Abstract
In the last two decades, there has been a rapid development in the research of the physiological brain mechanisms underlying human motor learning and memory. While conventional memory research performed on animal models uses intracellular recordings, microfusion of protein inhibitors to specific brain areas and direct induction of focal brain lesions, human research has so far utilized predominantly behavioural approaches and indirect measurements of neural activity. Repetitive transcranial magnetic stimulation (rTMS), a safe non-invasive brain stimulation technique, enables the study of the functional role of specific cortical areas by evaluating the behavioural consequences of selective modulation of activity (excitation or inhibition) on memory generation and consolidation, contributing to the understanding of the neural substrates of motor learning. Depending on the parameters of stimulation, rTMS can also facilitate learning processes, presumably through purposeful modulation of excitability in specific brain regions. rTMS has also been used to gain valuable knowledge regarding the timeline of motor memory formation, from initial encoding to stabilization and long-term retention. In this review, we summarize insights gained using rTMS on the physiological and neural mechanisms of human motor learning and memory. We conclude by suggesting possible future research directions, some with direct clinical implications.
Nitzan Censor received PhD in Neurobiology from Weizmann Institute of Science (Rehovot, Israel) before joining Human Cortical Physiology Section, NINDS in 2009. Is interested in mechanisms underlying neural plasticity in human brain involved in memory formation, consolidation and modification. Research focuses on how these brain processes enable improvement of perceptual and motor abilities through procedural learning, and involves behavioural paradigms, non-invasive brain stimulation techniques and brain imaging. Further interests are clinical applications of this research. Leonardo G. Cohen received MD from University of Buenos Aires followed by neurology residency at Georgetown University and postdoc training in clinical neurophysiology at Department of Neurology, University of California (Irvine) and in motor control and movement disorders at Human Motor Control Section, NINDS. Became chief of Human Cortical Physiology Section, NINDS in 1998. Received prestigious Humboldt Research Award from Germany (1999) and is elected member of American Neurological Association.
 |
The human brain has remarkable capabilities to improve motor performance with practice. Repetitive transcranial magnetic stimulation (rTMS) has become a widely used, safe (Wassermann, 1998; Rossi et al. 2009) non-invasive technique that applied to discrete brain areas can help identify neural substrates of human motor learning and memory. By evaluating the behavioural consequences of disruption of activity in specific cortical regions with rTMS, it is possible to identify a cause–effect link between such activity and function, a powerful approach which complements brain imaging studies (Reis et al. 2008).
TMS operates by creating a pulse magnetic field, which induces focal current flow and neural activation in the targeted cortical brain area (Hallett, 2005). Recently, it has been proposed that rTMS can stimulate deeper brain areas as well (Zangen et al. 2005). If reproduced, this approach may have an important impact on future research, further contributing to the study of the involvement of subcortical regions in motor learning. Single pulse TMS has been an important tool to study the mechanisms of motor learning and memory as reviewed before (see for example Bütefisch et al. 2004; Hadipour-Niktarash et al. 2007). Here, we will focus on the unique contribution of rTMS to the understanding of these mechanisms. Generally, low-frequency rTMS (i.e. 1 Hz) induces inhibitory effects on motor cortical excitability allowing creation of a reversible ‘virtual lesion’ (Chen et al. 1997). This approach, somewhat resembling ‘gene knockout’ in genetic research (though the direct effects induced by rTMS are temporal and reversible), enables the functional role of the specific targeted brain area on motor learning to be studied. High-frequency rTMS (5–20 Hz) usually increases cortical excitability (Pascual-Leone et al. 1994; Beradelli et al. 1998). We will not discuss other invasive or non-invasive brain stimulation techniques, addressed in previous reviews (see for example Reis et al. 2008; Bolognini et al. 2009).
