Abstract
Maternal salivary cortisol was measured at weekly intervals from 24 to 38 weeks gestation. The total sample consisted of 120 women enrolled in staggered intervals in such a way as to generate weekly measures of salivary cortisol during the latter half of pregnancy. Hierarchical linear modeling revealed the expected increase in unbound maternal cortisol during this period, with a slight deceleration in rate of increase at 33 weeks gestation. Women carrying male fetuses had higher levels of salivary cortisol initially as compared to women carrying female fetuses; at 30 weeks gestation there was cross-over such that higher maternal cortisol was observed in women carrying female fetuses beyond this time and through term. Results highlight the importance of considering fetal sex as a moderator of contemporaneous and predictive associations between maternal cortisol and prenatal or postnatal development.
Keywords: cortisol, pregnancy, sex differences, male vulnerability, HPA axis
The changing neuroendocrine milieu of human pregnancy is complex and involves products of maternal, fetal and placental origin. Interest in documenting the pathophysiology of maternal hypothalamic-pituitary-adrenal (HPA) axis function in pregnant women has been stimulated by accumulating evidence that its products, in concert with those of feto-placental origin, contribute to pregnancy outcome and offspring development. Maternal cortisol has been of particular interest as a potential pathway linking maternal psychological distress to observed outcomes.
Within this context, fetal sex is emerging as an important moderator of observed effects of prenatal stress on pregnancy and offspring outcome. It has been long recognized that male fetuses tend to be more vulnerable to prenatal and perinatal adversity (Gualtieri & Hicks, 1985). Efforts to elucidate mechanisms in animal models (Mueller & Bale, 2008) and in the human placenta (Clifton, 2010) are more recent and implicate sex specific variation in a number of physiological pathways, including the HPA axis.
Developmental studies designed to examine antecedents of neurobehavioral outcomes have reported unexplained inconsistencies in associations between maternal cortisol and outcomes when analyzed by fetal sex and gestational period. For example, higher maternal cortisol has been associated with more vigorous fetal motor activity for male, but not female fetuses earlier in gestation with the reverse true near term (DiPietro, Kivlighan, Costigan, & Laudenslager, 2009) and early cortisol has predicted poorer neuromaturation at birth for boys but with higher cortisol later in gestation predicting better neuromaturation for girls (Ellman et al., 2008). Prenatal exposures, including exposure to maternal psychological stress, have been found to generate differential effects of male and female offspring that vary as a function of gestational timing of the exposure in both animal (e.g., Mueller & Bale, 2008) and human studies (e.g., de Bruijn, van Bakel, & van Baar, 2009).
There is speculation that the moderating influence of fetal sex may be based on variation in developmental rates or trajectories resulting in different windows of vulnerability. There is at least one report of a sex by fetal age interaction in plasma cortisol levels of the guinea pig that extends from the late gestational to early postnatal periods (Owen & Matthews, 2003). The focus of the current analysis is to determine whether fetal sex is associated with variation in maternal cortisol during the second half of human pregnancy. To do so, we relied on a unique data set which includes salivary cortisol data at each of the 15 gestational weeks from 24 through 38 weeks, thereby allowing examination of cortisol trajectories throughout the second half of pregnancy.
Methods
Participants
Participants were 120 non-smoking women with normally progressing pregnancies carrying singleton fetuses. The sample was drawn from healthy volunteers who were recruited from local university and hospital-based advertisements. Accurate dating of the pregnancy, based on early first trimester pregnancy testing or examination and generally confirmed by early ultrasound was required (M gestational age at pregnancy detection = 4.8 weeks; sd = 1.2). The sample represents a relatively stable population of well-educated (M years education = 17.1 years, sd = 2.1), mature (M age = 31.1, sd = 4.5), married (91%), and primiparous (72.5%) women. Most were non-Hispanic white (80.8%); the remainder was African-American (12.5%), Hispanic or Asian (6.7%). Sixty-two (51.7%) of the fetuses were female. The study was approved by the local Institutional Review Board and participants provided written informed consent.
