Abstract
Variability in the affective and cognitive symptom response to antidepressant treatment has been observed in geriatric depression. The underlying neural circuitry is poorly understood. This study evaluated the cerebral glucose metabolic effects of citalopram treatment and applied multivariate, functional connectivity analyses to identify brain networks associated with improvements in affective symptoms and cognitive function. Sixteen geriatric depressed patients underwent resting positron emission tomography (PET) studies of cerebral glucose metabolism and assessment of affective symptoms and cognitive function before and after 8 weeks of selective serotonin reuptake inhibitor treatment (citalopram). Voxel‐wise analyses of the normalized glucose metabolic data showed decreased cerebral metabolism during citalopram treatment in the anterior cingulate gyrus, middle temporal gyrus, precuneus, amygdala, and parahippocampal gyrus. Increased metabolism was observed in the putamen, occipital cortex, and cerebellum. Functional connectivity analyses revealed two networks which were uniquely associated with improvement of affective symptoms and cognitive function during treatment. A subcortical‐limbic‐frontal network was associated with improvement in affect (depression and anxiety), while a medial temporal‐parietal‐frontal network was associated with improvement in cognition (immediate verbal learning/memory and verbal fluency). The regions that comprise the cognitive network overlap with the regions that are affected in Alzheimer's dementia. Thus, alterations in specific brain networks associated with improvement of affective symptoms and cognitive function are observed during citalopram treatment in geriatric depression. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: selective serotonin reuptake inhibitors, citalopram, serotonin, positron emission tomography (PET), glucose metabolism, functional connectivity, partial least squares (PLS), depression, aging
INTRODUCTION
Depression in the elderly is associated with disability, a dramatic increase in the rate of completed suicide, greater mortality in the medically ill, and greater risk of Alzheimer's dementia (AD; [Alexopoulos et al., 1996; Bruce and Leaf, 1989; Conwell et al., 1996; Hendriksson et al., 1995; Jorm, 2001; Ownby et al., 2006]. Even though there are effective antidepressant agents, more than half of geriatric depressed patients are partial responders or treatment refractory [Dew et al., 1997]. Despite affective symptom remission, persistent cognitive impairment is commonly observed [Alexopoulos et al., 1993; Bhalla et al., 2006]. The most consistent cognitive deficits observed in nondemented, geriatric depressed patients include slowed speed of processing and deficits in executive function, verbal fluency, confrontation naming, explicit sequence learning, verbal and spatial memory, and visual‐spatial function [Aizenstein et al., 2005; Alexopoulos et al., 2005; Butters et al., 2000; de Asis et al., 2001; Kramer‐Ginsberg et al., 1999; Lockwood et al., 2000, 2002; Nebes et al., 2003]. An understanding of the mechanisms underlying treatment resistance, of affective and cognitive symptoms, is among the central priorities in geriatric depression research [Smith et al., 2007].
Over the past decade, serial clinical, neuropsychological, and neuroimaging methods have been integrated into antidepressant treatment studies in geriatric depressed patients to understand the course of symptom remission with respect to affective and cognitive outcomes, as well as changes in neural circuitry. Such information could potentially inform the development of more effective intervention and prevention strategies. Positron emission tomography (PET) studies of cerebral glucose metabolism have identified the functional neural circuitry underlying geriatric depression symptom remission [Smith et al., 1999, 2002a, b, c, 2009a, b]. These studies have demonstrated that glucose metabolism is sensitive to detecting the neural circuitry involved in geriatric depression, as well as that affected by antidepressant interventions. The study of the relationship between the alterations in regional cerebral glucose metabolism and the improvement of affective symptoms and cognitive deficits is a logical next step to identifying the neural circuitry that underlies depressive symptom remission.
The focus of this study was to identify the network of brain regions associated with improvements in affective symptoms, as well as the network associated with improvements in cognitive function during antidepressant treatment. Cerebral glucose metabolism, affective symptom severity, and cognitive function were measured prior to and after 8 weeks of treatment with the SSRI, citalopram. As shown in prior studies of total sleep deprivation (TSD) and antidepressant treatment [see Buchsbaum et al., 1997; Mayberg, 2003; Mayberg et al., 2000; Smith et al., 2002a, c], it was hypothesized that 8 weeks of citalopram treatment would be associated with decreased metabolism in the anterior cingulate cortex (ACC, BA 24/32), superior and middle prefrontal, superior temporal, and parietal cortices (precuneus), as well as increased metabolism in the basal ganglia (putamen) and occipital cortex.
After evaluating the cerebral metabolic effects of citalopram treatment, functional connectivity analyses were performed using partial least squares [PLS; McIntosh and Lobaugh, 2004; McIntosh et al., 1996, 2004] to relate the regional glucose metabolic alterations to changes in affective symptoms and cognitive function. With respect to the functional connectivity patterns, improved affect was hypothesized to be associated with decreased metabolism in the ACC, medial frontal cortex, and amygdala. Improved cognition was hypothesized to be associated with decreased metabolism in superior frontal, medial temporal regions (including hippocampus, parahippocampal gyrus), and parietal cortices (precuneus). The hypothesis that improved affective and cognitive symptoms would be associated with decreased glucose metabolism is based on previous studies in geriatric depression [Smith et al., 2002a, c, 2009a, b]. In unmedicated, geriatric depressed patients relative to controls, cortical glucose metabolism is increased and the magnitude of increase in glucose metabolism in geriatric depressed patients is correlated with greater affective symptom severity [Smith et al., 2009c]. Acute and chronic treatment is associated with reduced metabolism in areas that are “hypermetabolic” at baseline [Smith et al., 2002a, b, c, 2009a].
MATERIALS AND METHODS
Study Design
The geriatric depressed patients underwent two PET scans on two consecutive days to measure the glucose metabolic effects of acute, intravenous citalopram compared to saline administration [Smith et al., 2009a]. Then, patients began a 12 week treatment trial with oral citalopram. The patients were rescanned after 8 weeks of citalopram. Based on other antidepressant trials in geriatric depression, the 8 week treatment interval was chosen for the follow up scan as this interval might serve as an early biomarker of response, and in addition, this is the time by which patients should have demonstrated significant clinical improvement [Sackeim et al., 2006]. The present report describes the effects of 8 weeks of citalopram treatment on cerebral glucose metabolism in the same patient sample as reported in the prior publication of the cerebral metabolic effects of acute (single dose) citalopram administration [Smith et al., 2009a].
Subject Screening and Selection
Geriatric depressed patients were recruited from the Geriatric Psychiatry Outpatient Clinic at the Zucker Hillside Hospital and also from advertisements in the community. Prior to the PET scans, the patients underwent screening procedures including a psychiatric evaluation (a structured clinical interview [SCID, First et al., 1995]), laboratory testing (CBC and blood chemistry including glucose levels and thyroid function tests), toxicology screening, and Magnetic Resonance (MR) imaging scans (GE 1.5T Magnetom Vision).
The inclusion criteria for the study were: (i) 60 years of age and older; (ii) DSM‐IV diagnosis of major depressive disorder (nonbipolar, nonpsychotic) without lifetime diagnosis of another Axis I disorder; (iii) no current treatment with antidepressant medication or other psychotropic drugs (including benzodiazepines and antipsychotic drugs); (iv) no past history of electroconvulsive therapy (ECT) treatment; (v) a score of 17 or higher on the Hamilton Depression Rating Scale [HDRS, Hamilton, 1959]; (vi) a score of 26 or higher on the 30‐item Mini Mental Status Examination (MMSE, [Folstein et al., 1975]); (vii) no diagnosis of a cognitive or neurological disorder including mild cognitive impairment or dementia, movement disorder (including Parkinson's Disease) or traumatic brain injury; (viii) no contraindication for MR scanning or focal finding on the MR scan including a tumor or stroke; (ix) no alcohol or drug abuse within the last 6 months; (x) not at eminent risk for suicide or a serious suicide attempt within the past year; (xi) no significant clinical laboratory abnormality that would be a contraindication for citalopram treatment or that would influence interpretation of the PET scan (including hyponatremia, a positive toxicology screen for centrally acting medications or elevated glucose level or); and (xii) no current or past history of significant medical illness (including active cancer, or insulin‐dependent diabetes) requiring current pharmacologic treatment with potential central nervous system effects (including beta blockers).