Motor learning and memory
The brain is constantly changing in response to environmental challenges. Training leads to learning of visual-perceptual (Karni & Sagi, 1993; Stickgold et al. 2000; Fahle, 2004; Censor et al. 2006) and motor (Brashers-Krug et al. 1996; Walker et al. 2002; Korman et al. 2003; Robertson et al. 2004) skills. Improving motor functions through efficient practice has an important impact on daily living activities of healthy subjects as well as patients with neurological disorders. Memories acquired during practice may be strengthened through consolidation after training finished. Such offline improvements in performance have been shown in the framework of different skill types, with studies showing that sleep plays an important role in strengthening of motor memories (Walker et al. 2002; Korman et al. 2007). rTMS enables the study of the mechanisms underlying consolidation (see Fig. 1), which refers to the process by which acquired memories become stable or strengthened over time and resistant to interference by chemical, electrical or behavioural interventions (Brashers-Krug et al. 1996; McGaugh, 2000; Dudai, 2004). Reactivation of previously consolidated memories turns them transiently labile to subsequent degradation, stabilization or further strengthening, a process referred to as reconsolidation (Walker et al. 2003; Dudai & Eisenberg, 2004; Stickgold & Walker, 2005; Nader & Hardt, 2009; Censor et al. 2010). Applying rTMS during reactivation of a motor memory enables the mechanisms underlying reconsolidation to be studied (Censor et al. 2010, see Fig. 1). One of the important features of rTMS has been its exquisite time resolution. Therefore, it is possible to apply rTMS at different stages during the preparation, execution and consolidation of a memory. Evaluation of the behavioural consequences of focal disruption or facilitation of excitability at each stage provides the opportunity to study specific spatiotemporal patterns of involvement of cortical areas associated with learning.
Figure 1.
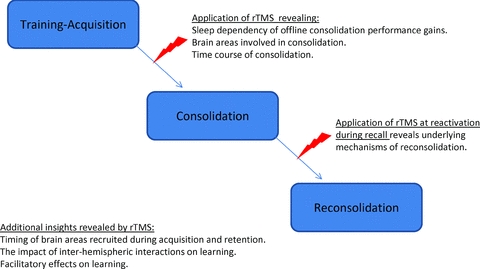
Schematic illustration summarizing some uses of rTMS in motor learning and memory research.
Primary motor cortex
Consolidation and resistance to interference
Following their initial acquisition through training, motor skills are consolidated into a more stable state, resistant to interference (Brashers-Krug et al. 1996). Muellbacher and colleagues (2002) applied 15 min of 1 Hz rTMS over the primary motor cortex (M1) immediately following practice of a ballistic finger movement task, which disrupted the retention of behavioural improvements as opposed to stimulation of other control brain areas. When rTMS to M1 was applied 6 h after practice, retention of the newly acquired motor skill was not disrupted. These results demonstrated that M1 is specifically engaged during the early stage of motor memory consolidation and are in line with psychophysical studies, showing that an acquired motor memory becomes resistant to interference several hours after practice (Brashers-Krug et al. 1996). Another study (Baraduc et al. 2004) has replicated these results and additionally showed that rTMS had no effect on retention of dynamic force-field adaptation. Therefore the authors suggested that unlike the learning of simple ballistic tasks, the learning of dynamics may be stored outside M1 in a more distributed manner. Interestingly, learning a motor task by observation has also been shown to rely to some extent on M1 function since rTMS to M1 is capable of disrupting it (Brown et al. 2009) consistent with previous reports of its involvement in this task (Stefan et al. 2005). These findings raised the question of possible different roles of M1 in consolidation of different forms of learning.
