Abstract
Interferon (IFN)-γ plays an important role in immunity and anti-tumor activity. It is produced by lymphocytes, but was recently shown to be also produced by human myeloid dendritic cells (DCs). We have shown that human mature t(9;22) acute lymphoblastic leukemia-derived (ALL) DCs induced autologous cytotoxic T cell responses and therefore asked whether t(9;22) ALL-DC secreted IFN-γ. IFN-γ varied among three cell line-derived ALL-DCs; median production from seven patient ALL-DCs was 3450pg/ml (range 1450-8675). IFN-γ production was dependent on maturation of ALL-DCs. This is the first demonstration of IFN-γ production by t(9;22) ALL-DCs.
Keywords: acute lymphoblastic leukemia, dendritic cells, interferon-γ
Introduction
Interferon (IFN)-γ is critical for immunity against pathogens and tumors. It is produced by natural killer and natural killer T cells as part of the innate immune response and by TH1 CD4 and cytotoxic CD8 T cells as part of the adaptive immune response. IFN-γ secretion from these cells is mediated in part by IL-12 released from regulatory immune cells including dendritic cells (DC) and macrophages. Recently, IFN-γ was shown to be produced by murine myeloid, lymphoid and IFN-producing killer dendritic cells (IKDCs) (1). In humans, IFN-γ has only been found to be secreted by myeloid DCs. We have shown that human mature t(9;22) acute lymphoblastic leukemia-derived dendritic cells (ALL-DC) induced autologous cytotoxic T cell responses against unmodified blasts in a patient who achieved remission (2). This is achieved partly by up-regulation of two components of the antigen processing machinery (APM), HLA class I heavy chain antigen (HLA-HC) and tapasin, in these t(9;22) ALL-DC compared to the unmodified blasts (3). The cytokine profile secreted by these cells is largely unknown. We asked whether t(9;22) ALL-DC secreted IFN-γ to assist in leukemia cell killing via T cell stimulation, polarization or increased expression of the APM components.
Materials and Methods
Cell lines and patient samples
Human t(9;22) ALL cell lines were cultured as previously described (2). The K562 cell line was purchased from the American Type Culture Collection (Manassas, Virginia). Bone marrow samples from seven consecutive t(9;22) ALL patients (containing >85% blasts) and peripheral blood mononuclear cells from four normal volunteers were used after approval by the Institute’s Scientific Review Committee and Institutional Review Board.
Cytokines
Interleukin (IL)-1β, IL-3, IL-7, tumor necrosis factor (TNF)-α and stem cell factor (SCF) were purchased from R & D, Minneapolis, MN. Type B CpG oligodeoxynucleotide (ODN) 2006 was purchased from Coley Pharmaceutical Group, Kanata, Canada and CD40 ligand (L) was provided by Amgen, Thousand Oaks, CA.
Antibodies
Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAb) against CD40, CD54, CD80, CD86 and IFN-γ peridinin chlorophyll (PerCP)-conjugated mAb against CD8 and Phycoerythrin (PE)-conjugated mAb against IL-4 were from BD Pharmingen, San Diego, CA. The Allophycocyanin (APC)-conjugated mAb against CD3 was from Caltag (Burlingame, CA), Alexa-647-conjugated HLA-DR antibody was from BioLegend (San Diego, CA); anti-tapasin mAb TO-3 and the mAb HC-10 which recognizes a determinant expressed on all β2m free HLA-B and C heavy chains and on β2m free HLA-A10, -A28, -29, -A30, -A31, -A32, and -A33 heavy chains (4) were developed and characterised as described (5). PE goat anti-mouse IgG (Caltag) was used as a secondary antibody. The neutralizing goat anti-human IFN-γ antibody and goat IgG isotype were purchased from Sigma (St. Louis, MO).
Generation of ALL-DC
Cytokine concentrations in conditioned media
Supernatants from triplicate day eight cultures were tested for IL-12 and IFN-γ using enzyme-linked immunosorbent assay, (R & D).
Flow cytometric analysis
Analysis of cell surface and intracellularly stained cells was performed as previously described (2, 3).
Tapasin and HLA-HC detection
Intracellular analysis of proteins was performed as previously described (3).
Allogeneic-Mixed Lymphocyte Reaction (allo-MLR)
Allo-MLR was conducted as previously described (2).