Sixteen depressed patients (10 females/6 males, age 65.3 ± 9.1 years) who met these inclusion criteria were enrolled in the study. The years of education was 14.3 ± 2.9 and the MMSE was 28.8 ± 1.0. The age at onset of depression was 60.0 ± 4.4 years and the duration of the index episode was 7.1 ± 2.8 months. Twelve of the patients had never been treated with psychotropic (including antidepressants and antipsychotics) drugs. Of the remaining four patients, three were treated with sertraline prior to study entry (the treatment ended from 6 months to 2 years prior to study enrolment). The fourth patient had been treated with nortriptyline for 2 years up until 2 weeks prior to the PET scan at which time the plasma nortriptyline concentrations were undetectable. As described in the results section, the neuropsychological data for the patients was compared with 13 nondepressed control subjects (who met the inclusion criteria described above except ii). The controls did not differ significantly from the patients in age, education, or MMSE score (8 females/6 males, age 67.4 ± 7.4 years, education 13.8 ± 2.7 years and mean MMSE 28.8 ± 1; P > 0.05).
After a complete description of the study to the subjects, written informed consent was obtained according to procedures established by the Institutional Review Board and the Radiation Safety Committee of the North Shore‐Long Island Jewish Health System.
Citalopram Treatment
Two days after the acute, intravenous administration of a single dose of citalopram [as reported in Smith et al., 2009a], the patients began treatment with the oral medication at a dose of 10 mg per day for 3 days. The dose was increased to 20 mg on the 4th day. If significant clinical improvement was not observed at the 20 mg dose after 4 weeks of treatment (measured as a rating of ≥3 on the Clinical Global Impression Scale [CGI; Guy, 1976]), the dose was incrementally increased to 30 mg, and then to 40 mg. The average citalopram dose at the time of the second PET scan was 35.0 mg ± 7.3. Patients were monitored on a weekly basis in the Geriatric Outpatient Clinic of the Zucker Hillside Hospital, at which time clinical ratings (depressive and anxiety symptoms) were administered and side effects were assessed. The clinical ratings included the Hamilton Depression Rating Scale‐24 item (HDRS; [Hamilton, 1960]) and Hamilton Anxiety Rating Scale (HARS; [Hamilton, 1959]). The clinical ratings were also performed on the day of the PET scans. All patients who began treatment completed the 12 week course of treatment and were included in this report. The side effects primarily included transient headache and nausea. These side effects were observed in 20% of subjects within the first few days of treatment, and subsided within the first week of treatment.
Neuropsychological Testing
All patients underwent a neuropsychological battery prior to starting citalopram treatment (baseline measure) and after 8 weeks of treatment (treatment measure), within 1 week of the PET scans. The neuropsychological battery included tests that had shown sensitivity to detecting differences between depressed patients and nondepressed controls, as well as tests sensitive to mild cognitive impairment or dementia [Kramer‐Ginsberg et al., 1999; Lockwood et al., 2000]. The battery included an established dementia rating scale (Mattis Dementia Rating Scale, [DRS, Mattis, 1976]), as well as neuropsychological tests that evaluated specific domains of cognition (psychomotor speed, executive function, verbal and spatial memory, verbal fluency, confrontation naming). As described, 13 nondepressed controls, which were demographically matched to the patients, underwent the same neuropsychological battery over the same time interval as the patients. The neuropsychological tests included in the PLS analyses were the tests that showed the most significant improvement over time in patients compared with controls.
As described in the Results section, the tests that showed the most significant differences included tests of verbal fluency (Controlled Oral Words Association Test, COWAT; [Benton and Hamsher, 1978]) and a measure of immediate verbal learning/memory (the sum of the total words recalled in the first five trials of the California Verbal Learning Test‐First Edition [CVLT; Delis et al., 1988]). The pattern of regional correlations between these variables and the PET data were evaluated for each of the two affective or cognitive variables to determine the similarities and differences between the regional pattern of correlations and whether there was a rationale for combining the affective/cognitive variables in the seed PLS analysis.
PET Imaging Procedures
The PET scans were performed on a GE Advance Tomograph at the Center for Neurosciences, Feinstein Institute for Medical Research, as described previously [Smith et al., 2002a, b, 2009a]. Upon arrival at the PET facility, one catheter was placed in a vein in the left arm for radiotracer administration and a second catheter was placed in a vein in the right arm to obtain a blood sample for radiotracer quantification (glucose concentrations and radioactivity). Five mCi of [18F]‐2‐deoxy‐2‐fluoro‐D‐glucose ([18F]‐FDG) was injected as an intravenous bolus. During the uptake interval, the subjects sat in a darkened, quiet room with eyes open, and ears unoccluded. Twenty‐five minutes after radiotracer injection, subjects were positioned in the PET scanner. A 10‐min transmission scan and a 5‐min two‐dimensional emission scan were first acquired to perform photon attenuation correction. A three‐dimensional emission scan began 40 min after radiotracer injection, and lasted for 10 min. At the end of the PET scan, each subject was debriefed as to her/his perception of the study.
PET Image Quantification and Processing
Glucose metabolic rates were calculated (in ml/100 g/min) on a pixel by pixel basis according to validated methods [Smith et al., 2002a, b; Takikawa et al., 1993]. The method used was a modification of the procedures developed by Phelps et al. [ 1979]. A single venous blood sample was obtained at 40 min postinjection (prior to the 3D emission scan) and was scaled to a population‐derived standardized input function [Takikawa et al., 1993]. A venous sample was used as after 15 min postinjection, the arterial and venous samples have been shown to be linear with respect to count rates and glucose concentrations [Takikawa et al., 1993]. This simplified method to derive the input function has been validated against standard methods to derive the arterial input function and also shows similar test‐retest variability compared to such quantification methods [Robeson et al., 1993; Takikawa et al., 1993].
PET data processing (coregistration, normalization, smoothing) was performed on the quantitative glucose metabolism images using the statistical parametric mapping program (SPM5, Institute of Neurology, London [Friston, 2007]). The images were smoothed with an isotropic Gaussian kernel (FWHM 8 mm for all directions).
PET Data Analysis Overview
The objectives of the PET data analysis were (i) to determine the regional glucose metabolic effects of citalopram treatment and (ii) to relate the regional cerebral metabolic alterations to changes in affective symptoms and cognitive function with citalopram treatment in order to identify the neural networks underlying improvement in these two symptom domains.
Voxel‐wise Analysis of the Citalopram Effect on Cerebral Glucose Metabolism (SPM)
The effects of citalopram treatment on cerebral glucose metabolism were evaluated on a voxel‐wise basis using SPM5 [Friston, 2007]. A within‐subject comparison of the baseline to 8 weeks of citalopram treatment was performed, using the flexible factorial option (paired T‐test) in SPM5. The glucose metabolic rates were normalized by scaling to a common mean value (50) for all scans, after establishing that the global means did not differ significantly between conditions (baseline versus treatment; P > 0.05). The data were normalized to the global mean value because test‐retest variability is greater for absolute compared to relative metabolic values [Bartlett et al., 1988]. The comparisons were considered significant at a t threshold greater than 3.51 (z > 2.98, P < 0.001; uncorrected for multiple independent comparisons, cluster size [K E] greater than 50 voxels).
Analysis of Affective and Cognitive Networks Associated with Citalopram Treatment (PLS)
Behavior PLS [McIntosh and Lobaugh, 2004] was used to correlate the treatment‐related changes in cerebral glucose metabolism with improvement in the affective and cognitive symptom measures. PLS was used to determine the distributed network of brain areas that correlated with either improvement in affect or with improvement in cognition. Based on this analysis, the key regions that correlated with improvement in affective symptoms, and not cognitive function (and vice versa), were determined. The seed (or region of interest) that was consistently identified in the behavior PLS analysis within each of the affective or cognitive symptom domains was used in the functional connectivity analyses.
Functional connectivity analysis using PLS (seed PLS) was then performed to delineate the distributed network of brain areas associated with improvements in affective symptoms from those associated with improvements in cognitive function. The purpose of the functional connectivity analysis was to determine the network of brain regions that correlated with affective and cognitive improvement during treatment that was measured by behavioral testing performed “outside of the scanner.” Thus, the behavioral measures were also included in the functional connectivity analysis (the method has also been applied to the analysis of scans acquired in the “activated” state as in event‐related functional magnetic resonance imaging (fMRI), [e.g., Diaconescu et al., 2010]).
Implementation of PLS
Behavior PLS
PLS analyzes the relationship between two matrices, one contained the brain imaging data and the other contained the affective (HARS and HDRS) and the cognitive measures (COWAT and CVLT). The method extracted spatial patterns that optimally represent the relationship between two blocks of data: (i) regional glucose metabolism before and after citalopram treatment and (ii) the outcome variable (affective or cognitive measures). Correlation images were then computed between each scan and each behavioral measure yielding eight correlation images (two scans by four behavior measures). Normalization of the voxel‐wise glucose metabolic rates to a global mean is not performed in PLS.
