Abstract
Helicobacter species are common laboratory pathogens which induce intestinal inflammation and disease in susceptible mice. Since in vitro studies indicate that Helicobacter products activate macrophages, we hypothesized that in vivo Helicobacter infection regulates the inflammatory response of intestinal muscularis macrophages from C57Bl/6 mice. Helicobacter hepaticus infection increased surface expression of macrophage markers F4/80, CD11b, and MHC-II within whole intestinal muscle mounts. However, constitutive cytokine and chemokine production by macrophages isolated from infected mice significantly decreased compared to macrophages from uninfected mice despite no detectable bacterial products in the cultures. In addition, muscularis macrophages from infected mice up-regulated FIZZ-1 and SK-1 gene expression, suggesting the macrophages had an anti-inflammatory phenotype. Corresponding with increased anti-inflammatory gene expression, macrophages from infected mice were more phagocytic but did not produce cytokines after stimulation with LPS and IFN-γ or immune complexes and IL-4. Therefore, the presence of Helicobacter infection matures intestinal muscularis macrophages, modulating the constitutive macrophage response to become more anti-inflammatory and resistant to secondary stimulation.
Keywords: Helicobacter, macrophage, intestine, inflammation, infection
Introduction
A common intestinal pathogen of laboratory mice, Helicobacter infects >85% of mice from commercial and academic sources worldwide, with H. hepaticus and H. rodentium as two of the most common species found in research-related rodents.21 In susceptible mice, H. hepaticus induces cecal inflammation and hepatitic lesions and necrosis.16 In contrast, H. rodentium appears to be part of the normal intestinal flora and does not induce clinical symptoms unless co-infected with H. hepaticus or H. bilus.19; 26 Co-infection exacerbates hepatitis and gallstones and increases NO, TNF-α, IP-10, IFN-γ, and IL-10 gene expression in immunocompromised mice.14 In addition, chronic, subclinical H. hepaticus infection changes the response to a secondary Citrobacter infection.10 Although Helicobacter sp. infect the mucosal cells, it is unknown if the bacteria or bacterial products alter the intestinal muscularis macrophages in vivo.
As sentinels of the innate immune response, macrophages are found throughout the body, and bacteria or bacterial products activate and mature macrophages. The primary resident leukocytes within the intestinal muscularis, macrophages form a regularly distributed network which is F4/80, CD11b, and MHC Class II (MHC-II) positive.11 In response to surgical manipulation, these cells actively secrete chemokines and cytokines, including MCP-1, NO, TNF-α, IL-1β, and IL-6.3; 15; 24 In response to E. coli LPS, intestinal muscularis macrophages up-regulate cyclooxygenase-2, subsequently releasing prostaglandin E2 (PGE2)7, and regulate cellular immunity by increasing MHC Class II (MHC-II) expression.11–12 However, during chronic infection, macrophages may become resistant to further stimulation.
To test the hypothesis that chronic Helicobacter infection matures intestinal muscularis macrophages to become anti-inflammatory and tolerant to secondary stimuli, we compared muscularis macrophages obtained from uninfected and infected mice. We demonstrate that Helicobacter infection matures intestinal muscularis macrophages, increasing F4/80, CD11b, and MHC-II surface expression, characteristic anti-inflammatory gene expression, and phagocytosis. However, treatment with either LPS and IFN-γ or immune complexes (IC) and IL-4 induced a significantly decreased cytokine response by muscularis macrophages from infected mice compared to similar cells from uninfected mice. Thus, Helicobacter infection induces a mature, anti-inflammatory but very phagocytic macrophage phenotype which does not respond to further stimulation.
Materials and Methods
Mice
C57Bl/6J mice were purchased (Jackson Laboratory, Bar Harbor, Maine) then bred and maintained in the Kansas State University Division of Biology rodent facility (Manhattan, KS). Uninfected mice were housed under specific pathogen free conditions (Helicobacter species, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Sendai virus, murine norovirus, Mycoplasma pulmonis, Theiler’s murine encephalomyelitis virus, and endo- and ectoparasites). All procedures were approved by the Kansas State University Institutional Animal Care and Use Committee and conducted in compliance with the Animal Welfare Act.
Helicobacter Infection
Some mice were naturally colonized with H. hepaticus either by being reared by an infected female or by contact with infected feces during normal grooming. The presence of H. hepaticus was verified by polymerase chain reaction (PCR) analysis of the feces from each infected mouse (data not shown). Fecal DNA was purified using the Qiagen DNA Stool mini kit according to the manufacturer’s protocol and PCR amplified for 35 cycles at 54°C using Helicobacter-specific 16s rRNA primers: forward 5′ATG GGT AAG AAA ATA GCA AAA AGA TTG CAA3′ and reverse 5′CTA TTT CAT ATC CAT AAG CTC TTG AGA ATC3′. The PCR products were imaged using AlphaImager (Alpha Innotech) and semiquantitative analysis performed using Image J (National Institutes of Health). Each mouse was infected for a minimum of 4 to 8 weeks before treatment. Feces from uninfected mice were also analyzed by PCR with 100% negative results. Liver, cecum, and colon DNA was purified by TRIzol according to the manufacturer’s protocol, and a similar PCR analysis was performed. Preliminary data indicated a constant level of shed bacteria at 1 to 2 months post-infection (data not shown). In addition, H. hepaticus DNA also detectable in the liver, cecum, and colon of all infected mice.
