Abstract
BACKGROUND
Single-dose nevirapine is the cornerstone of the regimen for prevention of mother-to-child transmission of human immunodeficiency virus (HIV) in resource-limited settings, but nevirapine frequently selects for resistant virus in mothers and children who become infected despite prophylaxis. The optimal antiretroviral treatment strategy for children who have had prior exposure to single-dose nevirapine is unknown.
METHODS
We conducted a randomized trial of initial therapy with zidovudine and lamivudine plus either nevirapine or ritonavir-boosted lopinavir in HIV-infected children 6 to 36 months of age, in six African countries, who qualified for treatment according to World Health Organization (WHO) criteria. Results are reported for the cohort that included children exposed to single-dose nevirapine prophylaxis. The primary end point was virologic failure or discontinuation of treatment by study week 24. Enrollment in this cohort was terminated early on the recommendation of the data and safety monitoring board.
RESULTS
A total of 164 children were enrolled. The median percentage of CD4+ lymphocytes was 19%; a total of 56% of the children had WHO stage 3 or 4 disease. More children in the nevirapine group than in the ritonavir-boosted lopinavir group reached a primary end point (39.6% vs. 21.7%; weighted difference, 18.6 percentage-points; 95% confidence interval, 3.7 to 33.6; nominal P = 0.02). Baseline resistance to nevirapine was detected in 18 of 148 children (12%) and was predictive of treatment failure. No significant between-group differences were seen in the rate of adverse events.
CONCLUSIONS
Among children with prior exposure to single-dose nevirapine for perinatal prevention of HIV transmission, antiretroviral treatment consisting of zidovudine and lamivudine plus ritonavir-boosted lopinavir resulted in better outcomes than did treatment with zidovudine and lamivudine plus nevirapine. Since nevirapine is used for both treatment and perinatal prevention of HIV infection in resource-limited settings, alternative strategies for the prevention of HIV transmission from mother to child, as well as for the treatment of HIV infection, are urgently required. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT00307151.)
Single-dose nevirapine has become a cornerstone of the regimen to prevent perinatal transmission of human immunodeficiency virus (HIV) in resource-limited settings, whether it is used alone or as part of combination regimens.1,2 Both the simplicity of the regimen and the long plasma half-life of the drug contribute to the success of single-dose nevirapine therapy.3-5 However, the low threshold of the drug for the selection of viral resistance results in the development of resistance to non-nucleoside reverse-transcriptase inhibitors (NNRTIs) in a large proportion of HIV-infected mothers who have received the drug and in children who have been infected with HIV despite prophylaxis.6-9 Resistance to NNRTIs is particularly worrisome in resource-limited settings, since many first-line regimens for maternal and infant antiretroviral therapy currently incorporate an NNRTI drug (nevirapine or efavirenz). Among postpartum women who have received single-dose nevirapine to prevent mother-to-child transmission of HIV and who subsequently require initiation of antiretroviral therapy for their own health, receipt of nevirapine-based therapy is associated with a poorer virologic outcome than is receipt of non–NNRTI-based therapy.10-12 One study involving a small number of children has suggested that the outcomes are similar with nevirapine-based treatment in infants previously exposed to single-dose nevirapine.11
The performance of nevirapine as compared with the protease inhibitor lopinavir boosted by ritonavir has never been established in children. Given the use of nevirapine for both prevention and treatment strategies, establishment of its relative performance is essential. The Pediatric AIDS Clinical Trials Network (PACTG) conceived and implemented the P1060 trial, which comprised two parallel, randomized, clinical trials — one involving HIV-infected children who had previously been exposed to single-dose nevirapine (cohort 1) and the other involving children who had not had prior exposure to single-dose nevirapine (cohort 2). The results from cohort 1 are reported here, following the recommendation by the data and safety monitoring board that enrollment in this cohort be closed; the trial involving cohort 2 is ongoing.
METHODS
STUDY DESIGN AND PATIENT POPULATION
In cohort 1 of the P1060 trial, we enrolled HIV-infected children between 6 and 36 months of age who had had documented exposure to a single dose of nevirapine for perinatal prevention of HIV transmission. According to version 1.0 of the protocol, the child could be eligible for inclusion if either the mother or the child had received nevirapine, but the protocol was subsequently amended to require specifically that the child had received nevirapine. The protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org. The authors attest that the study was performed in accordance with the protocol and the statistical analysis plan.