Consolidation and off-line gains in performance
In addition to the definition of a consolidated memory as one that implies resistance to interference as described above, consolidation has also been referred to as memory improvements that take place after the end of the training session (off-line gains, Walker et al. 2002; Korman et al. 2007). An interesting study by Robertson and colleagues (2005) has shown that 1 Hz rTMS of M1 applied immediately following practice of a sequential serial reaction time task (SRTT) blocks off-line improvements over the day but not overnight. This study suggested that different mechanisms and possibly brain areas are engaged during daytime and during overnight consolidation (as evident by differential effects on off-line gains), the latter involving an additional brain-state of sleep. Implicit and explicit motor sequence learning are influenced by sleep in different manners. It was shown that while explicit off-line learning is sleep dependent and correlates with the amount of non-rapid eye movement, implicit off-line learning does not depend on sleep (Robertson et al. 2004). As an example, the amount of slow wave sleep correlated with the learning of a visuomotor rotation adaptation task (Huber et al. 2004). It has been proposed that slow oscillations during sleep may produce synaptic downscaling and an increase in signal-to-noise ratios in the relevant trained neural circuits allowing improved performance (Tononi & Cirelli, 2003). Another study (Hotermans et al. 2008) has shown that rTMS to M1 immediately before testing an explicit finger-tapping task disrupts only the early off-line improvements (30 min after practice) but not the delayed off-line gains (observed 48 h later). rTMS has also been used in order to study learning of movement dynamics in adaptation paradigms, showing that 1 Hz disruption of M1 for 15 min immediately before learning reaching movements in a force field does not impair performance in the learning epoch itself but rather in the re-test of the following day (Richardson et al. 2006). Therefore it was suggested that M1 function contributes substantially to the early stages of memory consolidation (see also Cothros et al. 2006). On the other hand, Iezzi et al. (2010) have shown that inhibitory continuous theta-burst stimulation (cTBS, see Huang et al. 2005) over M1 interferes with early motor learning and retention of a finger movement task, but does not interfere with consolidation measured on the day following practice. Such differences between studies may arise from the use of different rTMS techniques and types of motor tasks (Iezzi et al. 2010).
Reconsolidation
A recent study (Censor et al. 2010) has shown that 1 Hz rTMS applied to M1 during reactivation of an already consolidated motor memory consisting of an explicit finger-tapping sequence blocks further memory modification (reconsolidation). In addition to animal studies proposing models according to which reactivated memories may be modified while being temporarily in their active state (Lewis, 1979; Nader & Hardt, 2009), the results of this study enabled the authors to suggest a model for human motor memory modification. The model differentiates between an executing storage domain (M1) which upon memory reactivation interacts with the environment and updates the core storage domain, which may include the cerebellum, striatum and/or other motor-related cortical areas and the hippocampus (shown to be involved in the generation of procedural memories, Shadmehr & Holcomb, 1997; Doyon et al. 2002; Albouy et al. 2008; Debas et al. 2010).
Non-primary motor cortices
Non-primary motor areas and the cerebellum are strongly involved in skill acquisition. The cerebellum contributes to the timing of motor movements (for example externally paced rhythmic movements of the right index finger, Del Olmo et al. 2007): a transient virtual lesion using 1 Hz rTMS of the cerebellum ipsilateral to the movement in a finger-tapping task or of the contralateral premotor cortex results in an increase in the variability of the intertap interval but only for movements at 2 Hz. These data have been interpreted as indicative of the involvement of a cerebellar-premotor network in event-related timing in the subsecond range (Del Olmo et al. 2007).
Other studies demonstrated that disruption of activity with 5 Hz rTMS applied over the right dorsolateral prefrontal cortex (DLPFC) resulted in impairments in procedural learning (Pascual-Leone et al. 1996). On the other hand, supporting the hypothesis that declarative and procedural consolidation processes interfere with each other under certain conditions (Brown & Robertson, 2007a,b;), Galea and colleagues demonstrated that intermittent theta-burst stimulation (iTBS, see Huang et al. 2005) over DLPFC lead to offline daytime improvements in the SRTT (Galea et al. 2010). rTMS over DLPFC may also interfere with performance of a visuomotor task containing a sequence to which subjects were previously exposed by observational learning, whereas rTMS applied over the cerebellum interfered with the performance of a newly presented sequence (Torriero et al. 2007).
rTMS studies unveiled the involvement of the supplementary motor area (SMA) in intermanual transfer of procedural motor learning (Perez et al. 2008) and in processes leading to successful motor memory recall, dependent on practice structure (Tanaka et al. 2009), which may also rely to some extent on DLPFC function (Kantak et al. 2010). Interestingly, it has been shown that 1 Hz rTMS over the primary somatosensory cortex (S1) reduces the magnitude of motor learning by reducing performance accuracy in a visuomotor tracking task (Vidoni et al. 2010). These findings are intriguing given the scarcity of reports in humans trying to separate the involvement of M1 and S1 in motor learning, an issue extensively explored in animal models. In one of these rTMS reports, it was demonstrated that M1 contributes to anticipatory grip-force scaling while S1 contributes to object manipulation in a precision grasping task (Schabrun et al. 2008).
