Abstract
OBJECTIVE
To determine whether there are any age-related disparities in the frequency of provision of counseling and education for diabetes care in a large HMO in Central Texas.
METHODS
EMR search from 13 primary care clinics on patients aged ≥18 years (n=1300) who had been diagnosed with type 2 diabetes.
RESULTS
There were no significant age differences in the frequency of provision of counseling about HBGM, diet, smoking or diabetes education. However, there were significant age differences in the provision of exercise counseling. Patients aged ≥75 were significantly less likely to have been provided exercise counseling than those aged <65 (adjusted OR=0.60; 95% CI=0.37–0.98). The mean HbA1c for patients aged ≥75 and 65–74 were significantly lower than that of patients aged <65 (8.9 vs. 9.0 vs. 9.7; P<.001).
CONCLUSION
While age-related variations in self-management protocols were not found, the provision of formal diabetes education was low (29.4%). The persistence of key risk factors in later life (e.g., obesity) underscores the need for better self-management protocols for older adults.
PRACTICE IMPLICATIONS
Additional efforts on strategies to increase counseling about lifestyle habits and diabetes self-management care by appropriate health care providers is needed. Diabetes counseling should be individually tailored in older population.
Keywords: Age-related disparities, health disparities, type 2 diabetes
1. INTRODUCTION
The prevalence of diabetes has been increasing continuously over the years. It also increases with age (1). From 1980 through 2006, the percentage of diagnosed diabetes increased in all age groups. Throughout this time period, however, people aged 65 years or older had a higher percentage increase than those aged younger than 65 years (2). The prevalence of complications of diabetes also increases with age (3). Statistics show that people aged 65 years or older represent the most rapidly growing age group in the U.S. (4). Representing 13% of the total U.S. population in 2005, the proportion of people aged 65 years or older is projected to reach 17% in 2020 and it is estimated that by the year 2050 one in five persons will be aged 65 years or older. In spite of this aging trend, older people with diabetes have received relatively little attention from investigators (5, 6, 7, 8).
Profound disparities in the prevalence and complications of type 2 diabetes (T2DM) have been the subject of multiple investigations. Some of these studies have addressed racial and/or ethnic (9, 10), gender (11, 12), and rural-urban (13, 14) disparities related to diabetes care. For example, two Australian studies demonstrated the rural-urban disparities in cardiovascular risk levels, risk management, and impact on patients with T2DM (13, 14). Growing disparities in diabetes-related mortalities in the U.S. have also been documented (15). However, there is sparse data on age-related disparities in diabetes care (16, 17), despite the documentation of age-related disparities in many health outcomes, particularly chronic diseases (18, 19). For example, some studies have described age-related disparities in cancer screening (20, 21) as well as treatment protocols (22, 23). In a Canadian study of age-related disparity in physician recommendation for colorectal cancer screening, patient age, along with other characteristics, was found to influence physicians' delivery of colorectal cancer screening and choice of modality (20). Upon finding receipt of sub-optimal therapy for breast cancer in older women, Owusu et al demonstrated that receipt of recommended guideline therapy in older women with early stage breast cancer is associated with improved outcomes, particularly among the very old. They further suggested that age should not be used as a criterion for excluding older women from the recommended guideline therapy (22).
Diabetes self-management involves teaching patients how to manage their diabetes (24). Although few studies have focused on age-related disparities in diabetes care (16, 17) as well as age-related changes in clinical outcomes (25), to the best of our knowledge, no study has investigated age-related variations in the provision of diabetes self-management counseling or education for persons with diabetes in multiple primary care clinics. This study evaluated the extent of age-related variations in self-management protocols as well as differences in clinical outcomes in diabetes care among patients with T2DM. We were particularly interested in assessing any in the provision of counseling about diet, exercise, home blood glucose monitoring (HBGM), and smoking cessation as well as provision of formal diabetes education classes, laboratory measures, and HbA1c values.
2. METHODS
2.1. Study Design and Setting
We performed an electronic medical records (EMR) search/chart review and data abstraction on baseline data on selected patients from 13 primary care clinics of a large university-affiliated, multi-specialty group practice, Scott & White. A large healthcare institution located in Central Texas, Scott & White is comprised of the Scott & White Clinic, the Scott & White Memorial Hospital, and a 250,000-member health maintenance organization (HMO) called the Scott & White Health Plan. Scott & White serves as the clinical component of the Texas A&M Health Science Center College of Medicine.
2.2. Study Subjects
Study subjects comprised 1,300 patients aged ≥18 years who had been diagnosed with T2DM with a HbA1c of >8.0% and identified via an exhaustive EMR search using the ICD code 250.xx as having been seen by a family physician at any of the 13 clinics between December 2006 and November 2007. These subjects were selected from a total of 4,351 patients using a random sampling scheme with over-sampling of racial/ethnic minorities and those seen at clinics located in rural settings. The Scott & White Institutional Review Board reviewed and approved the study protocol.