To ensure that the children were infected with HIV during the time of exposure to single-dose nevirapine — and not later during breast-feeding — the inclusion criteria specified that the children had to have received the diagnosis of HIV infection or to have had an acquired immunodeficiency syndrome (AIDS)–defining event by 60 days of age or that they had to have been formula-fed exclusively from birth. In addition, children had to be eligible for treatment according to World Health Organization (WHO) criteria and to have baseline plasma HIV type 1 (HIV-1) RNA levels of more than 5000 copies per milliliter. Children who were undergoing treatment for tuberculosis were ineligible. Enrollment was stratified according to age (<12 months vs. ≥12 months), and children were randomly assigned, in a 1:1 ratio, to an NNRTI-based regimen of nevirapine, zidovudine, and lamivudine or a protease-inhibitor–based regimen of ritonavir-boosted lopinavir, zidovudine, and lamivudine. In the event of a toxic reaction or contraindication to zidovudine, stavudine was substituted.
Children were enrolled at nine sites in Africa: four in South Africa and one each in Zimbabwe, Zambia, Malawi, Uganda, and Tanzania. The study was approved by the ethics review committee at each local site, the institutional review board at each partner U.S. institution, and the Ministry of Health, where needed. Written informed consent was obtained from the children’s parents or legal guardians. Study visits were conducted 2 and 4 weeks after the initiation of treatment, every 4 weeks until week 16, at week 24, and every 12 weeks thereafter. Plasma HIV-1 RNA levels were measured at site laboratories with the use of the standard Roche Amplicor v1.5 test kit (eight sites) or the Abbott RealTime HIV-1 assay (one site), with all the laboratories participating in the Division of Acquired Immunodeficiency Syndrome (DAIDS) Quality Assurance Program. Tests for HIV resistance were performed retrospectively on plasma samples at a single laboratory with the use of the ViroSeq HIV-1 Genotyping System, versions 2.7 and 2.8 (Celera), and ViroSeq software (in which lower-end sensitivity requires that approximately 20% of the virus population possess a resistance mutation for detection).
STUDY END POINTS
The primary study objective was to compare the rates of treatment failure by 24 weeks in the two study groups. Treatment failure was defined as permanent discontinuation of the treatment regimen for any reason, including death, toxic effects, and virologic failure. Virologic failure was defined as a confirmed plasma HIV-1 RNA level of less than 1 log10 copies per milliliter below the study-entry level at 12 to 24 weeks after the initiation of treatment or a confirmed plasma HIV-1 RNA level of more than 400 copies per milliliter at 24 weeks. The need for treatment of tuberculosis during the course of the study was a reason for discontinuation of the study treatment.
Secondary end points included confirmed virologic failure or death by week 24, confirmed virologic failure (as defined above until week 24 or defined as a confirmed viral rebound of more than 4000 copies per milliliter after week 24) or death at any time during the follow-up period, and a composite of virologic failure or discontinuation of study treatment at any time during the follow-up period. Data for children who were lost to follow-up were censored at the date of the last available measurement of the HIV-1 RNA level; children whose last measurement showed virologic failure but for whom no confirmatory value was obtained were classified as having had virologic failure.
STATISTICAL ANALYSIS
We estimated that with a sample of 288 participants, the study would have more than 90% power to detect an absolute difference of 20 percentage points in the rate of the primary end point between the two treatment groups. The P1060 trial was monitored at 6-month intervals by an independent data and safety monitoring board chartered by the National Institute of Allergy and Infectious Diseases. Stopping guidelines according to a Peto–Hay-bittle rule were proposed, with a two-sided nominal P value of less than 0.001 required before closure of enrollment in a cohort would be considered.
Reported P values have not been adjusted for interim analyses or multiple comparisons. The rate of treatment failure at week 24, with associated standard errors, was calculated from Kaplan–Meier curves for each treatment group and each age stratum separately. Rates of treatment failure and differences between study groups were calculated for the primary end point, weighted by the inverse of the variance in each age stratum. Unweighted rates (i.e., without stratification according to age) are also reported. Similar methods were used for the secondary end point of virologic failure or death at 24 weeks. We performed between-group comparisons of the time to each primary and secondary end point using Cox proportional-hazards models stratified according to age and based on the discrete version of the likelihood function, with P values calculated by means of the Wald test. Subgroup analyses were performed to assess whether the difference between treatments varied among subgroups of children; we tested for interactions of treatment with subgroup and compared treatment differences within subgroups, recognizing that the large number of statistical tests performed could result in some significant values (P<0.05) being observed by chance. Data from children who did not reach an end point were censored at the time of the last available measurement of the HIV-1 RNA level at or before the last follow-up assessment that was performed before April 20, 2009.
