survival of precursors and immature oligodendrocytes. J. Neuroimmunol. 1999;97:37–42. doi: 10.1016/s0165-5728(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Carpenter WT., Jr. The deficit syndrome. Am. J. Psychiatry. 1994;151:327–9. doi: 10.1176/ajp.151.3.327. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Waldman IN, Kleinman JE. A postmortem quantitative study of iron in the globus pallidus of schizophrenic patients. Biol Psychiatry. 1990;27:143–9. doi: 10.1016/0006-3223(90)90644-h. [DOI] [PubMed] [Google Scholar]
- Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. J. Nutr. 2003;133:1700S–1708S. doi: 10.1093/jn/133.5.1700S. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Smith MA, Nunomura A, Harris PL, Perry G. Is increased redox-active iron in Alzheimer disease a failure of the copper-binding protein ceruloplasmin? Free. Radic. Biol. Med. 1999;26:1508–12. doi: 10.1016/s0891-5849(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Catts SV, Shelley AM, Ward PB, Liebert B, McConaghy N, Andrews S, Michie PT. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am. J. Psychiatry. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13:171–81. doi: 10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol. Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, Kim HJ, Rodriguez-Iturbe B, Vaziri ND. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am J Physiol Renal Physiol. 2009;297(1):F106–13. doi: 10.1152/ajprenal.00126.2009. [DOI] [PubMed] [Google Scholar]
- Choi YB, Lipton SA. Redox modulation of the NMDA receptor. Cell Mol. Life. Sci. 2000;57:1535–41. doi: 10.1007/PL00000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Pavlick G, Karli D, Menzies SL, Palmer C. A histochemical study of iron-positive cells in the developing rat brain. J. Comp. Neurol. 1995;355:111–123. doi: 10.1002/cne.903550112. [DOI] [PubMed] [Google Scholar]
- Connor JR. Iron acquisition and expression of iron regulatory proteins in the developing brain: manipulation by ethanol exposure, iron deprivation and cellular dysfunction. Dev. Neurosci. 1994;16:233–47. doi: 10.1159/000112115. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res. Brain Res. Rev. 2000;32:476–509. doi: 10.1016/s0165-0173(00)00018-7. [DOI] [PubMed] [Google Scholar]
- Coward JK, Slixz EP, Wu FY. Kinetic studies on catechol O-methyltransferase. Product inhibition and the nature of the catechol binding site. Biochemistry. 1973;12:2291–7. doi: 10.1021/bi00736a017. [DOI] [PubMed] [Google Scholar]
- Coward JK, D’Urso-Scott M, Sweet WD. Inhibition of catechol-O-methyltransferase by S-adenosylhomocysteine and S-adenosylhomocysteine sulfoxide, a potential transition-state analog. Biochem. Pharmacol. 1972;21:1200–3. doi: 10.1016/0006-2952(72)90114-1. [DOI] [PubMed] [Google Scholar]
- Coward JK, Sweet WD. Analogs of S-adenosylhomocysteine as potential inhibitors of biological transmethylation. Synthesis and biological activity of homocysteine derivatives bridged to adenine. J. Med. Chem. 1972;15:381–4. doi: 10.1021/jm00274a013. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 2006:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3(5):241–53. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Craddock RM, Lockstone HE, Rider DA, Wayland MT, Harris LJ, McKenna PJ, Bahn S. Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS One. 2007;2:e692. doi: 10.1371/journal.pone.0000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Nishiguchim N, Yanagi M, Fukutake M, Mouri K, Kitamura N, Hashimoto T, Shirakawa O, Hishimoto A. A putative cis-acting polymorphism in the NOS1 gene is associated with schizophrenia and NOS1 immunoreactivity in the postmortem brain. Schizophr Res. 2010 doi: 10.1016/j.schres.2010.05.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dadheech G, Mishra S, Gautam S, Sharma P. Evaluation of antioxidant deficit in schizophrenia. Indian J. Psychiatry. 2008;50:16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology (Berl) 2005;182:494–8. doi: 10.1007/s00213-005-0117-1. [DOI] [PubMed] [Google Scholar]
- Dakhale G, Khanzode S, Khanzode S, Saoji A, Khobragade L, Turankar A. Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–9. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem. Soc. Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- Dean O, Bush AI, Berk M, Copolov DL, den Buuse MV. Interaction of glutathione depletion and psychotropic drug treatment in prepulse inhibition in rats and mice. Pharmacol Biochem Behav. 2010 doi: 10.1016/j.pbb.2010.08.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dean OM, van den Buuse M, Bush AI, Copolov DL, Ng F, Dodd S, Berk M. A role for glutathione in the pathophysiology of bipolar disorder and schizophrenia? Animal models and relevance to clinical practice. Curr. Med. Chem. 2009a;16:2965–76. doi: 10.2174/092986709788803060. [DOI] [PubMed] [Google Scholar]
- Dean O, Bush AI, Berk M, Copolov DL, van den Buuse M. Glutathione depletion in the brain disrupts short-term spatial memory in the Y-maze in rats and mice. Behav. Brain Res. 2009b;198:258–62. doi: 10.1016/j.bbr.2008.11.017. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Abrahamson EE, Taffe KM, Dixon CE, Kochanek PM, Ikonomovic MD. Effects of post-injury hypothermia and nerve growth factor infusion on antioxidant enzyme activity in the rat: implications for clinical therapies. J. Neurochem. 2004;90:998–1004. doi: 10.1111/j.1471-4159.2004.02575.x. [DOI] [PubMed] [Google Scholar]
- Delanty N, Dichter MA. Antioxidant therapy in neurologic disease. Arch. Neurol. 2000;57:1265–70. doi: 10.1001/archneur.57.9.1265. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Is immune dysfunction associated with schizophrenia? A review of the data. Psychopharmacol. Bull. 1984;20:509–13. [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dietrich-Muszalska A, Olas B. Isoprostenes as indicators of oxidative stress in schizophrenia. World J. Biol. Psychiatry. 2009;10:27–33. doi: 10.1080/15622970701361263. [DOI] [PubMed] [Google Scholar]