2.3. Assessment and Outcome Measures
We used a standardized form to abstract data electronically from the HMO's data warehouse via the exhaustive EMR search and manually from individual patient records via a chart review. Data abstracted electronically included patient demographic data, laboratory measures, healthcare utilization data, and HbA1c values. The demographic data included age, sex, race/ethnicity, zip code of residence, insurance type, and diabetes duration. Diabetes duration was defined, for the purpose of this study, as the length of time from when a patient entered the HMO's system with a diagnosis of diabetes until the time of data collection. The laboratory measures included total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, creatinine kinase, creatinine kinase myocardial band isoenzyme, and urine micro-albumin. The healthcare utilization data abstracted included hospital admissions, emergency room visits, and specialty care referrals as well as visits to ophthalmology and podiatry. The clinical outcome extracted, HbA1c value, was the most recent in the patients' EMR.
Data abstracted manually included systolic and diastolic blood pressures, height, and weight to compute body mass index (BMI). Two types of diabetes education activities were noted: 1) professional counseling about diet, exercise, home blood glucose monitoring (HBGM), and smoking cessation (as assessed by text that indicated the presence of one or more discussions between providers and their patient about these topics); and 2) ADA-approved diabetes self-management education (DSME). In the Scott & White Healthcare System, patients with diabetes are offered DSME classes by certified diabetes educators. These classes include education on pathophysiology and complications of diabetes, preventive practices (diet, exercise, smoking, monitoring blood glucose, and foot care), medications, heart care, stress management, current American Diabetes Association treatment recommendations, and traveling guidelines. Patients who attended one or more of these classes were considered as having been provided “diabetes education”. Counseling could be provided by primary care physicians, or in some cases by endocrinology or medical nutrition therapy nurses or other health care providers. Clinics were classified as urban or rural using clinic zip code and the Census Bureau definition. Patient residence was similarly categorized as urban or rural.
2.4. Statistical Analysis
Data management and analysis were performed using Stata IC 10 (StataCorp LP, College Station, TX) on a personal computer. Descriptive analyses were performed to determine frequencies, proportions, means, standard errors, and standard deviations. Subjects were categorized into three age groups: ≥75 years, 65 to 74 years, and <65 years. Chi-square tests, Fisher's exact tests, and one-way analysis of variance (ANOVA) tests were used to assess the relationships among the three age groups and the demographic, behavioral, and laboratory measures. Due to small numbers, the variables `smoking behavior' and `provision of counseling on smoking' were excluded from further analyses.
Multivariable logistic regression analyses were used to model diet, exercise, and HBGM counseling, and provision of diabetes education. Estimated adjusted odds ratios and 95% confidence intervals were calculated. The independent variables entered into the modeling were gender, race/ethnicity, BMI (categorized as <25.0, 25.0 to 29.9, and ≥30.0), insurance type, residence (rural vs. urban), LDL-cholesterol (categorized as <130 mg/dL, 130 to 159 mg/dL, and ≥160 mg/dL), systolic blood pressure (dichotomized as <130 mm Hg and ≥130 mm Hg), diastolic blood pressure (dichotomized as <80 and ≥80 mm Hg), HbA1c, and diabetes duration. Model fit was assessed using the Hosmer and Lemeshow chi-square with ten bins (26). Over-dispersion was checked by computing the phi coefficient for each model.
Ordinary least squares regression analyses were also used to model the main clinical outcome, HbA1c values, controlling for potential confounders. Normality was assessed using a combination of kernel density estimation and Royston's modification of D'Agostino's skewness/kurtosis test as implemented in Stata's “sktest” routine (27, 28). Because both methods indicated that HbA1c values were not normally distributed, the following strategy was employed. First, HbA1c values were transformed using a zero-skewness Box-Cox transformation and three models were fit to the data: 1) using the untransformed HbA1c variable; 2) using the transformed HbA1c variable; and 3) using non-parametric median regression. Then, the results of the three models were compared and, if the results of the three models were in agreement, the results of the untransformed linear regression model was reported to allow for simpler interpretation of the parameter estimates.
Variable selection in the model building phase proceeded by considering all predictor variables that had a bivariate relationship of P-value <0.025 and forward selection of these variables was conducted manually by retaining variables with likelihood ratio test p-values of less than 0.05. All P-values reported are two-sided and no adjustment was made for multiple comparisons.
3. RESULTS
3.1. Characteristics of Study Subjects
The study sample comprised 163 subjects aged ≥75 years, 239 aged 65 to 74 years, and 898 aged <65 years. Half were white, 29.8% were Hispanics, and 20.2% were African Americans. The majority were females (53.1%) and approximately 14.7% lived in rural areas. Their mean diabetes duration was 7.1 years. One third (37.8%) had at least one diabetes complication including retinopathy, neuropathy, nephropathy, or an amputation.