PLS uses singular value decomposition (SVD) to identify a set of latent variables (LVs) that characterize similarities and differences in the correlations between scans. All correlation images were stacked into a single matrix that is then subjected to an SVD. This produces three new matrices comprising of: (i) singular value, which represented the amount of brain‐behavior covariance the given LV accounts for; (ii) behavior LV, which identified the brain‐behavior correlation pattern between the two conditions; and (iii) voxel saliences that reflected the corresponding spatial pattern identified by the behavior LV. Voxel saliences were displayed as an image, which showed voxels weighted in proportion to the strength and direction (positive or negative) of their brain‐behavior correlations [McIntosh and Lobaugh, 2004]. Considered another way, the behavior LV codes a contrast of the pattern of correlations across behavioral measures and scans and the image derived from that shows how that contrast is expressed.
Functional connectivity analysis (seed PLS)
The main difference between behavior PLS and seed PLS lies in the additional use of regions of interest (or seeds) with the behavioral measures. The purpose here was to find the distributed pattern that both shows robust functional connections with the seed region and relates to behavior.
In the present application, the brain regions that reliably correlated with improvement in affective symptoms or cognitive function were used as seeds for the subsequent functional connectivity analysis (seed PLS).
The left anterior cingulate cortex (ACC, BA 24) (MNI coordinates = −1, −34, 43) was chosen as the seed for the affective network. The right parahippocampal gyrus (PHG, BA 36) (MNI coordinates = 22, −39, −4) was chosen as the seed for the cognitive network. The seeds were selected based on their differential correlation pattern to the affective and cognitive measures (see Results Section, Tables IV, V, VI), and the documented involvement of these brain regions in affect and cognitive functions, respectively. Altered activity in the ACC has been reported in neuroimaging studies of depressed patients [Canli et al., 2004; Gotlib et al., 2005; Keedwell et al., 2005; Lawrence et al., 2004; Mayberg et al., 1997; Surguladze et al., 2005]. PHG is activated during tasks involving verbal fluency and immediate verbal learning/memory [Daselaar et al., 2001; Pihlajamaki et al., 2000].
Table IV.
Local maxima from brain‐behavior PLS analysis between the affective measures (HDRS/HARS) and brain glucose metabolism (LV1)
| X (mm) | Y (mm) | Z (mm) | BSR | Cluster Size (voxels) | Laterality | Regions | BA |
|---|---|---|---|---|---|---|---|
| −23 | −3 | −19 | 3.56 | 81 | Left | Amygdala | |
| 33 | −1 | −26 | 4.94 | 55 | Right | Amygdala | |
| −5 | −41 | 55 | 9.53 | 8,621 | Left | Anterior cingulate cortex | BA 24 |
| 6 | −43 | 32 | 4.66 | 271 | Right | Anterior cingulate cortex | BA 24 |
| −17 | 22 | 64 | 4.45 | 148 | Left | Superior frontal gyrus | BA 9 |
| 20 | 30 | 55 | 7.46 | 660 | Right | Superior frontal gyrus | BA 6 |
| −43 | 53 | 12 | 4.4 | 345 | Left | Middle frontal gyrus | BA 46 |
| 39 | 1 | 65 | −4.62 | 194 | Right | Middle frontal gyrus | BA 46 |
| 42 | −11 | 6 | 3.81 | 79 | Right | Insula lobe | BA 13 |
| −64 | −23 | 10 | 5.82 | 1,502 | Left | Superior temporal gyrus | BA 22 |
| −3 | −45 | 27 | 4.46 | 304 | Left | Posterior cingulate cortex | BA 31 |
| −28 | −64 | 67 | −9.47 | 545 | Left | Superior parietal lobule | BA 39 |
Table V.
Local maxima from brain‐behavior PLS analysis between cognitive measures (COWAT/CVLT) and brain glucose metabolism (LV2)
| X (mm) | Y (mm) | Z (mm) | BSR | Cluster Size (voxels) | Laterality | Regions | BA |
|---|---|---|---|---|---|---|---|
| −25 | −15 | −18 | 3.9 | 27 | Left | Hippocampus | |
| 37 | −32 | −8 | 3.81 | 19 | Right | Hippocampus | |
| −25 | −22 | −20 | 5.3 | 566 | Left | Parahippocampal gyrus | BA 36 |
| 24 | −10 | −27 | 4.78 | 384 | Right | Parahippocampal gyrus | BA 36 |
| −3 | 43 | 49 | 6.42 | 1,673 | Left | Superior medial frontal gyrus | BA 10 |
| 11 | 37 | 45 | 3.61 | 51 | Right | Superior medial frontal gyrus | BA 10 |
| −31 | 20 | 30 | 3.52 | 192 | Left | Middle frontal gyrus | BA 46 |
| 36 | 50 | 12 | 3.7 | 44 | Right | Middle frontal gyrus | BA 46 |
| −24 | 43 | −17 | 3.61 | 46 | Left | Orbitofrontal gyrus | BA 11 |
| 20 | 39 | −14 | 4.6 | 65 | Right | Orbitofrontal gyrus | BA 11 |
| −44 | −53 | 19 | 5.51 | 101 | Left | Middle temporal gyrus | BA 21 |
| 53 | −33 | 2 | 7.98 | 800 | Right | Middle temporal gyrus | BA 21 |
| −56 | −8 | −37 | 5.86 | 802 | Left | Inferior temporal gyrus | BA 20 |
| 55 | −8 | −40 | 6.39 | 791 | Right | Inferior temporal gyrus | BA 20 |
| −14 | −55 | 27 | 3.71 | 91 | Left | Precuneus | BA 7 |
| 10 | −59 | 19 | 3.7 | 64 | Right | Precuneus | BA 7 |
| −44 | −60 | 55 | 8.94 | 1,154 | Left | Inferior parietal lobule | BA 40 |
| 29 | −49 | 75 | 5.03 | 258 | Right | Superior parietal lobule | BA 39 |
| 33 | −82 | 43 | −4.67 | 288 | Right | Superior occipital gyrus | BA 19 |
| −17 | −32 | −17 | −4.75 | 1,104 | Left | Fusiform gyrus | BA 37 |
| 32 | 0 | 1 | −4.75 | 403 | Right | Putamen | |
| 43 | −57 | −26 | 7.02 | 3,800 | Right | Cerebellar declive | |
| −37 | −43 | −39 | 8.52 | 2,717 | Left | Cerebellum | |
| 11 | −38 | −15 | 6.73 | 2,226 | Right | Cerebellum |
Table VI.
Seed and behavioral measure correlations
| HDRS | HARS | COWAT | CVLT | |
|---|---|---|---|---|
| Left ACC seed | 0.72; P < 0.023 | 0.54; P < 0.04 | −0.07; P > 0.7 | 0.12; P > 0.4 |
| Right PHG seed | −0.08; P > 0.6 | −0.12; P > 0.3 | −0.42; P < 0.04 | −0.60; P < 0.001 |
PLS statistical evaluation
For the statistical assessment of the brain‐behavior correlations and the functional connectivity analyses, two complementary resampling techniques were used. The overall statistical significance of the relationship between brain and behavior measures or brain and seed voxels was determined using permutation tests on the singular value. Statistical control for multiple comparisons in PLS is addressed by permutation testing that is performed at the level of the singular value (i.e., the whole image level). The reliability of the voxels contributing to significant patterns was assessed using bootstrap estimation of standard errors. Bootstrapping was also used to derive reliability assessment for the correlation of each behavioral measure or seed voxel with the overall pattern of glucose metabolism in the brain. In this study, 500 permutations tests and 200 bootstrap iterations were computed [McIntosh and Lobaugh, 2004].
RESULTS
Affective and Cognitive Measures
After 8 weeks of citalopram treatment, the patients as a group showed significant improvement in affective symptoms, and significantly greater improvement on the cognitive tests relative to demographically matched controls tested over the same time interval. The means and standard deviations for these measures and the Dementia Rating Scale [DRS; Mattis, 1976] for the depressed patients are listed in Table I. The mean total DRS score and subscale scores were within the range observed in nondemented, control subjects [Mattis, 1976]. Repeated measures analysis of variance (ANOVA) for these measures in the patients revealed significant improvements in affect (depression and anxiety, respectively) as measured with HDRS [F (1,15) = 210.4, P < 0.001] and HARS [F (1,15) = 64.1, P < 0.001]. With respect to the cognitive tests, the greatest improvement in the patients relative to controls tested over the same time interval was observed in immediate verbal learning/memory (CVLT‐total recall first five trials) [F (1,15) = 19.2, P < 0.001], and verbal fluency (COWAT) [F (1,15) = 7.4, P <0.02]. The other cognitive tests did not show a significantly greater change in the patients relative to controls (including the Benton Visual Retention Test, and Boston Naming Test; data not shown).