- Do KQ, Gysin R, Kraftsik R, Boulat O, Bovet P, Conus P, Emily CK, Polari A, Steullet P, Preisig M, Teichmann T, Cuénod M. Genetic dysregulation of glutathione synthesis predicts alteration of plasma thiol redox status in schizophrenia. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3463. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 2009;19:220–30. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuénod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 2000;12:3721–8. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008;8:1955–62. doi: 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- Downen M, Zhao ML, Lee P, Weidenheim KM, Dickson DW, Lee SC. Neuronal nitric oxide synthase expression in developing and adult human CNS. J. Neuropathol. Exp. Neurol. 1999;58:12–21. doi: 10.1097/00005072-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J. Neurosci. Res. 2005;79:157–65. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000;62:649–71. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, Behrens MM. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MD, Cooke MS. Factors contributing to the outcome of oxidative damage to nucleic acids. Bioessays. 2004;26:533–42. doi: 10.1002/bies.20027. [DOI] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang KY, Huganir RL, Valle D, Pulver AE. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am. J. Hum. Genet. 2005;77:918–36. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendri C, Mechri A, Khiari G, Othman A, Kerkeni A, Gaha L. Oxidative stress involvement in schizophrenia pathophysiology: a review. Encephale. 2006;32:244–52. doi: 10.1016/s0013-7006(06)76151-6. [DOI] [PubMed] [Google Scholar]
- Féron F, Perry C, McGrath JJ, Mackay-Sim A. New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol Head Neck Surg. 1998;124(8):861–6. doi: 10.1001/archotol.124.8.861. [DOI] [PubMed] [Google Scholar]
- Fisher J, Devraj K, Ingram J, Slagle-Webb B, Madhankumar AB, Liu X, Klinger M, Simpson IA, Connor JR. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am. J. Physiol. Cell. Physiol. 2007;293:C641–9. doi: 10.1152/ajpcell.00599.2006. [DOI] [PubMed] [Google Scholar]
- Flaum M, Arndt S, Andreasen NC. The role of gender in studies of ventricle enlargement in schizophrenia: a predominantly male effect. Am. J. Psychiatry. 1990;147:1327–32. doi: 10.1176/ajp.147.10.1327. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P. Influence of gender in schizophrenia as related to other psychopathological syndromes. Schizophr. Bull. 1990;16:211–27. doi: 10.1093/schbul/16.2.211. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–95. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol. Neurosurg. Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2010;16:1–8. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- Genova ML, Pich MM, Biondi A, Bernacchia A, Falasca A, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. Mitochondrial production of oxygen radical species and the role of Coenzyme Q as an antioxidant. Exp Biol Med (Maywood) 2003;228(5):506–13. doi: 10.1177/15353702-0322805-14. [DOI] [PubMed] [Google Scholar]
- Gibbs SM. Regulation of neuronal proliferation and differentiation by nitric oxide. Mol. Neurobiol. 2003;27:107–20. doi: 10.1385/MN:27:2:107. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF. Exposure to infection and brain development: cytokines in the pathogenesis of schizophrenia. Schizophr. Res. 1997;24:365–7. doi: 10.1016/s0920-9964(96)00123-5. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: implications for psychiatric illness. Am. J. Psychiatry. 1993;150:546–53. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am. J. Psychiatry. 1992;149:1189–94. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory. In: Carroll BJ, Barrrett JE, editors. Psychopathology and the Brain. Raven Press; New York: 1991. pp. 1–23. [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr. Psychiatry Rep. 2010;12:335–44. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima G, Benz B, Parpura V, Cuénod M, Do KQ. Dopamine-induced oxidative stress in neurons with glutathione deficit: implication for schizophrenia. Schizophr. Res. 2003;62:213–24. doi: 10.1016/s0920-9964(02)00405-x. [DOI] [PubMed] [Google Scholar]
- Gysin R, Riederer IM, Cuénod M, Do KQ, Riederer BM. Skin fibroblast model to study an impaired glutathione synthesis: consequences of a genetic polymorphism on the proteome. Brain Res. Bull. 2009;79:46–52. doi: 10.1016/j.brainresbull.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P, Tosic M, Werge T, Cuénod M, Do KQ. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc. Natl. Acad. Sci. U S A. 2007;104:16621–6. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JC. Free Radicals in Biology and Medicine. Oxford Univ. Press; London: 1998. [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–23. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol. Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hashimoto K, Matsuzawa D, Shimizu E, Sekine Y, Inada T, Ozaki N, Iwata N, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Iyo M. A functional glutathione S-transferase P1 gene polymorphism is associated with methamphetamine-induced psychosis in Japanese population. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;135B:5–9. doi: 10.1002/ajmg.b.30164. [DOI] [PubMed] [Google Scholar]
- Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J. Neurochem. 1995;64:919–24. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Kemether E, Bloom R, Platholi J, Brickman AM, Shihabuddin L, Tang C, Byne W. Abnormal glucose metabolism in the mediodorsal nucleus of the thalamus in schizophrenia. Am J Psychiatry. 2004;161(2):305–14. doi: 10.1176/appi.ajp.161.2.305. [DOI] [PubMed] [Google Scholar]
- Heinecke J, Rosen H, Suzuki L, Chait A. The role of sulphurcontaining amino acids in superoxide production and modification of low density lipoprotein by arterial smooth muscle cells. J. Biol. Chem. 1987;262:10098–10103. [PubMed] [Google Scholar]
- Henneberg A, Riedl B, Dumke HO, Kornhuber HH. T-lymphocyte subpopulations in schizophrenic patients. Eur. Arch. Psychiatry Neurol. Sci. 1990;239:283–4. doi: 10.1007/BF01735051. [DOI] [PubMed] [Google Scholar]
- Herken H, Uz E, Ozyurt H, Söğüt S, Virit O, Akyol O. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol. Psychiatry. 2001;6:66–73. doi: 10.1038/sj.mp.4000789. [DOI] [PubMed] [Google Scholar]
- Hindley S, Juurlink BH, Gysbers JW, Middlemiss PJ, Herman MA, Rathbone MP. Nitric oxide donors enhance neurotrophin-induced neurite outgrowth through a cGMP-dependent mechanism. J. Neurosci. Res. 1997;47:427–39. [PubMed] [Google Scholar]
- Hirose K, Chan PH. Blockade of glutamate excitotoxicity and its clinical applications. Neurochem. Res. 1993;18:479–83. doi: 10.1007/BF00967252. [DOI] [PubMed] [Google Scholar]
- Hölscher C, Rose SP. An inhibitor of nitric oxide synthesis prevents memory formation in the chick. Neurosci. Lett. 1992;145:165–7. doi: 10.1016/0304-3940(92)90012-v. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, Cahn W, Collins DL, Evans AC, Kahn RS. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. Neuroimage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37(45):15835–41. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch. Gen. Psychiatry. 2008;65:1136–44. doi: 10.1001/archpsyc.65.10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Ueda J, Bundo M, Nakano Y, Kato T. Effect of a functional single nucleotide polymorphism in the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene on the expression of oligodendrocyte-related genes in schizophrenia. Psychiatry Clin. Neurosci. 2008;62:103–108. doi: 10.1111/j.1440-1819.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–95. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol. Med. Monogr. Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J. Nutr. 2002;132:2361S–2366S. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- Janáky R, Varga V, Oja SS, Saransaari P. Release of [3H]GABA evoked by glutamate agonists from hippocampal slices: effects of dithiothreitol and glutathione. Neurochem. Int. 1994;24:575–82. doi: 10.1016/0197-0186(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalogr. Clin. Neurophysiol. 1998;108:143–153. doi: 10.1016/s0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc. Natl. Acad. Sci. U S A. 1996;93:11962–7. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG., Jr. Impairment of early cortical processing in schizophrenia: an event-related potential confirmation study. Biol. Psychiatry. 1993;33:513–519. doi: 10.1016/0006-3223(93)90005-x. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, Kumar A, Nestler EJ, Akbarian S, Beckel-Mitchener AC. Epigenetics in the nervous system. J. Neurosci. 2008;28:11753–9. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Svensson L. Nitric oxide synthase inhibition blocks phencyclidine-induced behavioural effects on prepulse inhibition and locomotor activity in the rat. Psychopharmacology (Berl) 1997;131:167–73. doi: 10.1007/s002130050280. [DOI] [PubMed] [Google Scholar]
- Jones BG, Rose FA, Tudball N. Lipid peroxidation and homocysteine induced toxicity. Atherosclerosis. 1994;105:165–70. doi: 10.1016/0021-9150(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–8. doi: 10.1002/(sici)1098-1136(199804)22:4<371::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. J. Neurosci. 2003;23:269–76. doi: 10.1523/JNEUROSCI.23-01-00269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner J, Harel S, Granit R. Nitric oxide as an antioxidant. Arch. Biochem. Biophys. 1991;289:130–6. doi: 10.1016/0003-9861(91)90452-o. [DOI] [PubMed] [Google Scholar]
- Karry R, Klein E, Ben-Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol. Psychiatry. 2004;55:676–84. doi: 10.1016/j.biopsych.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Karson CN, Griffin WS, Mrak RE, Husain M, Dawson TM, Snyderm S,H, Moore NC, Sturner WQ. Nitric oxide synthase (NOS) in schizophrenia: increases in cerebellar vermis. Mol. Chem. Neuropathol. 1996;27:275–84. doi: 10.1007/BF02815109. [DOI] [PubMed] [Google Scholar]
- Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;80(3):685–96. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Kim E, Park DW, Choi SH, Kim JJ, Cho HS. A preliminary investigation of alpha-lipoic acid treatment of antipsychotic drug-induced weight gain in patients with schizophrenia. J. Clin. Psychopharmacol. 2008;28:138–46. doi: 10.1097/JCP.0b013e31816777f7. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J. Neurosci. 2007;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J. Neurosci. 2006;26:1604–15. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamer D, Engel JA, Svensson L. The nitric oxide synthase inhibitor, L-NAME, block phencyclidine-induced disruption of prepulse inhibition in mice. Psychopharmacology (Berl) 2001;156:182–6. doi: 10.1007/s002130100783. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–8. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Knutson MD, Walter PB, Ames BN, Viteri FE. Both iron deficiency and daily iron supplements increase lipid peroxidation in rats. 2000;130:621–8. doi: 10.1093/jn/130.3.621. [DOI] [PubMed] [Google Scholar]