Table 1 summarizes the comparison of the study subjects by age group. While there were no age differences by gender or residential status (P>.05), we found significant differences by race/ethnicity. Those aged ≥75 and 65 to 74 years were significantly more likely to be white (69.9% vs. 63.6% vs. 42.8%, respectively; P<.0001). Subjects in the two older age groups were significantly less likely to be current smokers, more likely to have quit smoking, and also less likely to have the HMO's insurance health plan (P<.0001). Both the older age groups had significantly longer diabetes duration (8.6 vs. 8.1 vs. 6.5 years, respectively; P<.0001) but smaller proportions of obese individuals as compared to age group <65 years. Obesity remains a significant risk factor for diabetes, with the rates of obesity ranging from 73.5% in the youngest age groups to 46.6% in the oldest age group. Subjects in the two older age groups had significantly more diabetes complications than those age <65 years (P<.001); 57.1% of those aged ≥75 and 56.1% of those aged 65 to 74 had at least one diabetes complication compared to 29.4% in those aged <65 years.
Table 1.
Selected Demographic and Behavioral Characteristics by Age Group*
| Characteristic | All (N=1300) | Age (years) |
P-value | ||
|---|---|---|---|---|---|
| ≥75 (n=163) | 65 to 74 (n=239) | <65 (n=898) | |||
| Race / ethnicity | |||||
| White | 50.0 | 69.9 | 63.6 | 42.8 | <.0001 |
| Hispanic | 29.8 | 18.4 | 19.7 | 34.6 | <.0001 |
| African American | 20.2 | 11.7 | 16.7 | 22.6 | <.001 |
| Gender | |||||
| Female | 53.1 | 52.8 | 51.9 | 53.5 | 0.91 |
| Male | 46.9 | 47.2 | 48.1 | 46.5 | 0.91 |
| Residence | |||||
| Urban | 85.3 | 87.3 | 87.0 | 84.5 | 0.46 |
| Rural | 14.7 | 12.7 | 13.0 | 15.5 | 0.46 |
| Insurance type | |||||
| SWHP | 47.5 | 3.1 | 14.6 | 64.4 | <.0001 |
| Other | 52.5 | 96.9 | 85.4 | 35.6 | <.0001 |
| Body mass index | |||||
| <25 | 8.5 | 19.5 | 11.2 | 6.1 | <.0001 |
| 25 to 29.9 | 23.1 | 33.9 | 26.6 | 20.4 | <.0001 |
| ≥30 | 68.4 | 46.6 | 62.2 | 73.5 | <.0001 |
| Smoking behavior | |||||
| Current smoker | 14.7 | 4.9 | 10.9 | 17.4 | <.0001 |
| Non-smoker | 56.3 | 55.8 | 56.9 | 56.3 | 0.98 |
| Quit smoking | 23.1 | 33.8 | 28.4 | 19.7 | <.0001 |
| Other / unknown | 5.9 | 5.5 | 3.8 | 6.6 | 0.25 |
Numbers represent column % for each characteristic.
3.2. Provision of Counseling
Counseling about smoking cessation was provided for 71.1% current smokers, while counseling about diet, exercise, home blood glucose monitoring (HBGM), and diabetes education was provided for 79.2%, 69.0%, 75.0%, and 29.4% of the subjects, respectively. It is important to note, however, that rates of smoking were very low, preventing further multivariate analyses of this variable in our current study.
Table 2 shows the unadjusted and adjusted odds ratios and 95% confidence intervals for provision of counseling and DSME with <65 years as the reference age group. Few significant age differences emerged in either the unadjusted or adjusted analyses. No significant differences were seen for counseling about diet and HBGM by age group. These findings persisted after controlling for gender, race, insurance type, residence (rural vs. urban), BMI, LDL-cholesterol, systolic and diastolic blood pressures, HbA1c, and diabetes duration. However, significant age differences were seen for counseling about exercise. Subjects aged 65 to 74 years did not differ significantly from the reference age group (<65 years) in terms of receiving exercise counseling both before and after adjustment. However, subjects aged ≥75 years were significantly less likely to receive exercise counseling than those aged <65 years (unadjusted OR=0.47; 95% CI=0.33–0.65). This trend persisted after controlling for cholesterol, LDL-cholesterol, sex, DSME, HbA1c, and race (adjusted OR=0.60; 95% CI=0.37–0.98).
Table 2.