Table I.
Changes in affective and cognitive symptoms during citalopram treatment (8 weeks)
| Baseline | Citalopram (8 weeks) Treatment | |
|---|---|---|
| HDRS | 25.6 ± 4.1 | 8.7 ± 6.1a |
| HARS | 14.4 ± 5.5 | 5.3 ± 3.6a |
| CVLT | 44.1 ± 7.7 | 51.3 ± 7.3a |
| COWAT | 38.0 ± 7.8 | 43.7 ± 8.2b |
| Dementia Rating Scale (DRS) Attention (37c) | 36.8 ± 0.4 | 36.7 ± 0.5 |
| DRS Initiation/ Perseveration (371) | 36.7 ± 0.9 | 36.8 ± 0.7 |
| DRS Memory (25c) | 23.8 ± 1.5 | 24.1 ± 0.9 |
| DRS Construction (39c) | 38.1 ± 1.5 | 38.3 ± 1.1 |
| DRS Total (144c) | 141.2 ± 2.6 | 141.5 ± 2.1 |
Significantly different from baseline (P ≤ 0.001).
Significantly different from baseline (P < 0.05).
Maximal Score on the respective DRS tests.
With respect to the inter‐correlations between the change scores for the affective and cognitive measures, the HDRS was significantly positively correlated with the HARS (r = 0.80, P <0.001; Table II) and significantly negatively correlated with the CVLT (r = −0.37, P < 0.042), but not the COWAT (−0.11, P > 0.5). In contrast, the HARS was not significantly correlated with any of the cognitive measures. The measures of verbal fluency and immediate verbal learning/memory (i.e., COWAT and CVLT) were not significantly correlated with each other (r = 0.22, P > 0.1; Table II). The COWAT and CVLT measures include a verbal retrieval component and activate similar neural circuitries, as shown by previous fMRI studies [Daselaar et al., 2001; Pihlajamaki et al., 2000]. In addition, both tests have shown impairment in geriatric depressed patients and improvements following antidepressant treatment [Butters et al., 2000], similar to the present study. Thus, the decision was made that including both tests would be more representative of the cognitive deficits observed in geriatric depression, as opposed to including either one of the measures.
Table II.
Correlations between affective and cognitive measures during citalopram treatment (8 weeks)
| HDRS | HARS | COWAT | CVLT | |
|---|---|---|---|---|
| HDRS | 1.00 | 0.80; P < 0.001 | −0.11; P > 0.5 | −0.37; P < 0.04 |
| HARS | 0.80; P < 0.001 | 1.00 | 0.026; P > 0.9 | −0.19; P > 0.3 |
| COWAT | −0.11; P > 0.5 | 0.026; P > 0.9 | 1.00 | 0.22; P > 0.1 |
| CVLT | −0.37; P < 0.04 | −0.19; P > 0.3 | 0.22; P > 0.1 | 1.00 |
Comparison of Cerebral Glucose Metabolism Prior to and During Citalopram Treatment
The results of the SPM analysis to compare glucose metabolism during citalopram treatment to baseline metabolism before treatment are shown in Table III (decreases and increases in metabolism, respectively). Decreased metabolism was observed in (i) limbic regions including the right ACC (BA 24), bilateral posterior cingulate cortex (PCC; BA 31), bilateral PHG (BA 36), left insula (BA 13), and bilateral amygdala, (ii) frontal cortical regions including the bilateral superior (BA 6) and medial frontal gyrus (BA 10), (iii) temporal cortical regions including left superior and right middle temporal gyrus (STG/MTG; BA 22/21), and (iv) left precuneus (BA 7).
Table III.
Decreases and increases in cerebral glucose metabolism during citalopram treatment (8 weeks)
| Left Hemisphere | Structure | Right Hemisphere | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | x (mm) | y (mm) | z (mm) | Z score | Cluster size | |
| Decreases in cerebral glucose metabolism during citalopram treatment (8 weeks) | ||||||||||
| Anterior cingulate (BA 24) | 8 | −8 | 41 | 3.78 | 27,907 | |||||
| −4 | 14 | 62 | 4.21 | 1,186 | Superior frontal gyrus (BA 6 ) | 16 | 57 | 31 | 3.08 | 315 |
| −9 | 29 | 37 | 3.32 | 7,646 | Medial frontal gyrus (BA 10) | 8 | 43 | 38 | 2.6 | 905 |
| −43 | −40 | 7 | 3.79 | 5,237 | Superior temporal gyrus (BA 22) | |||||
| Middle temporal gyrus (BA 21) | 57 | 1 | −25 | 3.9 | 15,647 | |||||
| −18 | −81 | 42 | 3.45 | 727 | Precuneus (BA 7) | |||||
| −5 | −46 | 18 | 3.47 | 723 | Posterior cingulate (BA 31) | 10 | −45 | 25 | 3.38 | 1,409 |
| −29 | 23 | 3 | 3.56 | 1,680 | Insula (BA 13) | |||||
| −27 | −18 | −24 | 3.08 | 1,884 | Parahippocampal gyrus (BA 36) | 22 | −39 | −10 | 3.19 | 1,582 |
| −19 | −8 | −7 | 4.49 | 776 | Amygdala | 23 | −5 | −22 | 3.58 | 188 |
| Increases in cerebral glucose metabolism during Citalopram treatment (8 weeks) | ||||||||||
| −30 | −7 | 7 | 3.06 | 1,181 | Putamen | 31 | −17 | −1 | 3.84 | 357 |
| Thalamus (pulvinar) | 15 | −27 | 9 | 3.59 | 1,960 | |||||
| −43 | −62 | 46 | 3.69 | 585 | Inferior parietal lobule (BA 40) | 34 | −59 | 44 | 3.19 | 3,477 |
| Cuneus (BA 7) | 16 | −74 | 30 | 3.53 | 1,716 | |||||
| −28 | −79 | −16 | 3.41 | 2,761 | Cerebellum (declive) | 16 | −77 | −21 | 3.76 | 1,224 |
Increased metabolism was observed in the (i) right thalamus (pulvinar and medial dorsal nuclei), (ii) dorsal striatum (bilateral putamen), (iii) the right cuneus (BA 19), (iv) left inferior parietal lobule (IPL BA 40), and (v) bilateral cerebellum.
Behavior PLS Results
Behavior PLS analysis produced two mutually orthogonal latent variables (LV1 = 32.48% crossblock covariance, P < 0.006; LV2 = 27.07% crossblock covariance, P < 0.002) that captured distinct brain‐behavior correlation patterns for the affective and the cognitive measures, respectively, following citalopram treatment.
Affective measures
The first LV captured the correlations between the affective measures (HDRS and HARS) and cerebral glucose metabolism following citalopram treatment (Fig. 1A). No stable brain‐behavior correlations were observed with the cognitive measures; the correlation patterns for the cognitive variables reflected by the first LV were negligible (close to zero; see Fig. 1A). The brain regions exhibiting stable brain‐behavior correlations for HDRS and HARS measures are listed in Table IV. Regions with positive bootstrap ratios (BSR) reflect positive correlations between the two measures, whereas regions with negative BSR reflect negative correlations. Improvements in mood and anxiety symptoms are reflected as lower scores on the HDRS and HARS measures.
Figure 1.
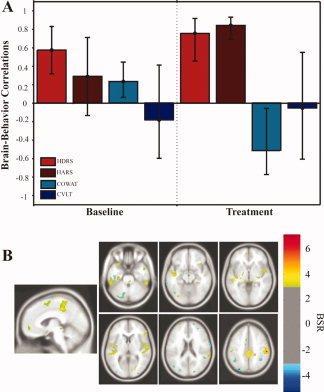
Behavior PLS results (LV1): Affective measures.