- Kodavali CV, Bamne MN, Nimgaonkar VL. Genetic Association Studies Of Antioxidant Pathway Genes And Schizophrenia. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3508. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002;30:620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Konat GW. H2O2-induced higher order chromatin degradation: a novel mechanism of oxidative genotoxicity. J. Biosci. 2003;28:57–60. doi: 10.1007/BF02970132. [DOI] [PubMed] [Google Scholar]
- Konat GW. Higher order chromatin degradation: implications for neurodegeneration. Neurochem. Res. 2002;27:1447–51. doi: 10.1023/a:1021688119574. [DOI] [PubMed] [Google Scholar]
- Köhr G, Eckardt S, Lüddens H, Monyer H, Seeburg PH. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 1994;12:1031–40. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Lange KW, Kruzik P, Rausch WD, Gabriel E, Jellinger K, Riederer P. Iron, copper, zinc, magnesium, and calcium in postmortem brain tissue from schizophrenic patients. Biol. Psychiatry. 1994;36:31–4. doi: 10.1016/0006-3223(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. 1999;26:463–71. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Anand A, Moghaddam B. Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch. Gen. Psychiatry. 2002;59:663–4. doi: 10.1001/archpsyc.59.7.663. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kuloglu M, Atmaca M, Ustündag B, Canatan H, Gecici O, Tezcan E. Serum iron levels in schizophrenic patients with or without akathisia. Eur. Neuropsychopharmacol. 2003;13:67–71. doi: 10.1016/s0924-977x(02)00073-1. [DOI] [PubMed] [Google Scholar]
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell. Biochem. Funct. 2002;20:171–5. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- Kunz M, Gama CS, Andreazza AC, Salvador M, Ceresér KM, Gomes FA, Belmonte-de-Abreu PS, Berk M, Kapczinski F. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol. Psychiatry. 2008;32:1677–81. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Lanté F, Meunier J, Guiramand J, Maurice T, Cavalier M, de Jesus Ferreira MC, Aimar R, Cohen-Solal C, Vignes M, Barbanel G. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic. Biol. Med. 2007;42:1231–45. doi: 10.1016/j.freeradbiomed.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuénod M, Buclin T, Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–99. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. Reduced plasma nitric oxide metabolites before and after antipsychotic treatment in patients with schizophrenia compared to controls. Shizophre. Res. 2008;104:36–43. doi: 10.1016/j.schres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–64. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta. Psychiatr. Scand. Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophreni as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Li HC, Chen QZ, Ma Y, Zhou JF. Imbalanced free radicals and antioxidant defense systems in schizophrenia: a comparative study. J. Zhejiang. Univ. Sci. B. 2006;7:981–6. doi: 10.1631/jzus.2006.B0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl. Acad. Sci. USA. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res. Brain Res. Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch. Gen. Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, Bankston LA. Cysteine regulation of protein function--as exemplified by NMDA-receptor modulation. Trends Neurosci. 2002;25:474–80. doi: 10.1016/s0166-2236(02)02245-2. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler DA, Connor JR, Juneau PL, Snyder BOS, Kanaley L, DeMaggio AJ, Nguyen H, Brickman CM, Lewitt PA. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J. Neurochem. 1995;65:710–724. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Hull EM. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport. 1993;5:87–9. doi: 10.1097/00001756-199310000-00024. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–91. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu. Rev. Neurosci. 2007;30:317–37. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S, Scheffer R, Correnti EE, Mahadik JS. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol. Psychiatry. 1998;43:674–9. doi: 10.1016/s0006-3223(97)00282-5. [DOI] [PubMed] [Google Scholar]
- Manowitz P, Gilmour DG, Racevskis J. Low plasma tryptophan levels in recently hospitalized schizophrenics. Biol. Psychiatry. 1973;6:109–18. [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Harris LW, Guest PC, Bahn S. The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3459. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Gattaz WF, Schmitt A, Novello JC, Marangoni S, Turck CW, Dias-Neto E. Proteome analysis of schizophrenia patients Wernicke’s area reveals an energy metabolism dysregulation. BMC Psychiatry. 2009;9:17. doi: 10.1186/1471-244X-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson E, O’Shea B. Smoking and malignancy in schizophrenia. Br. J. Psychiatry. 1984;145:429–32. doi: 10.1192/bjp.145.4.429. [DOI] [PubMed] [Google Scholar]
- Masukawa T, Sai M, Tochino Y. Methods for depleting brain glutathione. Life Sci. 1989;44(6):417–24. doi: 10.1016/0024-3205(89)90266-x. [DOI] [PubMed] [Google Scholar]
- Mathew SV, Law AJ, Lipska BK, Dávila-García MI, Zamora ED, Mitkus SN, Vakkalanka R, Straub RE, Weinberger DR, Kleinman JE, Hyde TM. Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum. Mol. Genet. 2007;16:2921–32. doi: 10.1093/hmg/ddm253. [DOI] [PubMed] [Google Scholar]