Unadjusted and Adjusted Odds Ratios with 95% Confidence Intervals for Provision of Counseling
| Variable | Unadjusted OR* (95%CI†) | P-value | Adjusted‡ OR (95% CI) | P-value |
|---|---|---|---|---|
| Diet counseling (Yes/No) | ||||
| <65 years | 1.00 (Reference) | 1.00 (Reference) | ||
| 65 to 74 years | 1.18 (0.82 – 1.70) | 0.37 | 1.00 (0.59 – 1.69) | 0.99 |
| ≥75 years | 0.91 (0.61 – 1.35) | 0.64 | 0.88 (0.48 – 1.64) | 0.70 |
| Exercise counseling (Yes/No) | ||||
| <65 years | 1.00 (Reference) | 1.00 (Reference) | ||
| 65 to 74 years | 1.04 (0.76 – 1.43) | 0.79 | 1.04 (0.67 – 1.62) | 0.86 |
| ≥75 years | 0.47 (0.33 – 0.65) | <0.01 | 0.60 (0.37 – 0.98) | 0.04 |
| HBGM counseling (Yes/No) | ||||
| <65 years | 1.00 (Reference) | 1.00 (Reference) | ||
| 65 to 74 years | 0.82 (0.59 – 1.13) | 0.22 | 0.95 (0.60 – 1.49) | 0.82 |
| ≥75 years | 0.79 (0.54 – 1.15) | 0.22 | 0.78 (0.46 – 1.33) | 0.36 |
| DSME (Yes/No) | ||||
| <65 years | 1.00 (Reference) | 1.00 (Reference) | ||
| 65 to 74 years | 0.73 (0.52 – 1.00) | 0.05 | 0.75 (0.50 – 1.13) | 0.17 |
| ≥75 years | 0.66 (0.45 – 0.97) | 0.04 | 0.82 (0.50 – 1.34) | 0.42 |
Odds Ratio
Confidence Interval
Adjusted for BMI, LDL, Diabetes Duration, Gender, Systolic & Diastolic Blood Pressures, HbA1c value, Race, Insurance Type and Residence (urban/rural).
HBGM = Home Blood Glucose Monitoring; DSME = Diabetes Self-Management Education.
With regard to DSME, subjects aged 65 to 74 years were less likely to receive DSME than subjects aged <65 years, (unadjusted OR = 0.73; 95% CI = 0.52–1.00). After adjustment, however, subjects aged 65 to 74 years were no longer less likely to receive DSME than those aged <65 years (adjusted OR = 0.75; 95% CI = 0.50–1.13). Similarly, subjects aged ≥75 years were significantly less likely to receive DSME (unadjusted OR = 0.66; 95% CI = 0.45–0.97). After adjustment, subjects in this age group did not differ significantly from those in the reference age group (adjusted OR = 0.82; 95% CI = 0.50–1.34).
Table 3 describes the unadjusted data on provision of counseling about diet, exercise, and HBGM by the type of counselor (e.g., primary care provider, other MD, or specialty nurse). There were no statistically significant differences in the dietary and HBGM counseling provided by primary care provider (PCP) across the three age groups. However, exercise counseling to subjects aged ≥75 years was less likely to be provided by a PCP compared with those aged <65 years. The two older age groups were less likely to have been provided diet (P=0.02), exercise (P=0.03), and HBGM (P=0.03) counseling by nurses compared to those aged <65 years.
Table 3.
Selected Counseling Characteristics by Age Group*
| Characteristic | All (N=1300) | Age (years) |
P-value | ||
|---|---|---|---|---|---|
| ≥75 (n=163) | 65 to 74 (n=239) | <65 (n=898) | |||
| Dietary counseling | |||||
| By PCP | 52.8 | 52.6 | 57.7 | 51.7 | 0.24 |
| By other MD | 12.0 | 14.7 | 13.8 | 11.0 | 0.26 |
| By nurse** | 14.4 | 10.4 | 10.1 | 16.2 | 0.02 |
| None / unknown | 20.8 | 22.7 | 18.4 | 21.1 | 0.54 |
| Exercise counseling | |||||
| By PCP | 47.6 | 32.5 | 51.9 | 49.2 | <.0001 |
| By other MD | 10.3 | 14.1 | 11.7 | 9.3 | 0.13 |
| By nurse** | 11.1 | 6.8 | 8.4 | 12.6 | 0.03 |
| None / unknown | 32.0 | 46.6 | 28.0 | 28.9 | <.0001 |
| HBGM counseling | |||||
| By PCP | 52.1 | 53.4 | 50.2 | 52.3 | 0.80 |
| By other MD | 10.2 | 11.7 | 10.9 | 9.8 | 0.73 |
| By nurse** | 12.7 | 6.7 | 11.3 | 14.2 | 0.03 |
| None / unknown | 25.02 | 28.2 | 27.6 | 23.7 | 0.28 |
Numbers represent column % for each characteristic.
Endocrinology/Medical Nutrition Therapy Nurse.