Procedure
To fully represent the gestational age span from 24 to 38 weeks gestation, participants were stratified into 3 cohorts with staggered entry into the protocol between 24 and 26 weeks gestation and tested in 3-week intervals. That is, data collection for the first cohort proceeded at 24, 27, 30, 33, and 36 weeks; the second at 25, 28, 31, 34, and 37 weeks; and the third at 26, 29, 32, 35, and 38 weeks. Prenatal visits were scheduled at 13:00 or 15:00 and were conducted at the same time for each subject throughout pregnancy. Cortisol data were collected as part of a larger protocol that included maternal-fetal monitoring, reported elsewhere (DiPietro et al., 2010).
Salivary Cortisol
Saliva Collection and cortisol assay
Saliva was collected approximately ½ hour after arrival to the laboratory at each visit. Participants were instructed to eat no more than 1.5 hours prior to arrival and to restrict fluid intake prior to collection. Participants moistened a filter paper strip (2.5 × 9.0 cm, Whatman Grade 42) which was subsequently air dried and stored at room temperature. This procedure has been used in a previous sample of pregnant women and described in detail (Kivlighan, DiPietro, Costigan, & Laudenslager, 2008). Dried filters were cut and extracted in assay buffer. Salivary cortisol concentration in the extraction buffer was determined using a commercial high sensitivity EIA kit (Salimetrics, LLC) that detects cortisol levels in the range of.003 – 3.0 μg/dl (0.083 – 82.77 nmol/L). Standard curves were fit by a weighted regression analysis using commercial software (Revelation 3.2) for the reader (Dynex MRX). The detection limit after accounting for the extraction dilution is 0.018 μg/dl (0.50 nmol/L). This kit shows minimal cross reactivity (4% or less) with other steroids present in the saliva. Inter-assay coefficients of variation were less than 9.0% for high and low range laboratory controls. Intra-assay coefficients of variation for duplicate determinations were less than 4.5% in this laboratory. Raw values were log transformed for data analyses.
Data analysis
Hierarchical linear models were estimated using the Mixed procedure in SAS (Version 9.2) to examine change in cortisol from 24 through 38 weeks gestation. Gestational age was centered at 38 weeks. Random effects specified in the model allowed cortisol at 38 weeks and its change over gestation to vary across subjects. Restricted maximum likelihood (REML) was used in reporting model parameters when assessing the significance of the random effects; degrees of freedom were estimated using the Satterthwaite method. The 95% confidence interval (CI) for random variation around each fixed effect was calculated as ± 2 standard deviations of its accompanying random variance term.
Results
The number of participants at each visit ranged from 114 to 92; the latter value reflects the final scheduled visit by which time 15 women had already delivered or were on bed rest. Cortisol values were out of range or there was inadequate sample for analyses for between 1 and 4 samples at each time point. Mean values (sd) in μg/dl at each visit were:.183 (.09);.204 (.10);.205 (.10);.237 (.12); and.252 (.11).
The HLM model revealed a significant increase in cortisol over time for the full sample, estimate = 0.0268, 95%CI: (0.0164, 0.0372), F=26.13, p<0.0001. Examination of whether there were any non-linear breaks in the slope was evaluated by insertion of a spline term which revealed a trend level change in slope at 33 weeks (p =.057) such that cortisol level continued to increase but at a slower rate. As expected due to diurnal rhythms, the timing of the sample collection (i.e. approximately at 13:30 or 15:30) modestly, but significantly, impacted cortisol levels, estimate = −0.00003, 95%CI: (−0.00005,−0.0000006) F = 4.60, p <.05 with lower cortisol detected later in the afternoon.
Inclusion of fetal sex in the model revealed a significant main effect, estimate = 0.3502, 95%CI: (0.1220, 0.5785), F = 9.25, p <.01; however, interpretation of that effect is mitigated by a significant Sex × Time interaction, estimate = 0.0232, 95%CI: (0.0027, 0.0437), F = 5.02, p < 0.05. The nature of this interaction is depicted in Figure 1 with data plotted at each gestational week. Removal of two outlier cases with persistently low and persistently high cortisol throughout the gestational period (both male fetuses) did not affect findings. There were no significant cohort (i.e., gestational age at entry into the protocol) effects. Data analysis was also conducted by visit (that is, by grouping 24 to 26 weeks, 27 to 29 weeks, etc) thereby increasing the sample size at each data point and reducing error. The same Sex by Time interaction was detected, estimate = 0.0677, 95%CI: (0.0063, 0.1291), F = 4.78, p < 0.05; these data are plotted in Figure 2. Timing of visits (13:00 versus 15:00) was equally distributed by sex and did not influence the results.