- Matigian N, Abrahamsen G, Sutharsan R, Cook AL, Vitale AM, Nouwens A, Bellette B, An J, Anderson M, Beckhouse AG, Bennebroek M, Cecil R, Chalk AM, Cochrane J, Fan Y, Féron F, McCurdy R, McGrath JJ, Murrell W, Perry C, Raju J, Ravishankar S, Silburn PA, Sutherland GT, Mahler S, Mellick GD, Wood SA, Sue CM, Wells CA, Mackay-Sim A. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech. doi: 10.1242/dmm.005447. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Matsuzawa D, Hashimoto K. Magnetic resonance spectroscopy study of the antioxidant defense system in schizophrenia. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3453. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, Takanashi J, Matsuda T, Shimizu E, Ikehira H, Iyo M, Hashimoto K. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS One. 2008;3:e1944. doi: 10.1371/journal.pone.0001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner HG, Rojewski MT, Schmitt A, Tumani H, Bechter K, Schmitt M. Flow cytometric analysis of T cell subsets in paired samples of cerebrospinal fluid and peripheral blood from patients with neurological and psychiatric disorders. Brain Behav. Immun. 2009;23:134–42. doi: 10.1016/j.bbi.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Mazzarello V, Cecchini A, Fenu G, Rassu M, Dessy LA, Lorettu L, Montella A. Lymphocytes in schizophrenic patients under therapy: serological, morphological and cell subset findings. Ital. J. Anat. Embryol. 2004;109:177–88. [PubMed] [Google Scholar]
- McCord JM, Day ED., Jr. Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978;86:139–42. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCreadie RG, MacDonald E, Wiles D, Campbell G, Paterson JR. The Nithsdale Schizophrenia Surveys. XIV: Plasma lipid peroxide and serum vitamin E levels in patients with and without tardive dyskinesia, and in normal subjects. Br. J. Psychiatry. 1995;167:610–7. doi: 10.1192/bjp.167.5.610. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr. Res. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy RD, Féron F, Perry C, Chant DC, McLean D, Matigian N, Hayward NK, McGrath JJ, Mackay-Sim A. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82(2-3):163–73. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr. Neurol. 2004;30:227–35. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J. Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr. Bull. 2009;35:959–72. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N, Sourgens H, Arolt V, Erfurth A. Severe tardive dyskinesia in affective disorders: treatment with vitamin E and C. Neuropsychobiology. 2002;46:28–30. doi: 10.1159/000068019. [DOI] [PubMed] [Google Scholar]
- Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans. 2003;31(Pt 6):1300–1. doi: 10.1042/bst0311300. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin. Investig. Drugs. 2002;11:1407–35. doi: 10.1517/13543784.11.10.1407. [DOI] [PubMed] [Google Scholar]
- Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363:2063–72. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- Mukerjee S, Mahadik SP, Scheffer R, Correnti EE, Kelkar H. Impaired antioxidant defense at the onset of psychosis. Schizophr. Res. 1996;19(1):19–26. doi: 10.1016/0920-9964(95)00048-8. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci. Signal. 2009;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Alho K, Reinikainen K, Sams M. Do event-related potentials reveal the mechanism of the auditory sensory memory in the human brain? Neurosci. Lett. 1989:98217–21. doi: 10.1016/0304-3940(89)90513-2. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Yoshimura R, Nakano H, Ikenouchi-Sugita A, Hori H, Umene-Nakano W, Ueda N, Nakamura J. Association between plasma nitric oxide metabolites levels and negative symptoms of schizophrenia: a pilot study. Hum. Psychopharmacol. 2010;25:139–44. doi: 10.1002/hup.1102. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Vass E. Iron, metalloenzymes and cytotoxic reactions. Cell. Mol. Biol. (Noisy-le-groand) 2000;4:637–47. [PubMed] [Google Scholar]
- Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–42. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, Andreasen NC. Sex differences in brain morphology in schizophrenia. Am. J. Psychiatry. 1997;154:1648–54. doi: 10.1176/ajp.154.12.1648. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Sano T, Toyoda H. Predominant contribution of IFN-beta expression to apoptosis induction in human uterine cervical fibroblast cells by influenza-virus infection. Biol. Pharm. Bull. 2004;27:1750–7. doi: 10.1248/bpb.27.1750. [DOI] [PubMed] [Google Scholar]
- Oja SS, Janáky R, Varga V, Saransaari P. Modulation of glutamate receptor functions by glutathione. Neurochem. Int. 2000;37:299–306. doi: 10.1016/s0197-0186(00)00031-0. [DOI] [PubMed] [Google Scholar]
- Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: Pharmacology, mechanisms, and prevention. J. Neurosci. 1993;13:1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T, Okochi T, Kishi T, Ikeda M, Kitajima T, Yamanouchi Y, Kinoshita Y, Kawashima K, Tsunoka T, Ujike H, Inada T, Ozaki N, Iwata N. No association between polymorphisms of neuronal oxide synthase 1 gene (NOS1) and schizophrenia in a Japanese population. Neuromolecular. Med. 2009;11:123–7. doi: 10.1007/s12017-009-8068-z. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol. Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr, Res. 1999;33:523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitatory amino acids and neuropsychiatric disorders. Biol. Psychiatry. 1989;26:505–25. doi: 10.1016/0006-3223(89)90072-3. [DOI] [PubMed] [Google Scholar]
- Ozturk E, Demirbilek S, Kadir But A, Saricicek V, Gulec M, Akyol O, Ozcan Ersoy M. Antioxidant properties of propofol and erythropoietin after closed head injury in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:922–7. doi: 10.1016/j.pnpbp.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Padurariu M, Ciobica A, Dobrin I, Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479(3):317–20. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch. Gen. Psychiatry. 1990;47:1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Parnas J, Bovet P, Innocenti GM. Schizophrenic trait features, binding, and cortico-cortical connectivity: a neurodevelopmental pathogenetic hypothesis. Neurol. Psychiatry Brain Res. 1996;4:185–196. [Google Scholar]