3.3. Laboratory Findings
Subjects aged ≥75 and 65 to 74 years were significantly more likely to have lower values of total cholesterol (164.2 vs. 168.3 vs. 188.8; P<.0001), LDL-cholesterol (88.4 vs. 90.2 vs. 106.1; P<.0001) and triglycerides (155.8 vs. 188.4 vs. 237.0; P<.01). Age group ≥75 had higher HDL-cholesterol than the youngest age group (44.0 vs. 41.8 vs. 42.0; P=0.35). There were no significant differences in mean values of systolic blood pressure, creatinine kinase, CK-myocardial band isoenzyme, troponin I, and urine microalbumin by age group. However, subjects aged ≥75 years had a lower mean diastolic blood pressure than the reference age group (68.1 vs. 70.8 vs. 75.7; P<.0001).
3.4. HbA1c Values
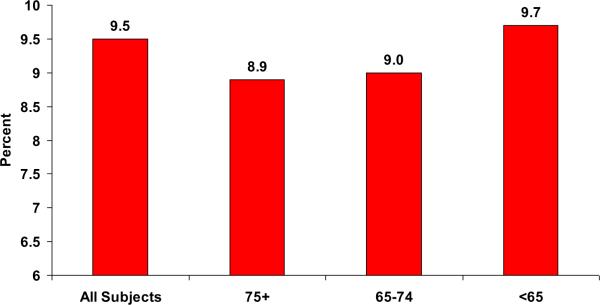
As shown in Figure 1, subjects aged ≥75 and 65 to 74 years had significantly lower mean HbA1c values than those aged <65 years (8.9 vs. 9.0 vs. 9.7; P<.0001), even after controlling for potential confounders. Table 4 shows the results of linear regression analyses used to model the clinical outcome HbA1c. Subjects aged 65 to 74 years had HbA1c values that were 0.691% less than those of subjects aged <65 years after controlling for race, BMI, gender, and diabetes duration (P<.0001). Similarly, subjects aged ≥75 years had HbA1c values that were 0.736% less than those of subjects aged <65 years after controlling for race, BMI, gender, and diabetes duration (P<.0001).
Figure 1. Mean HbA1c Levels for all Subjects and by Age Group*.
*Significant differences between 75+ and 65–74 age groups and <65 (P <.05)
Table 4.
Associations of Demographic and Laboratory Characteristics with HbA1c value: Results of Linear Regression Analysis (Coefficients Represent the Raw Change in HbA1c value Relative to the Referent Group).
| Characteristic | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| Coefficient (SE*) | P-value | Coefficient (SE*) | P-value | |
| Age (years) | ||||
| 65 to 74 | −0.773 (0.108) | <.0001 | − 0.691 (0.121) | <.0001 |
| ≥75 | −0.818 (0.126) | <.0001 | − 0.736 (0.149) | <.0001 |
| Race | ||||
| Hispanic | 0.393 (0.096) | <.0001 | 0.256 (0.107) | 0.02 |
| African American | 0.699 (0.111) | <.0001 | 0.607 (0.122) | <.0001 |
| BMI | ||||
| 25 to 29.9 | −0.013 (0.188) | 0.95 | − 0.124 (0.180) | 0.49 |
| ≥30 | −0.032 (0.170) | 0.85 | − 0.232 (0.165) | 0.16 |
| Gender | −0.056 (0.085) | 0.51 | 0.167 (0.092) | 0.07 |
| Diabetes duration (yrs) | −0.532 (0.136) | <.0001 | − 0.440 (0.222) | <0.01 |
SE: Standard error
4. DISCUSSION AND CONCLUSION
4.1. Discussion
In this study, we sought to evaluate age-related variations in self-management protocols as well as differences in clinical outcomes in diabetes care in a large healthcare institution in Central Texas. Few age-disparities emerged indicating limited age-bias in the provision of care given the identified behavioral and clinical risk factors (e.g., obesity, smoking, and CVD laboratory values). We did note areas for improvement in the general provision of DSME. Less than a third of the persons with type 2 diabetes had received formal DSME while approximately 70% of patients were receiving some provider counseling on at least one of the key self-management behaviors.
Considering that the ideal would be to have continual discussions about key self-management behaviors between providers and patients regardless of age, our rates of provision of counseling for diabetes care, while quantitatively high, are still sub-optimal, and consistent with those found by other investigators (29, 30, 31). For example, a recent study using a national dataset reported rates as low as 36% and 18%, respectively, for dietary and exercise counseling. In addition, the same study uncovered several factors that favored a patient being counseled or referred for lifestyle modification including being younger, having private insurance, and being diagnosed with an increased number of co-morbid illnesses (29). In our study, no significant differences in the provision of counseling about diet, home blood glucose monitoring, and DSME were found. However, our older patients (≥75 years) received significantly less counseling about exercise than the youngest age group.