Various motor tasks and practice schedules have been investigated in motor learning and memory research. rTMS enables the dissociation of the different brain mechanisms involved, which depend on practice type and schedule. For example, Tanaka and colleagues (2009) have shown that 1 Hz rTMS over SMA following block-designed training of a motor task reduced recall performance compared to sham and SMA stimulation applied 6 h after training, pointing to the involvement of SMA in motor memory consolidation. However, most interestingly, the study showed that when the same stimulation procedure was applied following random practice, there was no effect on recall, posing the hypothesis of an earlier involvement of this region in consolidation taking place as training evolved. The involvement of M1 in motor memory consolidation has also been shown to depend on practice type, with M1 being more involved in constant, repetitive-based learning (Karni et al. 1995; Classen et al. 1998; Bütefisch et al. 2000; Kantak et al. 2010). On the other hand, it has been proposed that error-based learning relies to a larger extent on cerebellar function (Tseng et al. 2007). Such studies show that the brain mechanisms underlying motor learning and memory highly depend on practice type and structure (see also Diedrichsen et al. 2010).
Timing of rTMS
The brain areas recruited during skill acquisition vary depending on the exact timing relative to performance of the training movements. rTMS has been used to study intermanual transfer of motor learning, defined as performance improvements in an untrained hand with training of the opposite hand (Perez et al. 2007b). Previous functional magnetic resonance imaging (fMRI) work documented activation of the SMA with successful intermanual transfer (Perez et al. 2007a). To evaluate the extent to which SMA activity actually contributed to successful transfer, rTMS was used to induce a transient virtual lesion of the SMA during training. Perez and colleagues (2008) showed that there was less intermanual transfer of learning when stimulation was applied at the premovement phase of training motions, compared to rTMS application in the movement phase or with sham stimulation. Studies like this document a direct causal link between the timing of activity in specific brain areas and specific stages of motor learning and memory processes.
As mentioned above, the importance of the exact timing at which rTMS is applied was also shown with regard to the cerebellum, with studies pointing to the involvement of the cerebellum in the timing of motor movements such as finger tapping (Del Olmo et al. 2007). Additionally, retention of visuomotor skills such as adaptation of arm movements to a visuomotor rotation was shown to depend on the exact timing at which M1 was disrupted (Hadipour-Niktarash et al. 2007).
Interaction between hemispheres
rTMS has been used to study interactions between right and left motor cortices and the impact of such interaction on motor learning. 1 Hz rTMS applied to M1 improved the performance of a sequential finger movement motor task when performed with the ipsilateral hand and was associated with increased intracortical excitability of the unstimulated M1 (Kobayashi et al. 2004, 2009; Schambra et al. 2003), possibly by releasing it from transcallosal inhibition by the stimulated M1. Furthermore, excitability changes in the ipsilateral M1 were shown to compensate for contralateral M1 dysfunction induced by rTMS (Strens et al. 2003). Other studies have used rTMS to explore cross-limb transfer of learning (Lee et al. 2010). An interesting approach for studying interhemispheric interactions using rTMS was introduced by Chiang and colleagues (2007) who used near infrared spectroscopy to show that the level of oxyhaemoglobin in the unstimulated M1 increased after 20 min of 1 Hz rTMS over the contralateral hemisphere, an increase which lasted 40 min after stimulation.
Beyond the knowledge gained by such studies regarding how the two motor cortices interact to produce motor output and motor learning, these studies provided a basis for the development of interventional approaches to ameliorate motor disability after stroke, presently under investigation (Floel et al. 2008).