- Paşca SP, Nemeş B, Vlase L, Gagyi CE, Dronca E, Miu AC, Dronca M. High levels of homocysteine and low serum paraoxonase 1 arylesterase activity in children with autism. Life Sci. 2006;78:2244–8. doi: 10.1016/j.lfs.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 2009;204:313–21. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol. Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- Perälä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsä E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppä T, Härkänen T, Koskinen S, Lönnqvist J. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch. Gen. Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- Perl O, Ilani T, Strous RD, Lapidus R, Fuchs S. The alpha7 nicotinic acetylcholine receptor in schizophrenia: decreased mRNA levels in peripheral blood lymphocytes. FASEB J. 2003;17:1948–50. doi: 10.1096/fj.03-0104fje. [DOI] [PubMed] [Google Scholar]
- Platt SR. The role of glutamate in central nervous system health and disease--a review. Vet. J. 2007;173:278–86. doi: 10.1016/j.tvjl.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Jr., Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc. Natl. Acad. Sci. USA. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol .Psychiatry. 2004;9:684–97. 643. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991;288:481–7. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Radonjić NV, Knežević ID, Vilimanovich U, Kravić-Stevović T, Marina LV, Nikolić T, Todorović V, Bumbaširević V, Petronijević ND. Decreased glutathione levels and altered antioxidant defense in animal model of schizophrenia: Long-term effects of perinatal phencyclidine administration. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.12.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rahman A, Azad MA, Hossain I, Qusar MM, Bari W, Begum F, Huq SM, Hasnat A. Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biol. Trace. Elem. Res. 2009;127:102–8. doi: 10.1007/s12011-008-8230-8. [DOI] [PubMed] [Google Scholar]
- Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–22. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1178–83. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Reddy R, Keshavan M, Yao JK. Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr. Res. 2003;62:205–12. doi: 10.1016/s0920-9964(02)00407-3. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot. Essent. Fatty Acids. 1996;55:33–43. doi: 10.1016/s0952-3278(96)90143-x. [DOI] [PubMed] [Google Scholar]
- Reddy R, Sahebarao MP, Mukherjee S, Murthy JN. Enzymes of the antioxidant defense system in chronic schizophrenic patients. Biol. Psychiatry. 1991;30:409–12. doi: 10.1016/0006-3223(91)90298-z. [DOI] [PubMed] [Google Scholar]
- Reif A, Herterich S, Strobel A, Ehlis AC, Saur D, Jacob CP, Wienker T, Töpner T, Fritzen S, Walter U, Schmitt A, Fallgatter AJ, Lesch KP. A neuronal nitric oxide synthase (NOS-I) haplotype associated with schizophrenia modifies prefrontal cortex function. Mol. Psychiatry. 2006;11:286–300. doi: 10.1038/sj.mp.4001779. [DOI] [PubMed] [Google Scholar]
- Rougemont M, Do KQ, Castagné V. New model of glutathione deficit during development: Effect on lipid peroxidation in the rat brain. J. Neurosci. Res. 2002;70:774–83. doi: 10.1002/jnr.10439. [DOI] [PubMed] [Google Scholar]
- Roy MA, Maziade M, Labbé A, Mérette C. The role of gender in studies of ventricle enlargement in schizophrenia: a predominantly male effect. Schizophr. Res. 2001;47:141–7. doi: 10.1016/s0920-9964(99)00231-5. [DOI] [PubMed] [Google Scholar]
- Rudolf S, Schlenke P, Broocks A, Peters M, Rothermundt M, Arolt V, Kirchner H. Search for atypical lymphocytes in schizophrenia. World J. Biol. Psychiatry. 2004;5:33–7. doi: 10.1080/15622970410029905. [DOI] [PubMed] [Google Scholar]
- Saadat M, Mobayen F, Farrashbandi H. Genetic polymorphism of glutathione S-transferase T1: a candidate genetic modifier of individual susceptibility to schizophrenia. Psychiatry Res. 2007;153:87–91. doi: 10.1016/j.psychres.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296(5568):692–5. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell. Biol. 2007;17:422–7. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Wagner JP, Yu X, Martin D. Bidirectional modulation of GABA-gated chloride channels by divalent cations: inhibition by Ca2+ and enhancement by Mg2+ J. Neurochem. 1994;62:916–22. doi: 10.1046/j.1471-4159.1994.62030916.x. [DOI] [PubMed] [Google Scholar]
- Selemon LD. Regionally diverse cortical pathology in schizophrenia: clues to the etiology of the disease. Schizophr. Bull. 2001;27:349–377. doi: 10.1093/oxfordjournals.schbul.a006881. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman RPS. Elevatedneuronal density in prefrontal area 46 in brains from schizophrenia patients: application of a three-dimensional, stereologic counting method. J. Comp. Neuro. 1998;392:402–412. [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Seybolt SE. Is it time to reassess alpha lipoic acid and niacinamide therapy in schizophrenia? Med Hypotheses. 2010 doi: 10.1016/j.mehy.2010.07.034. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Shahani N, Sawa A. Nitric oxide signaling and nitrosative stress in neurons: role for S-nitrosylation. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3580. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Butman M, Koistinaho J, Aardalen K, Nakki R, Massa SM, Swanson RA, Sagar SM. Phencyclidine induction of the hsp 70 stress gene in injured pyramidal neurons is mediated via multiple receptors and voltage gated calcium channels. Neuroscience. 1994;62:1079–92. doi: 10.1016/0306-4522(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol. Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- Shinkai T, Ohmori O, Hori H, Nakamura J. Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Mol. Psychiatry. 2002;7:560–3. doi: 10.1038/sj.mp.4001041. [DOI] [PubMed] [Google Scholar]