The brain regions that showed decreases in metabolism and correlated with a reduction in both depressive and anxiety symptoms included the bilateral amygdala, bilateral ACC (BA 24), left posterior cingulate gyrus (BA 31), frontal regions including the bilateral superior frontal gyrus (BA 9/BA 6), the left middle frontal gyrus (BA 46), right insula (BA 13), and left superior temporal gyrus (STG, BA 22). Negative correlations with the HDRS and HARS measures were noted in the right middle frontal gyrus (BA 46) and the left superior parietal lobule (BA 39) (Fig. 1B; Table IV). The brain‐behavior correlation patterns of the two clinical measures, HARS and HDRS, overlapped significantly, which is consistent with previous evidence of comorbidity of depressive and anxiety symptoms in geriatric depression [as reviewed by Lenze et al., 2001].
Cognitive measures
The second LV captured the correlations between the cognitive measures (COWAT and CVLT) and cerebral glucose metabolism following citalopram treatment (Fig. 2A). This LV reflects a lack of stable brain‐behavior correlations with the affective measures, as suggested by the large confidence intervals that are close to zero (see Fig. 2A). All regions with stable brain‐behavior correlations for the cognitive measures are listed in Table V. Regions with positive BSR reflect negative brain‐behavior correlations, while regions with negative BSR reflect positive correlations. Improvements in immediate verbal fluency and verbal learning/memory are reflected as increased scores on the COWAT and CVLT measures.
Figure 2.
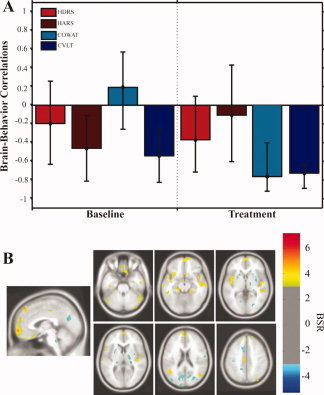
Behavior PLS results (LV2): Cognitive measures.
The brain regions that showed decreases in metabolism and correlated with an improvement in verbal fluency included the bilateral PHG (BA 36), frontal regions [left medial frontal gyrus (BA 10), right middle frontal gyrus (BA 46), left orbitofrontal cortex (BA 11)], temporal regions [left MTG (BA 21), right ITG (BA 20)], left precuneus (BA 7), and the bilateral cerebellum. Positive correlations with the COWAT measure were noted in the right lingual gyrus (BA 18), left fusiform gyrus (BA 37), and right putamen (Fig. 2B; Table V).
The brain regions that showed decreases in metabolism and correlated with improvement in immediate verbal learning/memory included: the bilateral hippocampus, right PHG (BA 36), frontal regions [bilateral orbitofrontal gyrus (BA 11), m edial frontal gyrus (BA 10), middle frontal gyrus (BA 46)], temporal regions [bilateral ITG (BA 20) and bilateral MTG (BA 21)], parietal regions [the left IPL (BA 40), right SPL (BA 39), and bilateral precuneus (BA 7)]. The putamen, right superior occipital gyrus (BA 19), and left fusiform gyrus correlated positively with the CVLT measure (Fig. 2B; Table V).
Functional Connectivity (seed PLS) Results
The results of the behavior PLS analyses were evaluated to identify the brain regions that reliably correlated with improvement in affective symptoms, and not cognitive function (and vice versa). Those brain regions were used as seeds for the subsequent seed PLS analysis.
ACC seed and improvement in affect (depressive mood and anxiety)
The left ACC showed decreased metabolism during treatment that correlated significantly with improved HDRS and HARS scores (r = 0.72 and r = 0.54, P < 0.05, respectively; Table VI), but not with the cognitive measures: COWAT and CVLT (Table VI). Thus, the left ACC (BA 24) (MNI = −1, −34, 43) was chosen as seed in the functional connectivity analysis to determine the network associated with improvement in affective symptoms.
The network of regions that correlated with the left ACC seed and with improved affect (LV1 = 52.91% cross‐block covariance, P < 0.01) was comprised of the left amygdala, frontal regions [right orbitofrontal cortex (BA 11), bilateral medial frontal gyrus (BA 10), bilateral middle frontal gyrus (BA 46), bilateral superior frontal gyrus (BA 6), and right inferior frontal gyrus (BA 45)], right ACC (BA 24), the bilateral insula (BA 13), and left midbrain. In contrast, the right inferior occipital gyrus and fusiform gyrus (BA 18/37) exhibited increases in glucose metabolism and correlated with improvements in mood/anxiety symptoms (Table VII). A schematic diagram of the functional network obtained is shown in Figure 3.
Table VII.
Local maxima from spatiotemporal seed PLS with the left ACC as seed
| X (mm) | Y (mm) | Z (mm) | BSR | Cluster Size (voxels) | Laterality | Region | BA |
|---|---|---|---|---|---|---|---|
| 0 | −27 | −5 | 3.08 | 7 | Left Midbrain | ||
| −28 | −6 | −18 | 3.39 | 42 | Left | Amygdala | |
| 5 | 33 | 36 | 3.39 | 94 | Right | Anterior cingulate cortex | BA 24 |
| −15 | 2 | 71 | 3.26 | 3,492 | Left | Superior frontal gyrus | BA 6 |
| 20 | 31 | 55 | 5.81 | 357 | Right | Superior frontal gyrus | BA 6 |
| −6 | 54 | −9 | 4.74 | 386 | Left | Superior‐medial frontal gyrus | BA 10 |
| 17 | 70 | 16 | 4.26 | 69 | Right | Superior‐medial frontal gyrus | BA 10 |
| −25 | 38 | 32 | 6.01 | 943 | Left | Middle frontal gyrus | BA 46 |
| 53 | 48 | 3 | 5.50 | 295 | Right | Middle frontal gyrus | BA 46 |
| 54 | 22 | 33 | 3.25 | 28 | Right | Inferior frontal gyrus | BA 45 |
| 6 | 53 | −22 | 4.56 | 605 | Right | Orbitofrontal cortex | BA 11 |
| −35 | −12 | 7 | 3.35 | 48 | Left | Insula | BA 13 |
| 44 | −11 | 7 | 3.39 | 102 | Right | Insula | BA 13 |
| 47 | −83 | −9 | −3.29 | 18 | Right | Inferior occipital gyrus | BA 18 |
| 39 | −57 | −10 | −3.59 | 45 | Right | Fusiform gyrus | BA 37 |
Figure 3.
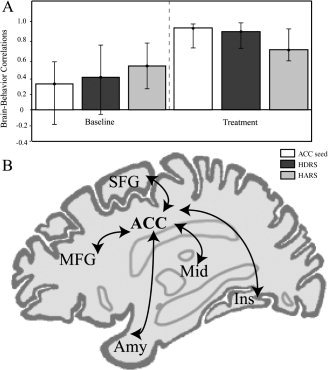
Functional network associated with affective symptom improvement.
PHG seed and improvement in cognitive function (verbal fluency and immediate verbal learning/memory)
The evaluation of the behavioral PLS results indicated that the right PHG (BA 36) (MNI = 22, −39, −4) showed decreased metabolism during treatment and correlated significantly with improved cognitive function as measured with COWAT and CVLT (r = −0.42, r = −0.60, P < 0.05, respectively; Table VIII). In contrast, glucose metabolism measured at the right PHG seed did not correlate with the affective measures (HDRS, HARS) (P > 0.3; Table VI).
Table VIII.