In its current standards of care in diabetes (32), the American Diabetes Association says, “People with diabetes should be advised to perform at least 150 min/week of moderate-intensity aerobic physical activity (50–70% of maximum heart rate). In the absence of contraindications, people with type 2 diabetes should be encouraged to perform resistance training three times per week. However, the patient's age and previous physical activity level should be considered.” For older population ADA further specifies the recommendations as - “Older adults that are functional, cognitively intact, and have significant life expectancy should receive diabetes treatment using goals developed for younger adults. Some older adults with diabetes are frail and have other underlying chronic conditions, substantial diabetes-related comorbidity, or limited physical or cognitive functioning. Other older individuals with diabetes have little comorbidity and are active. Life expectancies are highly variable for this population, but often longer than clinicians realize. Providers caring for older adults with diabetes must take this heterogeneity into consideration when setting and prioritizing treatment goals.” Based on expert opinion, a tool, Exercise and Screening Assessment for You has been developed to help patients and providers know how to tailor their physical activity appropriately for different conditions (http://www.easyforyou.info/index.asp).
In our study, subjects aged ≥75 and 65 to 74 years had significantly more diabetes complications (retinopathy, neuropathy, nephropathy, and amputations together) than younger age group (57.1% vs.56.1% vs. 29.4% respecively; P<.0001). Mobility limitations due to compromised physical and cognitive abilities should guide recommendations about exercise and physical activity, particularly to older individuals. Pratt et al (33) and Glasgow et al (34) demonstrated that older patients with diabetes can improve in self-care despite long-standing lifestyle habits and co-morbidities. They can also make significant lifestyle changes including increased physical activity if provided with appropriate tailored interventions. According to the diabetes guidelines for older people, older patients should be evaluated regularly for their level of physical activity and should be educated about the benefits of exercise and available resources of becoming more active (35, 36, 37). These guidelines also suggest that older patients should be evaluated for their diet and offered referral for appropriate medical nutrition therapy or diet counseling. Our study found that older patients are less likely to receive counseling from endocrinology/ medical nutrition therapy nurses than younger adults. Indeed, counseling about lifestyle modifications including dietary changes and being physically active have been shown to improve outcomes in patients with diabetes. It is therefore very important for all types of health care providers to be more proactive in counseling and referring all patients to appropriate counselors. In our study, we found that older patients with diabetes had significantly more specialty care visits (referrals) and hospital admissions, a finding similar to O'Connor et al (16).
In this study, HbA1c was found to be significantly lower in the older age groups. This result was consistent with some of the previous studies (14, 38) but contrary to the study by Shorr et al (24). Positive association between age and HbA1C levels has been reported (39). However, it has also been shown that BMI affects this age dependent increase in HbA1c (40). In our study, the lower BMI in older age groups than the younger subjects might explain the age differences in HbA1c. The fact that so many patients were obese reinforces the need for effective counseling and education that is tailored to the individual and reinforced in each clinical encounter over time. Counseling and education efforts should be based on behavioral skill training around goal setting, action planning, and effective use of social support, and combined with multilevel interventions to ensure access to safe physical environments and affordable healthy foods.
4.2. Limitations
There were a few limitations to this study. The study was cross-sectional and therefore no causal inferences may be made. The small sample may also have contributed to inability to uncover some important findings for some of the less prevalent variables due to low study power. This study involved only one health plan which may limit its generalization to other settings. Although patients with uncontrolled type 2 Diabetes Mellitus need counseling at every stage of the disease (34), we wanted to study the effect of diabetes duration on provision of counseling and glycemic control in patients with T2DM. This dataset did not have any information on the diabetes duration. However, it had the duration since the first mention of patients' diabetes in the Scott and White system. Our dichotomization of duration into less than 2 years and 2 or more years for the analysis purpose is admittedly a crude assessment of this important variable
Of major note, this study can only address the presence or absence of diabetes education and counseling and how this varies by age and other key covariates. Without information on patients' baseline self-management activities (physical exercise, diet, HBGM) which are important indicators for counseling and education, it is impossible to determine whether documented variations are justified or not. Absence of this information in our study limits assessing the need of counseling and exercise, although the presence of risk factors does suggest ongoing counseling and education would be valuable for many patients. This secondary analysis of medical charts should stimulate other primary research efforts to interview both providers and patients about the nature of diabetes self-management provided and received. It is critically important to go beyond documenting the presence of self-management protocols to understanding contextual, organizational and behavioral factors affecting the content and format of such educational activities. A secondary research question would be linking such activities to clinical outcomes for persons of different ages and functional abilities.
4.3. Conclusion
In summary, the sub-optimal rates of provision of counseling for diabetes care found in this study regardless of age indicate that there is still room for improvement in patient education and counseling.