- Shutara Y, Koga Y, Fujita K, Takeuchi H, Mochida M, Takemasa K. An event-related potential study on the impairment of automatic processing of auditory input in schizophrenia. Brain Topogr. 1996;8:285–289. doi: 10.1007/BF01184786. [DOI] [PubMed] [Google Scholar]
- Singh OP, Chakraborty I, Dasgupta A, Datta S. A comparative study of oxidative stress and interrelationship of important antioxidants in haloperidol and olanzapine treated patients suffering from schizophrenia. Indian J. Psychiatry. 2008;50:171–6. doi: 10.4103/0019-5545.43627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies J. The neurotoxicity of glutamate, dopamine, iron and reactive oxygen species: functional interrelationships in health and disease: a review-discussion. Neurotox. Res. 1999;1:27–39. doi: 10.1007/BF03033337. [DOI] [PubMed] [Google Scholar]
- Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, Dubois-Dauphin M, Trabace L, Krause KH. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30(34):11317–25. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperner-Unterweger B, Whitworth A, Kemmler G, Hilbe W, Thaler J, Weiss G, Fleischhacker WW. T-cell subsets in schizophrenia: a comparison between drug-naive first episode patients and chronic schizophrenic patients. Schizophr. Res. 1999;38:61–70. doi: 10.1016/s0920-9964(98)00175-3. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Barthwal MK, Dalal PK, Agarwal AK, Nag D, Srimal RC, Seth PK, Dikshit M. Nitrite content and antioxidant enzyme levels in the blood of schizophrenia patients. Psychopharmacology (Berl) 2001;158:140–5. doi: 10.1007/s002130100860. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol. Psychiatry. 2006;59:929–39. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, Cuenod M, Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 2010;30:2547–58. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Neijt HC, Cuénod M, Do KQ. Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience. 2006;137:807–819. doi: 10.1016/j.neuroscience.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Suboticanec K, Folnegović-Smalc V, Korbar M, Mestrović B, Buzina R. Vitamin C status in chronic schizophrenia. Biol. Psychiatry. 1990;28:959–66. doi: 10.1016/0006-3223(90)90061-6. [DOI] [PubMed] [Google Scholar]
- Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic. Biol. Med. 1996;21:335–48. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Nakaki T, Nakamura M, Miyaoka H. Plasma nitrate levels in deficit versus non-deficit forms of schizophrenia. J. Psychiatry Neurosci. 2003;28:288–92. [PMC free article] [PubMed] [Google Scholar]
- Tang W, Huang K, Tang R, Zhou G, Fang C, Zhang J, Du L, Feng G, He L, Shi Y. Evidence for association between the 5′ flank of the NOS1 gene and schizophrenia in the Chinese population. Int. J. Neuropsychopharmacol. 2008;11:1063–71. doi: 10.1017/S1461145708008924. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Holcomb HH. Phenotype of schizophrenia: a review and formulation. Mol. Psychiatry. 2005;10:27–39. doi: 10.1038/sj.mp.4001563. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Gender and schizophrenia. J. Clin. Psychiatry. 1997;58(Suppl 15):33–7. [PubMed] [Google Scholar]
- Tang LH, Aizenman E. The modulation of N-methyl-D-aspartate receptors by redox and alkylating reagents in rat cortical neurones in vitro. J. Physiol. 1993;465:303–23. doi: 10.1113/jphysiol.1993.sp019678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: application to schizophrenia. MAGMA. 2005;18:276–82. doi: 10.1007/s10334-005-0012-0. [DOI] [PubMed] [Google Scholar]
- Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F, Matthey ML, Parnas J, Preisig M, Saraga M, Solida A, Timm S, Wang AG, Werge T, Cuénod M, Do KQ. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am. J. Hum. Genet. 2006;79:586–92. doi: 10.1086/507566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007;8:355–67. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch. Gen. Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Upchurch GR, Jr., Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF, Jr., Loscalzo J. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J. Biol. Chem. 1997;272:17012–7. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Vikhreva OV, Zimina IS, Kolomeets NS, Orlovskaya DD. The role of oligodendrocyte pathology in schizophrenia. Int. J. Neuropsychopharmacol. 2007;10:537–545. doi: 10.1017/S1461145707007626. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: A study from the Stanley Neuropathology Consortium. Schizophr. Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- van Vliet J, Oates NA, Whitelaw E. Epigenetic mechanisms in the context of complex diseases. Cell. Mol. Life Sci. 2007;64:1531–8. doi: 10.1007/s00018-007-6526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendemiale G, Grattagliano I, Altomare E. An update on the role of free radicals and antioxidant defense in human disease. Int. J. Clin. Lab. Res. 1999;29:49–55. doi: 10.1007/s005990050063. [DOI] [PubMed] [Google Scholar]