Local maxima from spatiotemporal seed PLS with the right PHG as seed
| X (mm) | Y (mm) | Z (mm) | BSR | Cluster Size (voxels) | Laterality | Region | BA |
|---|---|---|---|---|---|---|---|
| −12 | −7 | −23 | 6.41 | 906 | Left | Hippocampus | |
| −27 | 44 | 21 | 4.48 | 225 | Left | Middle frontal gyrus | BA 46 |
| 33 | 47 | 11 | 3.58 | 204 | Right | Middle frontal gyrus | BA 46 |
| −1 | 32 | −14 | 5.55 | 227 | Left | Orbitofrontal cortex | BA 11 |
| 20 | 39 | −14 | 4.09 | 56 | Right | Orbitofrontal cortex | BA 11 |
| −26 | 28 | −22 | 4.29 | 147 | Left | Inferior Frontal Gyrus | BA 47 |
| −34 | 10 | 17 | −4.91 | 357 | Left | Insula | BA 13 |
| 65 | −9 | 6 | 3.68 | 37 | Right | Superior temporal gyrus | BA 22 |
| −66 | −7 | −19 | 3.91 | 58 | Left | Middle temporal gyrus | BA 21 |
| 53 | −59 | −17 | 4.64 | 93 | Right | Middle temporal gyrus | BA 21 |
| −48 | −52 | −13 | 5.25 | 520 | Left | Inferior temporal gyrus | BA 20 |
| 41 | −24 | 51 | 6.21 | 395 | Right | Postcentral gyrus | BA 2 |
| −47 | −55 | 49 | 5.74 | 614 | Left | Inferior parietal lobule | BA 40 |
| −12 | −91 | −11 | −4.86 | 459 | Left | Lateral occipital gyrus | BA 19 |
| 23 | −100 | 31 | −3.18 | 16 | Right | Lateral occipital gyrus | BA 19 |
| 24 | −87 | 17 | −3.23 | 22 | Right | Superior occipital gyrus | BA 39 |
| 27 | −48 | −16 | −4.67 | 431 | Right | Fusiform gyrus | BA 37 |
| −19 | −65 | −37 | 5.26 | 1,835 | Left cerebellum | Pyramis | |
| −27 | −54 | −49 | 4.52 | 843 | Left cerebellum | ||
| 10 | −50 | −46 | 4.77 | 878 | Right cerebellum |
The network of regions that correlated with the right PHG seed and with improved scores in the COWAT and CVLT measures (LV1 = 39.53% cross‐block covariance, P < 0.0001) included the left hippocampus, frontal [bilateral middle frontal gyrus (BA 46), bilateral orbitofrontal cortex (BA 11), and left inferior frontal gyrus (BA 47)], temporal regions [left ITG (BA 20), bilateral MTG (BA 21), and right STG (BA 22)], parietal regions [left IPL (BA 40) and right post‐central gyrus (BA 2)], and the bilateral cerebellum (Table VIII). In contrast, the bilateral insula and occipital areas [bilateral lateral occipital gyrus (BA 19), right superior occipital gyrus (BA 39), and right fusiform gyrus (BA 37)] showed increased metabolism and also correlated with improvements in the two cognitive measures. A schematic diagram of the functional network obtained is illustrated in Figure 4.
Figure 4.
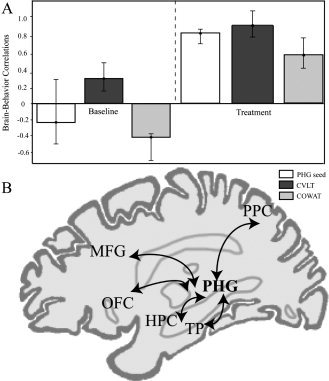
Functional network associated with cognitive improvement.
DISCUSSION
The Affective and Cognitive Effects of Chronic Citalopram Treatment
After 8 weeks of citalopram treatment, patients showed significant improvements in affective symptoms and cognitive function, as well as alterations in cerebral glucose metabolism. A significant reduction in depressive and anxiety symptoms was observed (HDRS and HARS), as well as improvements in immediate verbal learning/memory, and verbal fluency. Upon evaluating the inter‐correlations between the affective and cognitive variables, a significant correlation between the HDRS and HARS was observed, as expected, given that improvement of both depressive and anxiety symptoms is observed with antidepressant treatment [e.g., Lenze et al., 2003]. As performance on cognitive tests (including attention and memory) improves with depressive symptom remission, a significant correlation between the HDRS and the CVLT measures was also observed, as expected [cf. Butters et al., 2000]. In contrast, the HARS was not significantly correlated with any of the cognitive measures which might be explained by the moderate levels of anxiety in this patient sample. The lack of significant correlation between the COWAT and CVLT suggested that while improvement is observed on both tests, the magnitude of improvement may not be intercorrelated because performance on these tests is dependent on multiple aspects of cognition that overlap to some extent (e.g., the learning component of the CVLT, the retrieval of information in long term storage involved in the COWAT).
The Cerebral Metabolic Effects of Chronic Citalopram Treatment
Decreased glucose metabolism during citalopram treatment was observed in the ACC (BA 24), insula (BA 13), amygdala, PHG, superior and medial frontal, precuneus, medial and lateral temporal cortical regions. Previous PET studies have identified similar reductions in glucose metabolism with antidepressant treatment in anterior regions as observed in the present study, including bilateral anterior cingulate [Brody et al., 2001; Mayberg et al., 2000; Smith et al., 2002a; Vlassenko et al., 2004], superior frontal gyrus (BA 6) [Brody et al., 1999; Goldapple et al., 2004; Smith et al., 2002a], and medial frontal gyrus (BA 10) [Buchsbaum et al., 1997; Brody et al., 1999, 2001; Smith et al., 2002a; Vlassenko et al., 2004]. The regions that showed decreased cerebral glucose metabolism and that were associated with improved cognitive function in the present study (hippocampus and parahippocampal gyrus) have also shown reduced metabolism with antidepressant treatment in studies of mid‐life depressed patients [Goldapple et al., 2004; Kennedy et al., 2001; Mayberg et al., 1999, 2000].
The brain regions that showed decreased glucose metabolism during citalopram treatment in the present study have also shown altered functional brain activity to cognitive and affective stimuli in older depressed patients relative to controls. A PET study in geriatric depression found reduced hippocampal and bilateral cingulate activity during verbal fluency tasks [de Asis et al., 2001]. Another PET study investigating limbic‐frontal activity in response to negatively valenced autobiographical scripts found decreased activity in the subgenual cingulate and increased activity in the dorsolateral prefrontal and inferior parietal regions that were associated with recovery from depression in mid‐life patients [Mayberg et al., 1999].
The Relationship of the Alterations in Cerebral Glucose Metabolism to Changes in Affective Symptoms and Cognitive Function
The results of the behavior PLS analysis suggest distinct functional networks associated with affective symptoms and cognitive function during citalopram treatment. Studies in mid‐life depressed patients have implicated more rostral aspects of the ACC (BA 24/25), while in the present study, brain‐behavior correlations are observed with the more dorsal aspects of the ACC (see Fig. 1). The dorsal ACC also shows reduced glucose metabolism during acute citalopram administration [Smith et al., 2009b].
The regions exhibiting strong relationships with improved verbal fluency and immediate verbal learning/memory function (as measured with COWAT and CVLT, respectively) included the parahippocampal gyrus and the orbitofrontal cortex (see Fig. 2). These areas show reduced gray matter volumes in geriatric depression in some studies which suggests that these regions may be involved in a neurodegenerative disease process [Ashtari et al., 1999; Lai et al., 2000; Lee et al., 2003].
Functional connectivity analyses were performed in the interest of distinguishing the regional metabolic changes that relate to improvements in affective symptoms from those related to improvements in cognitive function. Two functional networks that were distinctly associated with improvements in affective symptoms and cognitive function during treatment were identified.
Functional Network Associated with Affective Symptom Improvement
A subcortical‐limbic‐frontal functional network was associated with improved affect (depression and anxiety) during citalopram treatment. The functional network was comprised of the ACC seed, left amygdala, bilateral middle (BA 46) and superior frontal (BA 6/9) cortical regions, and bilateral insula (BA 13) (Table VII; Fig. 3). In addition to frontal regions that have previously exhibited altered activity patterns for cognitive and affective stimuli in geriatric patients [Aizenstein et al., 2005], the amygdala and insula have also been associated with reductions in cerebral glucose metabolism during antidepressant treatment [Kennedy et al., 2001; Mayberg et al., 2000].
Recent fMRI studies have shown reduced prefrontal activity in sequence learning tasks [Aizenstein et al., 2005] in elderly patients with depression, and increased BOLD activity in the anterior cingulate (BA 24) [Gotlib et al., 2005] and amygdala [Canli et al., 2005] in response to negatively valenced stimuli in young and middle‐aged adults with depression. Studies in younger depressed patients using positively valenced stimuli reported decreased activation in a large cluster of regions including the left orbital‐frontal region [Keedwell et al., 2005; Lawrence et al., 2004], and pregenual and posterior cingulate cortices [Canli et al., 2004].
These activation studies have shown decreased responsiveness in the amygdala, cingulate, and frontal cortices in mid‐life depressed patients. The present study, however, suggests that depressive and anxiety symptom improvements are associated with decreased metabolism in cingulate and amygdala regions during citalopram treatment in older patients with depression, the majority of whom had not been treated previously with antidepressant medications. While these affective and cognitive activation studies generally showed blunted activation responses, when studied during the “resting” state, cerebral glucose metabolism is increased in geriatric depression and is reduced during treatment [Smith et al., 2009a, b]. This apparent discrepancy may suggest that metabolism may reflect a different physiological process that represents a compensatory response for the inability to engage these brain regions in affective or cognitive processing tasks.