Figure 1.
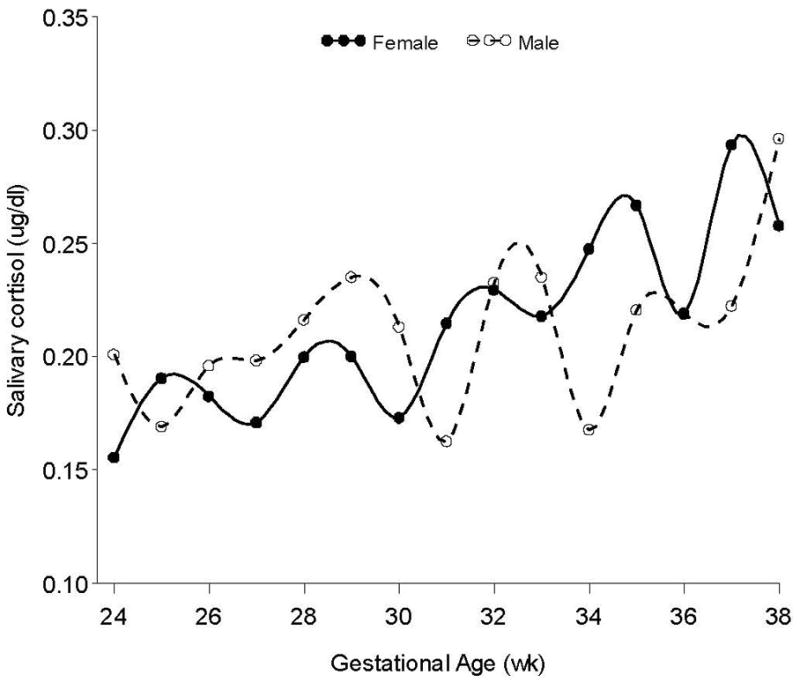
Unadjusted maternal salivary cortisol data from 24 to 38 week gestation plotted in weekly gestational intervals by fetal sex. There is a significant main effect for gestational age and a significant Sex by Gestational age interaction.
Figure 2.
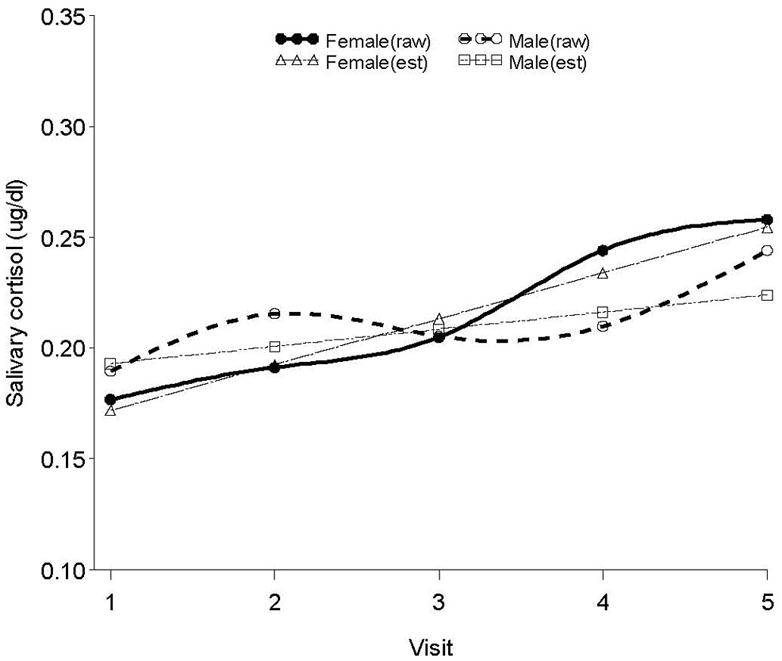
Unadjusted and modeled maternal salivary cortisol data and modeled data by fetal sex aggregated by visit. The main effect and Sex × Gestational age interactions remain significant.