T Cell Polarization
Multiparameter flow cytometry was used to detect intracellular levels of IFN-γ and IL-4 simultaneously with the expression of CD3 and CD8. ALL-DC were treated with medium, IgG isotype control, or IFN-γ neutralizing antibody at the ND50 and 100x the ND50 and co-cultured with stimulated allogeneic T cells for 48hrs at 37°C. Cells were stained and analyzed by flow cytometry as described (6).
Statistical analysis
Statistical analysis was performed by the Student’s paired two-tailed t test using Microsoft Excel.
Results and Discussion
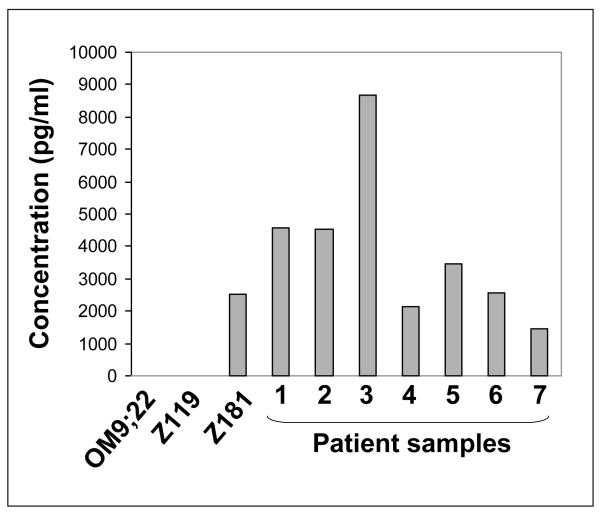
We recently demonstrated that ALL-DC undergo differentiation in two steps (2); the first step generates immature ALL-DC with low stimulatory potential and the second step generates mature, stimulatory ALL-DC. We studied cytokine secretion from these ALL-DCs to determine if IFN-γ aided in leukemia cell killing. As expected, immature ALL-DC secreted neither IL-12 nor IFN-γ. Unexpectedly, mature ALL-DC did not secrete IL-12, however, mature ALL-DC derived from one t(9;22) ALL cell line, Z181, and from primary blasts from seven t(9;22) ALL patients, secreted IFN-γ after treatment with the maturating agents CD40L and TNF-α (Figure 1). These data suggested that mature ALL-DC could produce IFN-γ independent of IL-12.
Figure 1. IFN-γ secretion from t(9;22) ALL-DC.
IFN-γ secretion was measured from ALL-DC generated from three cell lines and seven patient samples on day 8 of the standard cytokine culture.
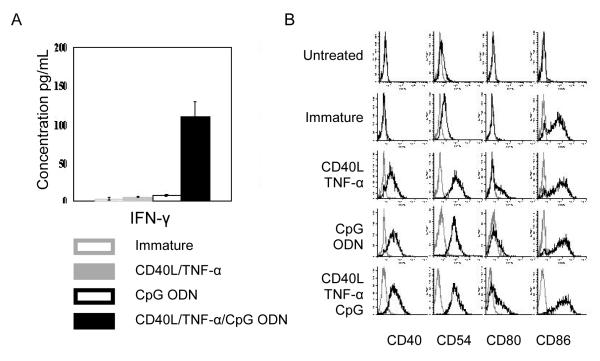
Interestingly, DCs generated from the other cell lines, OM9;22 and Z119 did not secrete significant amounts of IFN-γ even though these cells were determined to be mature by CD80+/CD86+ expression following culture with CD40L and TNF-α. We hypothesized that CD40L and TNF-α did not induce full maturation of the ALL-DCs generated from OM9;22 cells, and that further maturation was needed. Following the addition of CD40L, TNF-α and CpG ODN, IFN-γ (109.3 ± 20.23 pg/ml) was secreted from OM9;22-derived DCs (Figure 2).
Figure 2. IFN-γ secretion from immature and mature OM9;22 AML-DCs.
(A) IFN-γ secretion was measured by ELISA and (B) immunophenotype was measured by flow cytometry from immature OM9;22 ALL-DC treated with different maturating agent combinations.