- Wass C, Klamer D, Fejgin K, Pålsson E. The importance of nitric oxide in social dysfunction. Behav Brain Res. 2009;200(1):113–6. doi: 10.1016/j.bbr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Wass C, Svensson L, Fejgin K, Pålsson E, Archer T, Engel JA, Klamer D. Nitric oxide synthase inhibition attenuates phencyclidine-induced disruption of cognitive flexibility. Pharmacol. Biochem. Behav. 2008;89:352–9. doi: 10.1016/j.pbb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin. Neurosci. 2010;64:217–30. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weiser M, Levkowitch Y, Neuman M, Yehuda S. Decrease of serum iron in acutely psychotic schizophrenic patients. Int. J. Neurosci. 1994;78:49–52. doi: 10.3109/00207459408986045. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–82. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett. 1995;82-83:969–74. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
- Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv. Rev. Psychiatry. 2010;18:173–89. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218:267–77. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch. Gen. Psychiatry. 2004;61:649–57. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Yücel M, Pantelis C, Berk M. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann. Acad. Med. Singapore. 2009a;38:396–6. [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Wellard RM, Proffitt TM, McConchie M, Berk M, McGorry PD, Pantelis C. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis. 2009b;33(3):354–7. doi: 10.1016/j.nbd.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Wratten NS, Memoli H, Huang Y, Dulencin AM, Matteson PG, Cornacchia MA, Azaro MA, Messenger J, Hayter JE, Bassett AS, Buyske S, Millonig JH, Vieland VJ, Brzustowicz LM. Identification of a schizophrenia-associated functional noncoding variant in NOS1AP. Am J Psychiatry. 2009;166(4):434–41. doi: 10.1176/appi.ajp.2008.08081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO, Baccarelli A. Metals and neurotoxicology. J. Nutr. 2007;137:2809–13. doi: 10.1093/jn/137.12.2809. [DOI] [PubMed] [Google Scholar]
- Xi D, Keeler B, Zhang W, Houle JD, Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: application of laser microdissection technique. J. Neurosci. Methods. 2009;176:172–81. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Wratten N, Charych EI, Buyske S, Firestein BL, Brzustowicz LM. Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med. 2005;2(10):e263. doi: 10.1371/journal.pmed.0020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J. Biol. Chem. 2002;277:49446–52. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- Yanik M, Kocyigit A, Tutkun H, Vural H, Herken H. Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol. Trace Elem. Res. 2004;98:109–17. doi: 10.1385/BTER:98:2:109. [DOI] [PubMed] [Google Scholar]
- Yao JK, Dougherty GG, Jr., Reddy RD, Keshavan MS, Montrose DM, Matson WR, Rozen S, Krishnan RR, McEvoy J, Kaddurah-Daouk R. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.33. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis. Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr. Bull. 2004;30:923–34. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- Yao JK, Reddy RD, van Kammen DP. Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs. 2001;15:287–310. doi: 10.2165/00023210-200115040-00004. [DOI] [PubMed] [Google Scholar]
- Yao JK, Reddy R, McElhinny LG, van Kammen DP. Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J. Psychiatr. Res. 1998a;32:385–91. doi: 10.1016/s0022-3956(98)00028-4. [DOI] [PubMed] [Google Scholar]
- Yao JK, Reddy R, McElhinny LG, van Kammen DP. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr. Res. 1998b;32:1–8. doi: 10.1016/s0920-9964(98)00030-9. [DOI] [PubMed] [Google Scholar]
- Yao JK, Reddy R, van Kammen DP. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1998c;80:29–39. doi: 10.1016/s0165-1781(98)00051-1. [DOI] [PubMed] [Google Scholar]
- Yilmaz N, Herken H, Cicek HK, Celik A, Yürekli M, Akyol O. Increased levels of nitric oxide, cortisol and adrenomedullin in patients with chronic schizophrenia. Med. Princ. Pract. 2007;16:137–41. doi: 10.1159/000098367. [DOI] [PubMed] [Google Scholar]
- Yuan XM, Brunk UT, Olsson AG. Effects of iron- and hemoglobin-loaded human monocyte-derived macrophages on oxidation and uptake of LDL. Arterioscler Thromb Vasc Biol. 1995;15(9):1345–51. doi: 10.1161/01.atv.15.9.1345. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhao Z, He L, Wan C. A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci. 2010;53:112–24. doi: 10.1007/s11427-010-0013-8. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Chen da C, Xiu MH, Wang F, Qi LY, Sun HQ, Chen S, He SC, Wu GY, Haile CN, Kosten TA, Lu L, Kosten TR. The novel oxidative stress marker thioredoxin is increased in first-episode schizophrenic patients. Schizophr. Res. 2009;113:151–7. doi: 10.1016/j.schres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. The effect of risperidone treatment on superoxide dismutase in schizophrenia. J Clin Psychopharmacol. 2003;23:128–31. doi: 10.1097/00004714-200304000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Cao LY, Wu GY, Xu Q, Shen Y, Zhou DF. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr. Res. 2006;81:291–300. doi: 10.1016/j.schres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Su JM, Zhang PY. The effect of extract of ginkgo biloba added to haloperidol on superoxide dismutase in inpatients with chronic schizophrenia. J. Clin. Psychopharmacol. 2001a;21:85–8. doi: 10.1097/00004714-200102000-00015. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Zhang PY, Wu GY, Su JM, Cao LY. A double-blind, placebo-controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J. Clin .Psychiatry. 2001b;62:878–83. doi: 10.4088/jcp.v62n1107. [DOI] [PubMed] [Google Scholar]
- Zoroglu SS, Herken H, Yürekli M, Uz E, Tutkun H, Savaş HA, Bagci C, Ozen ME, Cengiz B, Cakmak EA, Dogru MI, Akyol O. The possible pathophysiological role of plasma nitric oxide and adrenomedullin in schizophrenia. J. Psychiatr. Res. 2002;36:309–15. doi: 10.1016/s0022-3956(02)00014-6. [DOI] [PubMed] [Google Scholar]