Safety analyses were limited to the period during which a child received a study treatment. For each type of adverse event, the highest-grade events were tabulated, and the time to a first grade 3 or higher event was compared between groups with the use of a stratified Cox proportional-hazards model.
We summarized the changes from baseline to each study visit in the percentage of CD4+ lymphocytes and in z scores for height and weight (using the Centers for Disease Control charts; www.cdc.gov/nchs/nhanes/growthcharts) according to treatment group, using all values obtained from children who were receiving the study treatment as well as from those who had discontinued treatment (an intention-to-treat approach). A multivariate Wilcoxon-test statistic was used to test for treatment differences across the first 96 weeks of the study.
RESULTS
STUDY PARTICIPANTS
A total of 164 of the targeted 288 children were enrolled in cohort 1 of the P1060 trial between November 9, 2006, and April 20, 2009 (Fig. 1 in the Supplementary Appendix, available at NEJM.org). On April 20, 2009, the data and safety monitoring board concluded that although the statistical significance of the between-group comparisons of the primary end point did not meet the protocol-specified stopping guideline, the observed superiority of the therapy that incorporated ritonavir-boosted lopinavir over the nevirapine-based therapy, combined with data from a similar trial involving women exposed to single-dose nevirapine,12 was sufficiently compelling evidence to recommend that enrollment in cohort 1 be closed. The recommendation of the data and safety monitoring board was accepted, and the parents and guardians of the participants and the study-site institutional review boards were quickly notified. The parents and guardians of the children in the nevirapine group were offered the option of switching their children to ritonavir-boosted lopinavir. The results reported here are based on the data collected until the date of the data and safety monitoring board’s recommendation to close enrollment.
Table 1 shows the baseline characteristics of the 164 children in cohort 1, of whom 123 were 6 to less than 12 months of age and 41 were 12 to 36 months of age (29 were 12 to less than 24 months and 12 were 24 to 36 months). Most children were not breast-fed and had advanced disease, as assessed on the basis of the baseline percentage of CD4+ lymphocytes (median, 19%), plasma HIV-1 RNA levels (median, 5.9 log10 copies per milliliter), and WHO stage (stage 3 or 4 in 56.1% of the children). A total of 95% of the children (141 of 148 tested) were infected with HIV-1 subtype C, as determined by phylogenetic analysis of HIV-1 pol region sequences. Written documentation of prior exposure to single-dose nevirapine was available for 74% of the children; for the remaining children, only an oral report of exposure was available. All but five children had received a single dose of nevirapine at birth; the five children who had not received single-dose nevirapine at birth were exposed to nevirapine as a result of their mothers’ having received nevirapine at delivery or as ongoing treatment during pregnancy (Table 1 in the Supplementary Appendix). As of April 20, 2009, a total of 146 children remained in the study (71 in the nevirapine group and 75 in the ritonavir-boosted lopinavir group) (Fig. 1 in the Supplementary Appendix). There were seven deaths — four in the nevirapine group and three in the ritonavir-boosted lopinavir group; none were attributable to the study treatment. Data were available for a median of 48 weeks (range, 0 to 125) from randomization until either early discontinuation of follow-up assessments or the last clinic visit before April 20, 2009.
Table 1.