Facilitatory effects of high-frequency rTMS
TMS application may result in increased corticomotor excitability (Pascual-Leone et al. 1994; Beradelli et al. 1998) as well as motor cortical plasticity in healthy subjects (Bütefisch et al. 2004). In healthy subjects, high-frequency rTMS applied just prior to the beginning of a sequential finger-tapping motor task enhanced the learning of a motor sequence compared to sham stimulation (Kim et al. 2004). In contrast, some studies have shown that despite this increase in cortical excitability of M1, neither subthreshold 5 Hz rTMS nor iTBS improved either motor performance or learning associated with rapid repetitive index finger abduction motions (Agostino et al. 2007, 2008) or synchronized co-contraction of the right abductor pollicis brevis and deltoid muscle compared to sham stimulation (Sczesny-Kaiser et al. 2009). These reports led to the proposal (Sczesny-Kaiser et al. 2009) that different motor tasks are affected differently by high-frequency rTMS to M1, with some motor tasks being more dependent on processing in non-primary cortical areas like the premotor cortex, posterior-parietal area and basal ganglia (Catalan et al. 1998; Mima et al. 1999). More importantly, they raised awareness that prediction of TMS effects on behaviour cannot be automatically extrapolated from its effects on motor cortical excitability.
High-frequency rTMS applied over the dorsal premotor cortex (PMd) elicited off-line gains in performance of a visuomotor tracking task compared to 1 Hz or sham stimulation under which no off-line gains were documented (Boyd & Linsdell, 2009). These findings support the hypothesis that PMd contributes to motor learning and off-line consolidation. It is important to note that the effects of high-frequency rTMS do not seem to be limited only to the motor domain, with studies showing that 5 Hz rTMS applied over the cortical representation of the right index finger of S1 improves tactile discrimination. Furthermore, fMRI showed that this stimulation resulted in larger representation of the right index finger in S1 (Tegenthoff et al. 2005).
In patients with stroke, it has been proposed that high frequency rTMS over the ipsilesional M1 could facilitate motor cortical excitability and motor performance in patients with chronic stroke (Kim et al. 2006, but see also Talelli & Rothwell, 2006).
Conclusion and future directions
rTMS studies over the last decade provided important insights into the mechanisms of motor learning and memory formation. In its inhibitory or excitatory forms, rTMS has been utilized to evaluate neural substrates of different stages of motor skill learning in health and disease. Proof of principle studies suggest that facilitating excitability in the ipsilesional motor cortex after brain lesions like stroke or inhibiting the unaffected motor cortex may improve motor performance, a hypothesis presently evaluated as an adjuvant to training-based rehabilitation protocols (Ward & Cohen, 2004; Khedr et al. 2005; Kim et al. 2006; Fregni et al. 2006; Talelli et al. 2007; Takeuchi et al. 2008; Emara et al. 2010) but larger well-controlled multicentre clinical trials are required before firmer conclusions on clinical usefulness can be drawn.
An additional exciting avenue for future research is the use of rTMS in the setting of multimodal investigations that include also functional and anatomical neuroimaging (O'Shea et al. 2007), electroencephalography (Hamidi et al. 2010), and positron emission tomography (Eisenegger et al. 2008; Conchou et al. 2009). Such combinations could be used in various ways, for example by applying rTMS and then exploring the reorganization of the stimulated or distant brain regions using techniques such as fMRI. It is also possible to identify the neural structures activated in association with a particular form of learning and then determine the behavioural consequences of rTMS application, which provides a cause–effect link between activation and function.
In summary, rTMS is already a heavily used technique in the study of mechanisms and modulation of motor skill learning. It is likely that future investigations will continue providing important information in this regard with meaningful clinical implications.