4.4. Practice Implications
There is a need for additional efforts on strategies to increase counseling given the less than universal rate of diabetes counseling and education seen. Older adults should be evaluated purposely for lifestyle habits and diabetes self-management care and should be offered counseling by appropriate health care providers. We recommend the use of electronic medical records that track comprehensive information on patient demographic, behavioral, and clinical characteristics. Such centralized record keeping is an important tool for tracking quality care processes for treating older patients at risk of or already needing to manage their diabetes. Additionally, more attention is needed to understand which patients should be referred to formal DSME classes, which might benefit from counseling from primary care providers, and how to best coordinate informal counseling with formal DSME classes to provide optimum care for persons with diabetes, regardless of age.
ACKNOWLEDGMENTS
This research was supported, in part, by a NIH grant # P20MD002295 awarded for the Program for Rural and Minority Health Disparities Research, which is a research collaborative between the Center for the Study of Health Disparities at the Texas A&M University and the Center for Community Health Development at the Texas A&M Health Science Center School of Rural Public Health. We thank Phyllis Davis for providing secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests: There is no financial conflict of interest by any author of this paper.
Prior Presentation: Portions of these data were presented at the 19th IAGG World Conference of Gerontology and Geriatrics, Paris, France, July 5–9, 2009.
REFERENCES
- 1.Wilson PW, Anderson KM, Kannel WB, et al. Epidemiology of diabetes mellitus in the elderly: The Framingham Study. Am J Med. 1986;80:3–9. doi: 10.1016/0002-9343(86)90532-2. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed on February 6, 2009]; http://www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- 3. [Accessed on February 6, 2009]; http://www.cdc.gov/diabetes/statistics/complications_national.htm.
- 4.Moody HR. Aging: Concepts and Controversies. Edition: 5 Pine Forge Press; 2006. pp. 51–54. [Google Scholar]
- 5.Selvin E, Coresh J, Brancati FL, et al. The burden and treatment of diabetes in elderly individuals in the U.S. Diabetes Care. 2006;29:2415–2419. doi: 10.2337/dc06-1058. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, Kannel WB. Obesity, diabetes, and risk of cardiovascular disease in the elderly. Am J Geriatr Cardiol. 2002;11:119–123. 125. doi: 10.1111/j.1076-7460.2002.00998.x. [DOI] [PubMed] [Google Scholar]
- 7.Adelman RD, Greene MG, Ory MG. Communication between older patients and their physicians. Clin Geriatr Med. 2000;16:1–24. doi: 10.1016/s0749-0690(05)70004-5. [DOI] [PubMed] [Google Scholar]
- 8.Ory MG, Hoffman M, Hawkins M, Sanner B, Mockenhaupt R. Challenging aging stereotypes: Designing and evaluating physical activity programs. Am J Prev Med. 2003;25(3S2):164–17. doi: 10.1016/s0749-3797(03)00181-8. [DOI] [PubMed] [Google Scholar]
- 9.Quandt SA, Bell RA, Snively BM, et al. Ethnic disparities in glycemic control among rural older adults with type 2 diabetes. Ethn Dis. 2005;15:656–663. [PMC free article] [PubMed] [Google Scholar]
- 10.Kirk JK, Graves DE, Bell RA, et al. Racial and ethnic disparities in self-monitoring of blood glucose among US adults: a qualitative review. Ethn Dis. 2007;17:135–142. [PubMed] [Google Scholar]
- 11.Sarafidis PA, McFarlane SI, Bakris GL, et al. Gender disparity in outcomes of care and management for diabetes and the metabolic syndrome. Curr Diab Rep. 2006;6:219–224. doi: 10.1007/s11892-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 12.Ren J, Ceylan-Isik AF. Diabetic cardiomyopathy: do women differ from men? Endocrine. 2004;25:73–83. doi: 10.1385/ENDO:25:2:073. [DOI] [PubMed] [Google Scholar]
- 13.Wan Q, Harris MF, Davies GP, et al. Cardiovascular risk management and its impact in Australian general practice patients with type 2 diabetes in urban and rural areas. Int J Clin Pract. 2008;62:53–58. doi: 10.1111/j.1742-1241.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 14.Wan Q, Harris MF, Powell-Davies G, et al. Cardiovascular risk levels in general practice patients with type 2 diabetes in rural and urban areas. Aust J Rural Health. 2007;5:327–333. doi: 10.1111/j.1440-1584.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 15.Miech RA, Kim J, McConnell C, et al. A growing disparity in diabetes-related mortality: U.S. trends, 1989–2005. Am J Prev Med. 2009;36:126–132. doi: 10.1016/j.amepre.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor PJ, Desai JR, Solberg LI, et al. Variation in diabetes care by age: opportunities for customization of care. BMC Fam Pract. 2003;4:16. doi: 10.1186/1471-2296-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persell SD, Zaslavsky AM, Weissman JS, et al. Age-related differences in preventive care among adults with diabetes. Am J Med. 2004;116:630. doi: 10.1016/j.amjmed.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Hendrix KH, Riehle JE, Egan BM, et al. Ethnic, gender, and age-related differences in treatment and control of dyslipidemia in hypertensive patients. Ethn Dis. 2005;15:1–2. [PubMed] [Google Scholar]