Baseline Characteristics of the Children.*
| Characteristic | Nevirapine Group | Ritonavir-Boosted Lopinavir Group | Total Cohort | ||||
|---|---|---|---|---|---|---|---|
| Children <12 mo (N = 60) | Children ≥12 mo (N = 22) | All Children (N = 82) | Children <12 mo (N = 63) | Children ≥12 mo (N = 19) | All Children (N = 82) | Total (N = 164) | |
| Age — yr | |||||||
| Median | 0.6 | 1.5 | 0.7 | 0.6 | 1.5 | 0.7 | 0.7 |
| 10th–90th percentile | 0.5 to 0.9 | 1.1 to 2.2 | 0.5 to 1.6 | 0.5 to 0.8 | 1.2 to 2.6 | 0.5 to 1.7 | 0.5 to 1.7 |
| Weight z score | |||||||
| Median | −1.2 | −1.5 | −1.3 | −0.9 | −1.1 | −1.1 | −1.1 |
| 10th–90th percentile | −3.2 to 0.8 | −5.5 to −0.2 | −3.7 to 0.5 | −2.6 to 0.5 | −3.4 to 0.2 | −2.7 to 0.4 | −3.3 to 0.4 |
| Height z score | |||||||
| Median | −1.3 | −1.8 | −1.5 | −0.9 | −1.3 | −1.0 | −1.2 |
| 10th–90th percentile | −3.1 to 0.3 | −3.1 to −0.2 | −3.1 to 0.2 | −2.5 to 0.4 | −2.8 to 0.1 | −2.5 to 0.1 | −2.7 to 0.2 |
| Percentage of CD4+ lymphocytes | |||||||
| Median | 19.2 | 16.9 | 18.9 | 21.1 | 15.2 | 19.7 | 19.0 |
| 10th–90th percentile | 12.0 to 31.4 | 9.8 to 27.1 | 10.5 to 30.7 | 14.0 to 30.6 | 7.3 to 33.0 | 9.7 to 30.6 | 10.4 to 30.7 |
| HIV-1 RNA — log10 copies/ml† | |||||||
| Median | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 |
| 10th–90th percentile | 5.1 to 5.9 | 5.4 to 5.9 | 5.2 to 5.9 | 5.2 to 5.9 | 5.4 to 5.9 | 5.2 to 5.9 | 5.2 to 5.9 |
| Male sex — no. (%) | 28 (46.7) | 8 (36.4) | 36 (43.9) | 31 (49.2) | 10 (52.6) | 41 (50.0) | 77 (47.0) |
| Breast-fed — no. (%) | 13 (21.7) | 2 (9.1) | 15 (18.3) | 18 (28.6) | 1 (5.3) | 19 (23.2) | 34 (20.7) |
| WHO disease stage — no. (%) | |||||||
| 1 | 5 (8.3) | 0 | 5 (6.1) | 16 (25.4) | 3 (15.8) | 19 (23.2) | 24 (14.6) |
| 2 | 23 (38.3) | 3 (13.6) | 26 (31.7) | 19 (30.2) | 3 (15.8) | 22 (26.8) | 48 (29.3) |
| 3 | 27 (45.0) | 11 (50.0) | 38 (46.3) | 25 (39.7) | 12 (63.2) | 37 (45.1) | 75 (45.7) |
| 4 | 5 (8.3) | 8 (36.4) | 13 (15.9) | 3 (4.8) | 1 (5.3) | 4 (4.9) | 17 (10.4) |
WHO denotes World Health Organization.
HIV-1 RNA levels were censored at the upper limit of the assay (5.9 log10 copies per milliliter).
PRIMARY END POINT
Kaplan–Meier plots of the time to the primary composite end point of treatment failure (i.e., virologic failure or treatment discontinuation) by week 24, according to treatment group and age stratum, are shown in Figure 1A and 1B. A total of 12 children in the nevirapine group, as compared with 4 in the ritonavir-boosted lopinavir group, had virologic failure; 15 children in the nevirapine group, as compared with 12 in the ritonavir-boosted lopinavir group, discontinued treatment; and 1 child in the nevirapine group, as compared with none in the ritonavir-boosted lopinavir group, reached both end points simultaneously (Table 2 in the Supplementary Appendix). The proportion of children, weighted across age strata, who reached the primary end point was 39.6% in the nevirapine group as compared with 21.7% in the ritonavir-boosted lopinavir group (Table 2), with a difference of 18.6 percentage points (95% confidence interval [CI], 3.7 to 33.6; nominal P = 0.02). These weighted estimates were very similar to the unweighted estimates (40.2% in the nevirapine group vs. 21.9% in the ritonavir-boosted lopinavir group; difference, 18.3 percentage points; 95% CI, 3.2 to 33.4; P = 0.02). Failure rates were higher among children in the younger age stratum than among those in the older age stratum (45.3% and 23.3% among children 6 to less than 12 months of age in the nevirapine group and the ritonavir-boosted lopinavir group, respectively, vs. 28.9% and 17.5% among children 12 months of age or older in the two groups, respectively). The magnitude of the between-group difference was also larger in the younger age stratum than in the older age stratum (22.0 percentage points vs. 11.4 percentage points), although this difference was not significant (P = 0.52 for interaction).
Figure 1. Times to Primary End Point and to Virologic Failure, According to Treatment and Age Stratum.
The time to the primary end point of virologic failure or discontinuation of treatment by study week 24 is shown among children 6 to less than 12 months of age (Panel A) and among children 12 months of age or older (Panel B) (P = 0.52 for interaction of age stratum with treatment at week 24); the time to virologic failure or death is also shown among children in the two age groups (Panels C and D, respectively) (P = 0.65 for interaction of age stratum with treatment at week 24).
Table 2.