Discussion
Results confirm the expected increase in unbound cortisol level as gestation progresses that has been documented elsewhere during a similar gestational period, (e.g., Davis & Sandman, 2010) but weekly sampling further revealed a mildly decelerating rate of increase commencing at 33 weeks gestation. In addition we show a differential trajectory of maternal cortisol on the basis of fetal sex. Women carrying male fetuses had higher levels of salivary cortisol from 24 to 30 weeks as compared to women carrying female fetuses. At 30 weeks gestation there was cross-over such that higher maternal cortisol was observed in women carrying female fetuses after this gestational period and this persisted into term. The narrowing of the gap as term approaches is consistent with a report of no sex differences in cortisol measured from umbilical vein blood at birth (Clifton, Bisits, & Zarzycki, 2007).
Examination of Figure 1 reveals that a one to two week shift to the right in the cortisol values of women carrying male fetuses would more closely align them with female levels, suggesting that the findings may be evidence of maturational delay. This supposition is supported by evidence of a sex differential in the developmental rate of behaviors indicative of neural maturation (Buss et al., 2009). However, it may also reflect downstream effects of sex specific adaptations across a broad array of physiological and molecular processes (Clifton, 2010).
This is not the first report of prenatal sex differences in the trajectory of products of complex physiologic processes. Earlier reports note sex by gestational age specific variation in cytokine production in amniotic fluid of human fetuses (Romero et al., 1994) as well as a marginal sex difference in the ratio of maternal β-endorphin to adrenocorticotropin levels (Sandman et al., 2003) during the third trimester. The findings of this study provide further impetus for closer examination of sex differences in studies that invoke the HPA axis or its constituents during pregnancy and may contribute to interpretation of observed sex differences in contemporaneous and predictive associations between maternal cortisol and prenatal or postnatal development.
Acknowledgments
Funding for this study was provided by NIH/NICHD grant R01 HD27592 to the first author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buss C, Davis E, Class Q, Gierczak M, Pattillo C, Glynn L, Sandman C. Maturation of the human fetal startle response: evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009;85:633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton V. Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;24:S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Clifton V, Bisits A, Zarzycki P. Characterization of human fetal cord blood steroid profiles in relation to fetal sex and mode of delivery using temperature-dependent inclusion chromatography and Principal Components Analysis (PCA) J Chromatogr B Analyt Technol Biomed Life Sci. 2007;855:249–254. doi: 10.1016/j.jchromb.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Davis E, Sandman C. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn A, van Bakel H, van Baar A. Sex differences in the relation between prenatal maternal emotional complaints and child outcome. Early Hum Dev. 2009;85:319–324. doi: 10.1016/j.earlhumdev.2008.12.009. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Laudenslager ML. Fetal motor activity and maternal cortisol. Dev Psychobiol. 2009;51:505–512. doi: 10.1002/dev.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson J, Pillion JP. Prenatal antecedents of newborn neurological maturation. Child Dev. 2010;81:115–130. doi: 10.1111/j.1467-8624.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri T, Hicks R. An immunoreactive theory of selective male affliction. Behav Brain Sci. 1985;8:427–441. [Google Scholar]
- Kivlighan KT, DiPietro JA, Costigan KA, Laudenslager ML. Diurnal rhythm of cortisol during late pregnancy: associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology. 2008;33:1225–1235. doi: 10.1016/j.psyneuen.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Romero R, Gomez R, Galasso M, Mazor M, Berry S, Quintero R, Cotton D. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol. 1994;171:912–921. doi: 10.1016/s0002-9378(94)70058-3. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Wadhwa P, Chicz-DeMet A, Porto M, Garite T. Maternal hypothalamic-pituitary-adrenal disregulation during the third trimester influences human fetal responses. Dev Neurosci. 2003;25:41–49. doi: 10.1159/000071467. [DOI] [PubMed] [Google Scholar]