- 19.Dunlop DD, Manheim LM, Song J, et al. Age and racial/ethnic disparities in arthritis-related hip and knee surgeries. Med Care. 2008;46:200–208. doi: 10.1097/MLR.0b013e31815cecd8. [DOI] [PubMed] [Google Scholar]
- 20.Sewitch MJ, Fournier C, Dawes M, et al. Do physician recommendations for colorectal cancer screening differ by patient age? Can J Gastroenterol. 2007;21:435–438. doi: 10.1155/2007/938978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerant AF, Franks P, Jackson JE, et al. Age-related disparities in cancer screening: analysis of 2001 Behavioral Risk Factor Surveillance System data. Ann Fam Med. 2004;2:481–487. doi: 10.1370/afm.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owusu C, Lash TL, Silliman RA, et al. Effect of undertreatment on the disparity in age-related breast cancer-specific survival among older women. Breast Cancer Res Treat. 2007;102:227–236. doi: 10.1007/s10549-006-9321-x. [DOI] [PubMed] [Google Scholar]
- 23.August DA, Rea T, Sondak VK, et al. Age-related differences in breast cancer treatment. Ann Surg Oncol. 1994;1:45–52. doi: 10.1007/BF02303540. [DOI] [PubMed] [Google Scholar]
- 24.Funnell MM, Brown TL, Childs BP, et al. National standards for diabetes self-management education. Diabetes Care. 2010;33(Suppl 1):S89–96. doi: 10.2337/dc10-S089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shorr RI, Franse LV, Resnick HE, et al. Glycemic control of older adults with type 2 diabetes: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. J Am Geriatr Soc. 2000;48:264–267. doi: 10.1111/j.1532-5415.2000.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed Wiley; New York, NY: 2000. [Google Scholar]
- 27.D'Agostino RB, Balanger A, D'Agostino RB., Jr A suggestion for using powerful and informative tests of normality. American Statistician. 1990;44:316–321. [Google Scholar]
- 28.Royston P. sg3.5: Comment on sg3.4 and an improved D'Agostino test. Stata Technical Bulletin 3: 19. Reprinted in Stata Technical Bulletin Reprints. 1991;1:110–112. [Google Scholar]
- 29.Peek ME, Tang H, Alexander GC, Chin MH. National prevalence of lifestyle counseling or referral among African-Americans and whites with diabetes. J Gen Intern Med. 2008;23:1858–1864. doi: 10.1007/s11606-008-0737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin SX, Larson E. Does provision of health counseling differ by patient race? Fam Med. 2005;37:650–654. [PubMed] [Google Scholar]
- 31.Kirk JK, Bell RA, Bertoni AG, et al. Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes. Ann Pharmacother. 2005;39:1439–1501. doi: 10.1345/aph.1E685. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratt C, Wilson W, Leklem J, et al. Peer support and nutrition education for older adults with diabetes. J Nutr Elder. 1987;6:31–43. doi: 10.1300/J052v06n04_04. [DOI] [PubMed] [Google Scholar]
- 34.Glasgow RE, Toobert DJ, Hampson SE, et al. Improving self-care among older patients with type II diabetes: the “Sixty Something…” Study. Patient Educ Couns. 1992;19:61–74. doi: 10.1016/0738-3991(92)90102-o. [DOI] [PubMed] [Google Scholar]
- 35.Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl Guidelines):S265–280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 36.Resnick B, Ory M, Coday N, Riebe D. Professional perspectives on physical activity screening practices: Shifting the paradigm. Crit Public Health. 2008;18:21–32. [Google Scholar]
- 37.Resnick B, Ory MG, Chodzko-Zajko WJ, Bazzarre T, Rogers M, Page P. Screening for and prescribing exercise for older adults. Geriatr and Aging. 2006;9:174–182. [Google Scholar]
- 38.Vijan S, Stevens DL, Herman WH, et al. Screening, prevention, counseling, and treatment for the complications of type II diabetes mellitus. Putting evidence into practice. J Gen Intern Med. 1997;12:567–580. doi: 10.1046/j.1525-1497.1997.07111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2008;31:1991–1996. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto Y, Futamura A, Ikushima M. Effect of aging on HbA1c in a working male Japanese population. Diabetes Care. 1995;18:1337–1340. doi: 10.2337/diacare.18.10.1337. [DOI] [PubMed] [Google Scholar]