Rates of the Primary End Point and One Secondary End Point at 24 Weeks, for Total Cohort and According to Age.*
| End Point | Nevirapine Group | Ritonavir-Boosted Lopinavir Group | Between-Group Difference (95% CI)† | P Value | ||
|---|---|---|---|---|---|---|
| Subjects | Rate of End Point | Subjects | Rate of End Point | |||
| no. | % | no. | % | percentage points | ||
| Virologic failure or discontinuation of treatment | ||||||
| Children <12 mo | 60 | 45.3 | 63 | 23.3 | 22.0 (3.9 to 40.0) | 0.02 |
| Children ≥12 mo | 22 | 28.9 | 19 | 17.5 | 11.4 (−15.3 to 38.0) | 0.40 |
| All children | ||||||
| Unweighted analysis | 82 | 40.2 | 82 | 21.9 | 18.3 (3.2 to 33.4) | 0.02 |
| Weighted analysis | 82 | 39.6 | 82 | 21.7 | 18.6 (3.7 to 33.6) | 0.02 |
| Virologic failure or death | ||||||
| Children <12 mo | 60 | 29.0 | 63 | 9.9 | 19.1 (3.4 to 34.8) | 0.02 |
| Children ≥12 mo | 22 | 24.4 | 19 | 12.0 | 12.3 (−12.1 to 36.8) | 0.32 |
| All children | ||||||
| Unweighted analysis | 82 | 27.4 | 82 | 10.4 | 17.1 (4.0 to 30.1) | 0.01 |
| Weighted analysis | 82 | 27.4 | 82 | 10.4 | 17.1 (3.9 to 30.3) | 0.01 |
The rates of the end points were estimated with the use of Kaplan–Meier methods. The weighted analysis was the primary study comparison. In the unweighted analysis, children were not stratified according to age.
The between-group difference is the rate in the nevirapine group minus the rate in the ritonavir-boosted lopinavir group.
RESISTANCE
Baseline nevirapine resistance was detected in 18 of 148 children tested (12%); 14 of the 18 children were less than 12 months of age (13% of the 108 children in that age stratum) and 4 were 12 months of age or older (10% of the 40 children in that age stratum); a Y181C mutation was responsible for the resistance in 15 children, and a K103N mutation in 3 children. Among the 18 children with resistance, the estimated proportion of children who reached a primary end point was 83.3% in the nevirapine group versus 18.2% in the ritonavir-boosted lopinavir group. Among the 130 children without resistance, the corresponding proportions were 35.8% and 20.3% (P = 0.02 for interaction) (Fig. 2).
Figure 2. Time to Primary End Point, According to Treatment and Resistance or Nonresistance to Nevirapine at Baseline.
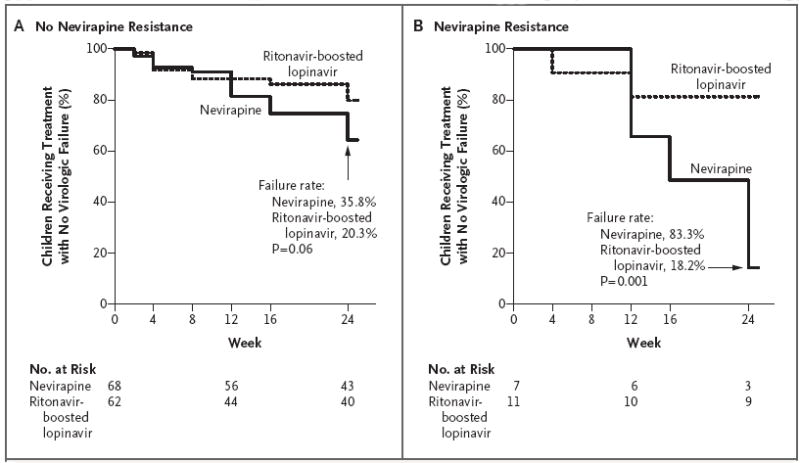
The primary end point was virologic failure or discontinuation of treatment by study week 24. P = 0.02 for interaction between treatment and baseline resistance to NNRTIs.
A total of 25 of the 27 children with virologic failure had plasma samples available for genotype resistance testing. Among the 5 children in the ritonavir-boosted lopinavir group for whom results of genotype testing were available, an M184V mutation (resulting in resistance to lamivudine) developed in 1 by the time of virologic failure. Among the 20 children with virologic failure in the nevirapine group, 3 had wild-type virus at both baseline and the time of virologic failure; mutations responsible for resistance to protease inhibitors, as well as an M184V mutation, developed in 1 child; mutations responsible for nevirapine resistance were detected in 4 children at both baseline and the time of virologic failure; and mutations responsible for nevirapine resistance developed in 12 children by the time of virologic failure (Table 3 in the Supplementary Appendix).