We next asked what role IFN-γ plays in this system. We tested three known mechanisms: T cell stimulation, T cell polarization and intracellular expression of the APM components tapasin and HLA-HC. We measured T cell stimulation by ALL-DC generated from three cell lines, Z119 (no IFN-γ secretion), Z181 (high IFN-γ secretion) and OM9;22 (IFN-γ secretion following exposure to CpG ODN), and two patient samples. There were no statistically significant changes in MLR responses after the addition of IFN-γ neutralizing antibodies. Subsequently, intracellular expression of tapasin and HLA-HC was tested in ALL-DC generated from Z119 (no IFN-γ secretion) and Z181 (high IFN-γ secretion) treated with neutralizing IFN-γ antibody; expression remained unchanged. Finally, no effect of IFN-γ neutralizing antibody was detected on T cell polarization by ALL-DC generated from Z181. To demonstrate that IFN-γ was functional, supernatants from Z119 and Z181 ALL-DC were added to the cell line K562, previously shown to up-regulate HLA-DR in response to IFN-γ exposure (7). Supernatants from Z181 but not Z119 ALL-DC induced increased expression of HLA-DR (Figure 3). These data suggest that IFN-γ is functional, but the role of IFN-γ production by ALL-DC is still unknown.
Figure 3. IFN-γ induces up-regulation of HLA-DR in the K562 cell line.
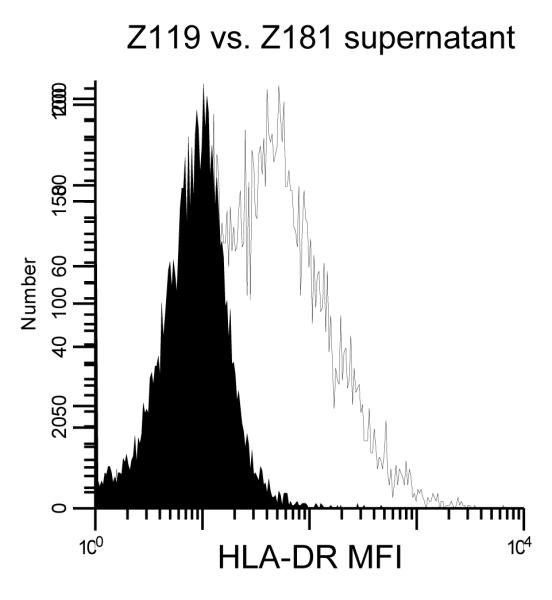
HLA-DR expression was measured by flow cytometry in the K562 cell line after exposure to supernatants from ALL-DC generated from the Z119 (solid) or Z181 (gray) cell lines.
In summary, we demonstrate for the first time that t(9;22) ALL-DCs secrete IFN-γ independent of IL-12. Though our studies did not elucidate the exact role of IFN-γ, we propose that similar to murine IKDCs (8), IFN-γ may enhance the ability of t(9;22) ALL-DC to induce direct or indirect cytotoxicity against unmodified blasts. These cells may be useful in the development of immunotherapeutic approaches for the treatment of t(9;22) ALL.
Acknowledgements
Supported partially by National Cancer Institute Grant CA 16056, the Szefel Foundation, Roswell Park Cancer Institute (ESW), the Leonard S. LuVullo Endowment for Leukemia Research (MW), the Nancy C. Cully Endowment for Leukemia Research (MW) and the Heidi Leukemia Research Fund, Buffalo, NY
Footnotes
Conflict of Interest All authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vremec D, O’Keeffe M, Hochrein H, Fuchsberger M, Caminschi I, Lahoud M, Shortman K. Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood. 2007;109:1165–1173. doi: 10.1182/blood-2006-05-015354. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Sait SN, Wetzler M. Characterization of dendritic-like cells derived from t(9;22) acute lymphoblastic leukemia blasts. Int Immunol. 2004;16:1377–1389. doi: 10.1093/intimm/dxh139. [DOI] [PubMed] [Google Scholar]
- 3.Claus JA, Brady MT, Lee J, Donohue KA, Sait SN, Ferrone S, Wetzler M. T-Cell activation by t(9;22) acute lymphoblastic leukemia-derived dendritic-like cells is associated with increased tapasin expression. Cancer Immunol Immunother. 2006;55:160–165. doi: 10.1007/s00262-005-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–1926. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 5.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 6.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O’Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ress SR, Rousseau J, Ratanjee B, Eidne K, Millar RP, Keraan M. HLA class II induction by interferon-gamma in K562 variant cell line: inhibition by serum lipid. Hum Immunol. 1991;31:57–66. doi: 10.1016/0198-8859(91)90049-f. [DOI] [PubMed] [Google Scholar]
- 8.Bonmort M, Ullrich E, Mignot G, Jacobs B, Chaput N, Zitvogel L. Interferon-gamma is produced by another player of innate immune responses: the interferon-producing killer dendritic cell (IKDC) Biochimie. 2007;89:872–877. doi: 10.1016/j.biochi.2007.04.014. [DOI] [PubMed] [Google Scholar]