With respect to the neurochemical substrates of the network, the cortical and midbrain regions involved suggest that interactions between sub‐cortical monoamine systems (serotonin and dopamine) and cortical glutamate systems that are modulated by citalopram treatment may underlie the cerebral metabolic alterations associated with the observed improvements in depression and anxiety [as reviewed by Pralong et al., 2002; Smith et al., 2002b, 2009a].
Functional Network Associated with Cognitive Improvement
A medial temporal‐parietal‐frontal functional network was related uniquely to improved cognitive function (measured with CVLT and COWAT) during citalopram treatment. The network was comprised of the right PHG seed, left hippocampus, middle frontal, bilateral orbitofrontal (BA 11), medial temporal, and posterior parietal cortices, such as the IPL (BA 40) (Table VIII; Fig. 4).
The medial temporal‐parietal‐frontal network identified has been previously associated with several processes including visuo‐spatial imagery, episodic memory retrieval, and self‐processing, as reviewed in [Cabeza and Nyberg, 2000]. Since medial temporal and parietal regions are associated with verbal fluency and immediate verbal learning/memory [Cabeza et al., 1997; Daselaar et al., 2001; Pihlajamaki et al., 2000], improvement in these specific tasks with citalopram treatment may be related to the modulation of the temporal‐parietal‐frontal network identified.
Verbal fluency and verbal learning/memory are cognitive processes that are specifically impaired in geriatric depression [Butters et al., 2000]. In some studies, deficits on such tests persist after remission of depression symptoms [Alexopoulos et al., 2005; Bhalla et al., 2006; Lockwood et al., 2000; Nebes et al., 2000]. The geriatric depressed patients in the present study were not as cognitively impaired as the subjects enrolled in the aforementioned studies (as shown by the MMSE and DRS scores of the patients in the present study that fall within the ranges reported in nondemented, healthy control subjects). In addition, the patients in this study were medically stable, and, over 70% of them, were not previously treated with antidepressant medications. These factors (i.e., later onset of depression and low medical comorbidity) may explain the differences in baseline cognitive function between the patients enrolled in the present study and patients with persistent cognitive impairment reported in previous studies of geriatric depression. However, geriatric depressed patients who demonstrated “reversible” cognitive impairment during the index depressed episode have been show to decline cognitively despite an initial improvement in cognition [Alexopoulos et al., 1996]. Longitudinal studies in geriatric depressed patients are critically needed to further understand the prognostic significance of the cognitive and cerebral metabolic responses to antidepressant treatment.
Considerations Regarding the Study Design and Data Interpretation
Several issues should be considered when interpreting the present findings. This was a complex study involving PET imaging, and clinical and cognitive assessments before and during treatment. The sample size is relatively small as it was extremely challenging to recruit never medicated, medically stable geriatric depressed patients. Thus, the study sacrifices the generalizability of the results for the purpose of attaining strong internal validity. The present findings should be replicated in a larger sample of patients. Another issue is that the study did not include a nondepressed comparison group evaluated and rescanned at the same time interval as the patients. Thus, the validity of the networks in control subjects over time cannot be evaluated. Another issue is the lack of a placebo control group. Changes in cerebral glucose metabolism similar to those observed with antidepressant treatment have been observed in placebo treated patients [Mayberg, 2002]. While the effects of placebo response cannot be ruled out, it is important to note the rate of treatment nonresponse observed in the study was about 25%, similar to that observed in placebo‐controlled antidepressant clinical trials in geriatric depression [e.g., Little et al., 1998].
SUMMARY
In summary, reductions in cerebral glucose metabolism were observed during 8 weeks of citalopram treatment in geriatric depressed patients that were more extensive with respect to decreases in regional cerebral glucose metabolism than that observed after acute antidepressant interventions [TSD and acute citalopram administration; Smith et al., 2002a, b, c, 2009a]. The application of the PLS method in the present study identified the functional networks associated with improvements in affective symptoms in contrast to those associated with improvements in cognitive function, as hypothesized. Many of the regions implicated in the PLS analysis also show metabolic effects in the SPM analyses in the present study, as well as previous studies in geriatric depression [e.g., Smith et al., 2002a, b, 2009a] and are consistent with regions implicated in affective and cognitive activation studies, as discussed in this section. The underlying mechanisms of the midbrain‐limbic‐frontal affective network may involve interactions between monoaminergic and glutamatergic systems. The regions involved in the medial temporal‐ parietal‐frontal cognitive network overlap with the regions affected in Alzheimer's dementia and may reflect neuronal vulnerability to a neurodegenerative processes [such as β‐amyloid deposition, Buckner et al., 2005]. An understanding of the cerebral metabolic networks associated with the affective and cognitive responses to antidepressant treatment is critical to the design of future mechanistic studies. The application of molecular neuroimaging methods to evaluate the pathophysiology of affective and cognitive symptoms may inform strategies for improved clinical management of affective symptoms and for prevention of neurodegenerative processes that might underlie progressive cognitive dysfunction in geriatric depression.
Acknowledgements
Citalopram medication was provided by Forest Laboratories. David Bjelke, CNMT, and Claude Margouleff, B.S. are gratefully acknowledged for their contribution to the conduct of the PET studies.
REFERENCES
- Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF III, Carter CS ( 2005): Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry 58: 290–296. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Young RC, Meyers BS ( 1993): Geriatric depression: Age of onset and dementia. Biol Psychiatry 34: 141–145. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Feder M, Einhorn A, Rosendahl E ( 1996): Recovery in geriatric depression. Arch Gen Psychiatry 53: 305–312. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning‐Dixon F ( 2005): Executive dysfunction and the course of geriatric depression. Biol Psychiatry 58: 204–210. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Greenwald BS, Kramer‐Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S ( 1999): Hippocampal/amygdala volumes in geriatric depression. Psychol Med 29: 629–638. [DOI] [PubMed] [Google Scholar]
- Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M ( 1988): Reproducibility of cerebral glucose metabolic measurements in resting human subjects. J Cereb Blood Flow Metab 8: 502–512. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K ( 1978): Multilingual Aphasia Examination. Iowa City: University of Iowa. [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF III, Becker JT ( 2006): Persistence of neuropsychologic deficits in the remitted state of late‐life depression. Am J Geriatr Psychiatry 14: 419–427. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR Jr ( 1999): Brain metabolic changes in major depressive disorder from pre‐ to post‐treatment with paroxetine. Psychiatry Res 91: 127–139. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR Jr ( 2001): Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: Preliminary findings. Arch Gen Psychiatry 58: 631–640. [DOI] [PubMed] [Google Scholar]
- Bruce ML, Leaf PJ ( 1989): Psychiatric disorders and 15‐month mortality in a community sample of older adults. Am J Public Health 79: 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C ( 1997): Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 41: 15–22. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA ( 2005): Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25: 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF III ( 2000): Changes in cognitive functioning following treatment of late‐life depression. Am J Psychiatry 157: 1949–1954. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FIM ( 1997): Age‐related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci 17: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield‐Gabrieli S, Gabrieli JD, Gotlib IH ( 2004): Brain activation to emotional words in depressed vs healthy subjects. Neuroreport 15: 2585–2588. [DOI] [PubMed] [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, Whitfield‐Gabrieli S, Gabrieli JD, Gotlib IH ( 2005): Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport 16: 1267–1270. [DOI] [PubMed] [Google Scholar]
- Conwell Y, Duberstein PR, Cox C, Herrmann JH, Forbes NT, Caine ED ( 1996): Relationships of age and axis I diagnoses in victims of completed suicide: A psychological autopsy study. Am J Psychiatry 153: 1001–1008. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Lazeron RH, Jonker C ( 2001): Parahippocampal activation during successful recognition of words: A self‐paced event‐related fMRI study. Neuroimage 13: 1113–1120. [DOI] [PubMed] [Google Scholar]
- de Asis JM, Stern E, Alexopoulos GS, Pan H, Van Gorp W, Blumberg H, Kalayam B, Eidelberg D, Kiosses D, Silbersweig DA ( 2001): Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry 158: 1321–1323. [DOI] [PubMed] [Google Scholar]
- Delis DC, Freeland J, Kramer JH, Kaplan E ( 1988): Integrating clinical assessment with cognitive neuroscience: Construct validation of the California Verbal Learning Test. J Consult Clin Psychol 56: 123–130. [DOI] [PubMed] [Google Scholar]
- Dew MA, Reynolds CF III, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ ( 1997): Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry 54: 1016–1024. [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Menon M, Jensen J, Kapur S, McIntosh AR ( 2010): Dopamine‐induced changes in neural network patterns supporting aversive conditioning. Brain Res 1313: 143–161. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J ( 1995): Structured clinical interview for DSM‐IV axis 1 disorders‐patient edition (SCID‐I/P). New York: New York Psychiatric Institute. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Friston KJ, editor ( 2007): Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press. [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H ( 2004): Modulation of cortical‐limbic pathways in major depression: Treatment‐specific effects of cognitive behavior therapy. Arch Gen Psychiatry 61: 34–41. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield‐Gabrieli S, Goldin P, Minor KL, Canli T ( 2005): Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport 16: 1731–1734. [DOI] [PubMed] [Google Scholar]
- Guy W ( 1976): An Assessment Manual for Psychopharmacology, Vol. 76. US Department of Health Education and Welfare Publication. p 336.