SECONDARY END POINTS
The results of analysis of the rate of virologic failure or death by 24 weeks, irrespective of whether a child continued to receive the study treatment, paralleled the results of the primary end-point analysis (Fig. 1C and 1D). The weighted failure rate (combined across age strata) was 27.4% in the nevirapine group as compared with 10.4% in the ritonavir-boosted lopinavir group, with a weighted difference of 17.1 percentage points (95% CI, 3.9 to 30.3; P = 0.01) (Table 2). Failure rates in the two treatment groups were similar across age strata.
The results of analyses of the rates of virologic failure or discontinuation of treatment (Fig. 1A and 1B) and of virologic failure or death throughout the follow-up period (Fig. 1C and 1D) were consistent with the results of the 24-week analyses (hazard ratio for virologic failure or discontinuation of treatment with nevirapine as compared with ritonavir-boosted lopinavir, 1.77; 95% CI, 1.01 to 3.12; P = 0.05; hazard ratio for virologic failure or death, 3.14; 95% CI, 1.44 to 6.87; P = 0.004).
ADVERSE EVENTS
Two children — one in each treatment group — discontinued the study treatment owing to hepatotoxic effects that met the criteria for a prespecified toxicity end point. An additional child in the nevirapine group discontinued treatment owing to grade 3 hepatotoxic effects that did not meet those criteria.
A total of 17 children in each treatment group had at least one new grade 3 or higher abnormal laboratory test result, and 5 in each group had at least one new grade 3 or higher sign or symptom (P = 0.59 for the between-group comparison of time to a new event of grade 3 or higher) (Table 4 in the Supplementary Appendix). There were no significant between-group differences in the changes in cholesterol levels from baseline to week 24 or 48. There was a marginally significant difference in the changes in triglyceride levels from baseline to week 48 (P = 0.08), with larger median declines among children in the nevirapine group than among children in the ritonavir-boosted lopinavir group (44 mg per deciliter [0.50 mmol per liter] vs. 19 mg per deciliter [0.21 mmol per liter]). The immune reconstitution syndrome was reported in 5 children (2 in the ritonavir-boosted lopinavir group and 3 in the nevirapine group), AIDS encephalopathy in 4 children (2 in each group), malaria in 4 children (2 in each group), and active tuberculosis in 10 children (5 in each group).
IMMUNOLOGIC AND GROWTH RESPONSES
Median increases in the percentage of CD4+ lymphocytes (Fig. 3, and Fig. 2 in the Supplementary Appendix) were larger among children in the nevirapine group than among children in the ritonavir-boosted lopinavir group at all study visits until week 84, although the differences were not significant (median increase in percentage of CD4+ lymphocytes at 48 weeks, 17.0 percentage points vs. 11.7 percentage points; P = 0.35 by a multivariate Wilcoxon test). Median increases in z scores for height and weight were also larger in the nevirapine group than in the ritonavir-boosted lopinavir group at all study visits up to week 96, but the differences were not significant (median increase in z score for weight at 48 weeks, 0.78 vs. 0.03; P = 0.46; median increase in z score for height at 48 weeks, 0.41 vs. 0.07; P = 0.15).