References
- Agostino R, Iezzi E, Dinapoli L, Gilio F, Conte A, Mari F, Berardelli A. Effects of 5 Hz subthreshold magnetic stimulation of primary motor cortex on fast finger movements in normal subjects. Exp Brain Res. 2007;180:105–111. doi: 10.1007/s00221-006-0838-3. [DOI] [PubMed] [Google Scholar]
- Agostino R, Iezzi E, Dinapoli L, Suppa A, Conte A, Berardelli A. Effects of intermittent theta-burst stimulation on practice-related changes in fast finger movements in healthy subjects. Eur J Neurosci. 2008;28:822–828. doi: 10.1111/j.1460-9568.2008.06373.x. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, Peigneux P, Luxen A, Maquet P. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58:261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Baraduc P, Lang N, Rothwell JC, Wolpert DM. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Curr Biol. 2004;14:252–256. doi: 10.1016/j.cub.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Currà A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Linsdell MA. Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neurosci. 2009;10:72. doi: 10.1186/1471-2202-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, Gribble PL. Repetitive transcranial magnetic stimulation to the primary motor cortex interferes with motor learning by observing. J Cogn Neurosci. 2009;21:1013–1022. doi: 10.1162/jocn.2009.21079. [DOI] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nat Neurosci. 2007a;10:148–149. doi: 10.1038/nn1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Off-line processing: reciprocal interactions between declarative and procedural memories. J Neurosci. 2007b;27:10468–10475. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain. 1998;121:253–264. doi: 10.1093/brain/121.2.253. [DOI] [PubMed] [Google Scholar]
- Censor N, Dimyan MA, Cohen LG. Primary cortical processing during memory reactivation enables modification of existing human motor memories. Curr Biol. 2010;20:1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Karni A, Sagi D. A link between perceptual learning, adaptation and sleep. Vision Res. 2006;46:4071–4074. doi: 10.1016/j.visres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chiang TC, Vaithianathan T, Leung T, Lavidor M, Walsh V, Delpy DT. Elevated haemoglobin levels in the motor cortex following 1 Hz transcranial magnetic stimulation: a preliminary study. Exp Brain Res. 2007;181:555–560. doi: 10.1007/s00221-007-0952-x. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Conchou F, Loubinoux I, Castel-Lacanal E, Le Tinnier A, Gerdelat-Mas A, Faure-Marie N, Gros H, Thalamas C, Calvas F, Berry I, Chollet F, Simonetta Moreau M. Neural substrates of low-frequency repetitive transcranial magnetic stimulation during movement in healthy subjects and acute stroke patients. A PET study. Hum Brain Mapp. 2009;30:2542–2557. doi: 10.1002/hbm.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cothros N, Köhler S, Dickie EW, Mirsattari SM, Gribble PL. Proactive interference as a result of persisting neural representations of previously learned motor skills in primary motor cortex. J Cogn Neurosci. 2006;18:2167–2176. doi: 10.1162/jocn.2006.18.12.2167. [DOI] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Tahar AH, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci U S A. 2010;107:17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol. 2007;98:145–152. doi: 10.1152/jn.01088.2006. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N. J Neurosci. 2010;30:5159–5166. doi: 10.1523/JNEUROSCI.5406-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci U S A. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Treyer V, Fehr E, Knoch D. Time-course of “off-line” prefrontal rTMS effects – a PET study. Neuroimage. 2008;42:379–384. doi: 10.1016/j.neuroimage.2008.04.172. [DOI] [PubMed] [Google Scholar]
- Emara TH, Moustafa RR, Elnahas NM, Elganzoury AM, Abdo TA, Mohamed SA, Eletribi MA. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010;17:1203–1209. doi: 10.1111/j.1468-1331.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: A case for early selection. J Vis. 2004;4:879–890. doi: 10.1167/4.10.4. [DOI] [PubMed] [Google Scholar]
- Floel A, Hummel F, Duque J, Knecht S, Cohen LG. Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:477–485. doi: 10.1177/1545968308316388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Galea JM, Albert NB, Ditye T, Miall RC. Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. J Cogn Neurosci. 2010;22:1158–1164. doi: 10.1162/jocn.2009.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci. 2007;27:13413–13419. doi: 10.1523/JNEUROSCI.2570-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2005;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Brain responses evoked by high-frequency repetitive transcranial magnetic stimulation: an event-related potential study. Brain Stimul. 2010;3:2–14. doi: 10.1016/j.brs.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur J Neurosci. 2008;28:1216–1221. doi: 10.1111/j.1460-9568.2008.06421.x. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Iezzi E, Suppa A, Conte A, Agostino R, Nardella A, Berardelli A. Theta-burst stimulation over primary motor cortex degrades early motor learning. Eur J Neurosci. 2010;31:585–592. doi: 10.1111/j.1460-9568.2010.07090.x. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Sullivan KJ, Fisher BE, Knowlton BJ, Winstein CJ. Neural substrates of motor memory consolidation depend on practice structure. Nat Neurosci. 2010;13:923–925. doi: 10.1038/nn.2596. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci Lett. 2004;367:181–185. doi: 10.1016/j.neulet.2004.05.113. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, Yoo WK, Hallett M. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Théoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Théoret H, Pascual-Leone A. Suppression of ipsilateral motor cortex facilitates motor skill learning. Eur J Neurosci. 2009;29:833–836. doi: 10.1111/j.1460-9568.2009.06628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–1213. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during acquisition of skilled performance. Proc Natl Acad Sci U S A. 2003;100:12492–12497. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Hinder MR, Gandevia SC, Carroll TJ. The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol. 2010;588:201–212. doi: 10.1113/jphysiol.2009.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86:1054–1083. [PubMed] [Google Scholar]
- McGaugh JL. Memory – a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Mima T, Sadato N, Yazawa S, Hanakawa T, Fukuyama H, Yonekura Y, Shibasaki H. Brain structures related to active and passive finger movements in man. Brain. 1999;122:1989–1997. doi: 10.1093/brain/122.10.1989. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Wassermann EM, Grafman J, Hallett M. The role of the dorsolateral prefrontal cortex in implicit procedural learning. Exp Brain Res. 1996;107:479–485. doi: 10.1007/BF00230427. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe HC, Willingham DT, Cohen LG. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007a;17:1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Willingham DT, Cohen LG. Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge. J Neurosci. 2008;28:9664–9669. doi: 10.1523/JNEUROSCI.3416-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci. 2007b;27:1045–1053. doi: 10.1523/JNEUROSCI.4128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, Wassermann E, Pascual-Leone A, Hummel F, Celnik PA, Classen J, Floel A, Ziemann U, Paulus W, Siebner HR, Born J, Cohen LG. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 2008;1:363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabré A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci. 2006;26:12466–12470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. J Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabrun SM, Ridding MC, Miles TS. Role of the primary motor and sensory cortex in precision grasping: a transcranial magnetic stimulation study. Eur J Neurosci. 2008;27:750–756. doi: 10.1111/j.1460-9568.2008.06039.x. [DOI] [PubMed] [Google Scholar]
- Sczesny-Kaiser M, Tegenthoff M, Schwenkreis P. Influence of 5 Hz repetitive transcranial magnetic stimulation on motor learning. Neurosci Lett. 2009;457:71–74. doi: 10.1016/j.neulet.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 2005;28:408–415. doi: 10.1016/j.tins.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Strens LH, Fogelson N, Shanahan P, Rothwell JC, Brown P. The ipsilateral human motor cortex can functionally compensate for acute contralateral motor cortex dysfunction. Curr Biol. 2003;13:1201–1205. doi: 10.1016/s0960-9822(03)00453-6. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Toshima M, Chuma T, Matsuo Y, Ikoma K. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranical magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008;40:298–303. doi: 10.2340/16501977-0181. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118:333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Talelli P, Rothwell J. Does brain stimulation after stroke have a future? Curr Opin Neurol. 2006;19:543–550. doi: 10.1097/WCO.0b013e32801080d1. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Honda M, Hanakawa T, Cohen LG. Differential contribution of the supplementary motor area to stabilization of a procedural motor skill acquired through different practice schedules. Cereb Cortex. 2009;20:2114–2121. doi: 10.1093/cercor/bhp276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Förster AF, Nicolas V, Dinse HR. Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol. 2005;3:e362. doi: 10.1371/journal.pbio.0030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. The what and how of observational learning. J Cogn Neurosci. 2007;19:1656–1663. doi: 10.1162/jocn.2007.19.10.1656. [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Acerra NE, Dao E, Meehan SK, Boyd LA. Role of the primary somatosensory cortex in motor learning: An rTMS study. Neurobiol Learn Mem. 2010;93:532–539. doi: 10.1016/j.nlm.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775–779. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]