- Hamilton M ( 1959): The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M ( 1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksson M, Marttunen I, Isometsa E ( 1995): Mental disorders in elderly suicide. Int Psychogeriatr 7: 275–286. [DOI] [PubMed] [Google Scholar]
- Jorm AF ( 2001): History of depression as a risk factor for dementia: An updated review. Aust N Z J Psychiatry 35: 776–781. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML ( 2005): A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry 58: 495–503. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ ( 2001): Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 158: 899–905. [DOI] [PubMed] [Google Scholar]
- Kramer‐Ginsberg E, Greenwald BS, Krishnan KR, Christiansen B, Hu J, Ashtari M, Patel M, Pollack S ( 1999): Neuropsychological functioning and MRI signal hyperintensities in geriatric depression. Am J Psychiatry 156: 438–444. [DOI] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR ( 2000): Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry 48: 971–975. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML ( 2004): Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55: 578–587. [DOI] [PubMed] [Google Scholar]
- Lee SH, Payne ME, Steffens DC, McQuoid DR, Lai TJ, Provenzale JM, Krishnan KR ( 2003): Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry 54: 529–533. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Mulsant BH, Shear MK, Alexopoulos GS, Frank E, Reynolds CF III ( 2001): Comorbidity of depression and anxiety disorders in later life. Depress Anxiety 14: 86–93. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Mulsant BH, Dew MA, Shear MK, Houck P, Pollock BG, Reynolds CF III ( 2003): Good treatment outcomes in late‐life depression with comorbid anxiety. J Affect Disord 77: 247–254. [DOI] [PubMed] [Google Scholar]
- Little J, Reynolds Cr, Dew M, Frank E, Begley A, Miller M, Cornes C, Mazumdar S, Perel J, Kupfer D ( 1998): How common is resistance to treatment in recurrent, nonpsychotic geriatric depression? Am J Psychiatry 155: 1035–1038. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG ( 2000): Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry 8: 201–208. [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, van Gorp WG ( 2002): Executive dysfunction in geriatric depression. Am J Psychiatry 159: 1119–1126. [DOI] [PubMed] [Google Scholar]
- Mattis S ( 1976): Mental status examination for organic mental syndrome in the elderly patient In: Bellak L, Karasu T, editors. Geriatric Psychiatry. New York: Grune and Stratton; pp 77–121. [Google Scholar]
- Mayberg HS ( 2002): Modulating limbic‐cortical circuits in depression: Targets of antidepressant treatments. Semin Clin Neuropsychiatry 7: 255–268. [DOI] [PubMed] [Google Scholar]
- Mayberg HS ( 2003): Modulating dysfunctional limbic‐cortical circuits in depression: Towards development of brain‐based algorithms for diagnosis and optimised treatment. Br Med Bull 65: 193–207. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT ( 1997): Cingulate function in depression: A potential predictor of treatment response. Neuroreport 8: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT ( 1999): Reciprocal limbic‐cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA ( 2000): Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry 48: 830–843. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ ( 2004): Partial least squares analysis of neuroimaging data: Applications and advances. Neuroimage 23 ( Suppl 1): S250–S263. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL ( 1996): Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3: 143–157. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB ( 2004): Spatiotemporal analysis of event‐related fMRI data using partial least squares. Neuroimage 23: 764–775. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, Reynolds CF III ( 2000): Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med 30: 679–691. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF III ( 2003): Persistence of cognitive impairment in geriatric patients following antidepressant treatment: A randomized, double‐blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res 37: 99–108. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D ( 2006): Depression and risk for Alzheimer disease: Systematic review, meta‐analysis, and metaregression analysis. Arch Gen Psychiatry 63: 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ ( 1979): Tomographic measurement of local cerebral glucose metabolic rate in humans with (18F) 2‐fluoro‐2‐deoxy‐d‐glucose. Ann Neurol 6: 371–388 [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Laakso M, Partanen K, Soininen H, Aronen HJ ( 2000): Verbal fluency activates the left medial temporal lobe: A functional magnetic resonance imaging study. Ann Neurol 47: 470–476. [PubMed] [Google Scholar]
- Pralong E, Magistretti P, Stoop R ( 2002): Cellular perspectives on the glutamate‐monoamine interactions in limbic lobe structures and their relevance for some psychiatric disorders. Prog Neurobiol 67: 173–202. [DOI] [PubMed] [Google Scholar]
- Robeson W, Dhawan V, Takikawa S, Babchyck B, Zanzi I, Margouleff D, Eidelberg D ( 1993): Superpett 3000: Time of flight PET tomograph: Optimization of factors affecting quantitation. IEEE Trans Nucl Sci 40: 135–142. [Google Scholar]
- Sackeim HA, Roose SP, Lavori PW ( 2006): Determining the duration of antidepressant treatment: Application of signal detection methodology and the need for duration adaptive designs (DAD). Biol Psychiatry 59: 483–492. [DOI] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF III, Pollock B, Derbyshire S, Nofzinger E, Dew MA, Houck PR, Milko D, Meltzer CC, Kupfer DJ ( 1999): Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry 156: 683–689. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Hermann CR, Goldberg S, Ma Y, Dhawan V, Barnes A, Chaly T, Belakhleff A, Laghrissi‐Thode F, Greenwald B, Eidelberg D, Pollock BG ( 2002a) Acute and chronic effects of citalopram on cerebral glucose metabolism in geriatric depression. Am J Geriatr Psychiatry 10: 715–723. [PubMed] [Google Scholar]
- Smith GS, Ma Y, Dhawan V, Gunduz H, Carbon M, Kirshner M, Larson J, Chaly T, Belakhleff A, Kramer E, Greenwald B, Kane JM, Laghrissi‐Thode F, Pollock BG, Eidelber D ( 2002b) Serotonin modulation of cerebral glucose metabolism measured with positron emission tomography (PET) in human subjects. Synapse 45: 105–112. [DOI] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF III, Houck PR, Dew MA, Ma Y, Mulsant BH, Pollock BG ( 2002c) Glucose metabolic response to total sleep deprivation, recovery sleep, and acute antidepressant treatment as functional neuroanatomic correlates of treatment outcome in geriatric depression. Am J Geriatr Psychiatry 10: 561–567. [PubMed] [Google Scholar]
- Smith GS, Gunning‐Dixon FM, Lotrich FE, Taylor WD, Evans JD ( 2007): Translational research in late‐life mood disorders: Implications for future intervention and prevention research. Neuropsychopharmacology 32: 1857–1875. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kahn A, Hanratty K, Sacher J, Meyer J, Rusjan P, Wilson A, Flint A, Mulsant B, Houle S ( 2008) Serotonin Transporter Occupancy by Citalopram Treatment in Geriatric Depression. Neuroimage 41 ( 2): T168. [Google Scholar]
- Smith GS, Kramer E, Hermann C, Ma Y, Dhawan V, Chaly T, Eidelberg D ( 2009a) Serotonin modulation of cerebral glucose metabolism in depressed older adults. Biol Psychiatry 66: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, Eidelberg D ( 2009b) The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry 24: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML ( 2005): A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 57: 201–209. [DOI] [PubMed] [Google Scholar]
- Takikawa S, Dhawan V, Spetsieris P, Robeson W, Chaly T, Dahl R, Margouleff D, Eidelberg D ( 1993): Noninvasive quantitative fluorodeoxyglucose PET studies with an estimated input function derived from a population‐based arterial blood curve. Radiology 188: 131–136. [DOI] [PubMed] [Google Scholar]
- Vlassenko A, Sheline YI, Fischer K, Mintun MA ( 2004): Cerebral perfusion response to successful treatment of depression with different serotoninergic agents. J Neuropsychiatry Clin Neurosci 16: 360–363. [DOI] [PubMed] [Google Scholar]