DISCUSSION
The P1060 trial was designed and implemented as a randomized clinical trial to address the prevailing uncertainty regarding the consequences of prior exposure to single-dose nevirapine with respect to the subsequent treatment of children with nevirapine-based regimens. Results involving cohort 1 of the P1060 trial provide evidence of the superiority of a treatment regimen based on a protease inhibitor (lopinavir boosted by ritonavir) over a nevirapine-based regimen for children who had previously been exposed to single-dose nevirapine for perinatal prevention of HIV infection. The study results were consistent for both primary and secondary efficacy end points. Similar outcomes were recently observed in a trial involving HIV-infected women who had been exposed to single-dose nevirapine, influencing the members of the data and safety monitoring board to call for the enrollment of children in cohort 1 to be closed.12 The results of the P1060 trial confirm the inferiority of nevirapine treatment in infants with prior exposure to single-dose nevirapine — an observation that was first suggested in the Mashi trial (ClinicalTrials.gov number, NCT00197587)11 — lending urgency to the WHO “conditional recommendation,” issued in April 2008, that children previously exposed to single-dose nevirapine treatment receive a protease-inhibitor–based antiretroviral regimen.13
The question of whether nevirapine can be initiated after sufficient time has elapsed since the exposure to single-dose nevirapine is of great interest. The results of several clinical trials involving HIV-infected women — among them the Optimal Combination Therapy after Nevirapine Exposure (OCTANE; NCT00089505), Perinatal HIV Prevention Trial 2 (PHPT-2; NCT00398684), and Mashi trials — have suggested that 12 months or longer after exposure to single-dose nevirapine would be a safe interval.10-12,14 The difference in the estimated failure rate in the P1060 trial was larger among children 6 to less than 12 months of age than among children 12 months of age or older (45.3% and 23.3% in the nevirapine group and ritonavir-boosted lopinavir group, respectively, vs. 28.9% and 17.5% in the two groups, respectively), but this difference was not significant and was limited by the relatively small sample in the older-age stratum (41 children [25%]). In addition, only 12 of those 41 children (7% of the total sample) were 24 to 36 months old. Thus, the study does not allow a clear identification of an age threshold beyond which the use of ritonavir-boosted lopinavir could be avoided. We believe that the study provides strong evidence that ritonavir-boosted lopinavir should be used as the initial therapy in children younger than 12 months of age who have had prior exposure to single-dose nevirapine, with moderate evidence that this recommendation should be extended to children 12 to 36 months of age.
Analyses of baseline NNRTI resistance showed that children with detectable mutations associated with resistance to nevirapine, as assessed by population sequencing, were at very high risk for a lack of response to nevirapine treatment. There was a 12% rate of baseline resistance to nevirapine detected in this cohort, possibly reflecting a combination of the relative insensitivity of population sequencing and a reversion to wild-type virus in children who were older than 6 months of age. Planned analyses with the use of an ultra-sensitive resistance assay may help to define threshold levels for resistance mutations associated with treatment failure. Most samples from children who had virologic failure with nevirapine showed either new nevirapine-resistance mutations or an evolution in resistance over time.
An unexpected, but nonsignificant, set of findings was the superior performance of nevirapine with respect to changes in the percentage of CD4+ lymphocytes and z scores for weight and height. These findings are reminiscent of suboptimal pediatric growth reported in the early trials of ritonavir15-18 and suggest the possibility that ritonavir-boosted lopinavir has a negative metabolic effect, owing either to ritonavir specifically or to protease inhibitors in general. Because the focus of the P1060 trial was the effect of potential pre-existing resistance to nevirapine, virologic failure within 24 weeks was part of the composite primary end point. We await the results involving cohort 2 of the P1060 trial (children without prior exposure to single-dose nevirapine) and longer-term follow-up data from cohort 1 to extend these immunologic and clinical analyses, which are of considerable value in assessing treatment efficacy.
Both the P1060 and A5208 studies provide evidence in randomized clinical trials that nevirapine-based treatment regimens are less effective than regimens that incorporate ritonavir-boosted lopinavir among subjects who have had prior exposure to single-dose nevirapine. These findings support the urgent need for alternative strategies for preventing mother-to-child transmission of HIV, including the use of antiretroviral “tails” (agents from alternative classes of drugs) — in both mothers and infants19-21 — and triple-drug maternal prophylactic regimens. Pending the results of the P1060 trial involving children and mothers without prior exposure to single-dose nevirapine, both the P1060 and the A5208 trials strongly support the use of ritonavir-boosted lopinavir for the treatment of women and children who have had prior exposure to single-dose nevirapine. A number of barriers to implementation exist, including the cost of the drugs and the lack of palatable, heat-stable pediatric drug formulations. Creative strategies for increasing the availability of ritonavir-boosted lopinavir (and other effective agents) for children — and advocacy for the dissemination of these drugs — are urgently needed in resource-limited settings.
Supplementary Material
Figure 3. Changes from Baseline in the Percentage of CD4+ Lymphocytes and Z Scores for Weight and Height.
Median changes in the percentage of CD4+ lymphocytes and z scores for weight and height are shown, with 95% confidence intervals, according to the week of treatment.
Acknowledgments
Supported by grants to the Pediatric AIDS Clinical Trials Group (PACTG) and the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group from the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. The Statistical and Data Analysis Center at the Harvard School of Public Health was supported by NIAID cooperative agreements with the PACTG (5 U01 AI41110) and the IMPAACT Group (1 U01 AI068616). Support of the sites was provided by the NIAID. Additional support was provided by the NIAID (contract no. HHSN272200800014C).
We thank the children, their families, and the care providers who agreed to participate in the P1060 trial and place their trust in the site study teams; the following P1060 study-team members for their contributions: Joan Coetzee, Emily Barr, Tamara Kuryla, Carrie Fry, Don Decker, Robert Bollinger, George Kafulafula, Namwinga Chintu, and Linda Barlow, as well as Sandi Lehrman; and the following pharmaceutical representatives and their companies for generous provision of the antiretroviral agents used in the study: Lauren Petrella and Peter Piliero, Boehringer Ingelheim Pharmaceuticals; Marisol Martinez, M.D., Abbott Laboratories; and Navdeep Thoofer and Wendy Snowden, GlaxoSmithKline.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
The views expressed in this article are solely those of the authors and do not necessarily represent the official views or policies of the Department of Health and Human Services or the National Institutes of Health.
References
- 1.Lallemant M, Jourdain G, Le Coeur S, et al. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;343:982–91. doi: 10.1056/NEJM200010053431401. [DOI] [PubMed] [Google Scholar]
- 2.Dabis F, Bequet L, Ekouevi DK, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19:309–18. [PMC free article] [PubMed] [Google Scholar]
- 3.Musoke P, Guay LA, Bagenda D, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006) AIDS. 1999;13:479–86. doi: 10.1097/00002030-199903110-00006. [DOI] [PubMed] [Google Scholar]
- 4.Mirochnick M, Siminski S, Fenton T, Lugo M, Sullivan JL. Nevirapine pharmacokinetics in pregnant women and in their infants after in utero exposure. Pediatr Infect Dis J. 2001;20:803–5. doi: 10.1097/00006454-200108000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Cressey TR, Jourdain G, Lallemant MJ, et al. Persistence of nevirapine exposure during the postpartum period after intrapartum single-dose nevirapine in addition to zidovudine prophylaxis for the prevention of mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2005;38:283–8. [PubMed] [Google Scholar]
- 6.Jackson JB, Becker-Pergola G, Guay LA, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14:F111–F115. doi: 10.1097/00002030-200007280-00001. [DOI] [PubMed] [Google Scholar]
- 7.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15:1951–7. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JA, Li JF, Morris L, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 9.Flys TS, Chen S, Jones DC, et al. Quantitative analysis of HIV-1 variants with the K103N resistance mutation after single-dose nevirapine in women with HIV-1 subtypes A, C, and D. J Acquir Immune Defic Syndr. 2006;42:610–3. doi: 10.1097/01.qai.0000221686.67810.20. [DOI] [PubMed] [Google Scholar]
- 10.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–40. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 11.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 12.Lockman S, Hughes MD, McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO Antiretroviral Therapy for Infants and Children 2008. Geneva: WHO Technical Reference Group; Apr, 2008. [Google Scholar]
- 14.Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21:957–64. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachman SA, Lindsey JC, Pelton S, et al. Growth in human immunodeficiency virus-infected children receiving ritonavir-containing antiretroviral therapy. Arch Pediatr Adolesc Med. 2002;156:497–503. doi: 10.1001/archpedi.156.5.497. [DOI] [PubMed] [Google Scholar]
- 16.Dreimane D, Nielsen K, Deveikis A, Bryson YJ, Geffner ME. Effect of protease inhibitors combined with standard anti-retroviral therapy on linear growth and weight gain in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 2001;20:315–6. doi: 10.1097/00006454-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Buchacz K, Cervia JS, Lindsey JC, et al. Impact of protease inhibitor-containing combination antiretroviral therapies on height and weight growth in HIV-infected children. Pediatrics. 2001;108(4):E72. doi: 10.1542/peds.108.4.e72. [DOI] [PubMed] [Google Scholar]
- 18.Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23:661–72. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntyre JA, Hopley M, Moodley D, et al. Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: a randomized clinical trial. PLoS Med. 2009;6(10):e1000172. doi: 10.1371/journal.pmed.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaix ML, Ekouevi DK, Rouet F, et al. Low risk of nevirapine resistance mutations in the prevention of mother-to-child transmission of HIV-1: Agence Nationale de Recherches sur le SIDA Ditrame Plus, Abidjan, Cote d’Ivoire. J Infect Dis. 2006;193:482–7. doi: 10.1086/499966. [DOI] [PubMed] [Google Scholar]
- 21.Chi BH, Sinkala M, Mbewe F, et al. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet. 2007;370:1698–705. doi: 10.1016/S0140-6736(07)61605-5. Erratum, Lancet 2008;371: 650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