Immunohistochemistry
Acetone fixed whole intestinal muscle mounts or methanol fixed cellular cytospins were blocked with 10% sera (Jackson ImmunoResearch) prior to addition of purified or biotin labeled primary F4/80, CD11b, MHC-II (eBioscience), or IL-6 (Biolegend) antibody overnight at 4°C. Slides were washed and incubated with fluorescent conjugated secondary antibodies (Jackson ImmunoResearch) or fluorescent Strepavidin. Fluorescence was visualized on a Nikon eclipse 80i microscope equipped with a CoolSnap CF camera (Photometrics) and analyzed using Metavue software (Molecular Devices). Relative staining intensity was quantified using Image J (NIH) setting the isotype control threshold to zero and measuring total positive area.
Macrophage Isolation and Tissue Culture
Intestinal macrophages were isolated from 2–4 mo old uninfected or infected mice using a method adapted from Kalff et. al.8 Jejunum and ileum were flushed with cold Ca2+ and Mg2+ free HBSS (Cellgro). Mesentery-free muscularis was circumferentially stripped from the mucosa and digested at 37°C in Ca2+ and Mg2+ free HBSS containing 1 mg/ml collagenase (Worthington) supplemented with 0.4 mM CaCl2 (Allied Chemical). The washed pellet was plated in DMEM (Atlanta Biologicals) containing 5% FBS (Atlanta Biologicals), 5% NuSerum (BD Biosciences), 10% OptiMEM (Gibco), 5 ng/ml recombinant mouse M-CSF (R&D Systems), 1% L-Glutamine (Gibco), 100 U/ml penicillin G, 100 μg/ml streptomycin, and 50 μg/ml gentamicin (Gibco) then incubated overnight at 37°C with 8% CO2. Non-adherent cells were removed and remaining cells cultured in fresh medium. Cultured macrophages from either uninfected or infected mice did not contain detectable Helicobacter DNA, and regardless of infection status unstimulated supernatants contained <18pg/ml LPS as determined by Limulus Amoebocytelysate Assay (Hycult Biotechnology).
Stimulation and secretions
Isolated macrophages pooled from 2–4 mice were stimulated immediately after isolation or cultured 6 days prior to stimulation. Some macrophage cultures were stimulated with 30 U/ml IFN-γ (Sigma) or 100 U/ml IL-4 (Antigenix) for 18–20h followed by an additional 18–20h with 10 μg/ml E. coli LPS O55:B5 (Sigma) or 150 μg/ml IgG-opsonized ovalbumin immune complexes (IC) made as previously described.4 Optimum concentrations were determined by dose response. Cell-free supernatants were harvested and stored at −80°C. Supernatants were assayed for IL-6, keratinocyte-derived cytokine (KC), monocyte chemoattractant protein-1 (MCP-1), and TNF-α production with a Milliplex kit (Millipore). Plates were read on a Luminex100 machine and results analyzed with MasterPlex QT software (MiraiBio).
RNA Isolation, cDNA synthesis, and PCR
Total RNA was isolated from 0.5–1×106 macrophages with TRIzol reagent (Invitrogen) and cDNA was synthesized using a Superscript III first strand synthesis kit (Invitrogen) according to manufacturer’s instructions. PCR was performed using the following primers from Integrated DNA Technologies: FIZZ-1 (Fwd 5′ TCC AGC TGA TGG TCC CAG TGA ATA 3′, Rev 5′ AAG CTG GGT TCT CCA CCT CTT CAT 3′), SK-1 (Fwd 5′ ATG GTC TGA TGC ATG AGG TGG TGA 3′, Rev 5′ ACC ACA GTC CAA TGA GAA GGC ACT 3′), Ym-1 (Fwd 5′ TTC CAA GGC TGC TAC TCA CTT CCA 3′, Rev 5′ AGA CCA CGG CAC CTC CTA AAT TGT 3′), and GAPDH (Fwd 5′ CCA TGG AGA AGG CCG GGG 3′, Rev 5′ CAA AGT TGT CAT GGA TGA CC 3′). Products were electrophoresed and relative band intensity was quantified using Image J then normalized to GAPDH.
Phagocytosis
Cultured macrophages and FITC-conjugated zymosan were incubated (10 particles per cell) in Ca2+ and Mg2+ free HBSS containing 5% normal mouse sera rotating at 37°C for 20 min prior to trypsinization to remove external zymosan. After preparing cytospins, fluorescence was visualized and analyzed as described above. Percent phagocytosis (cells ingesting at least 1 particle/total cells scored) and average number of particles ingested (total particles ingested/100 cells scored) of greater than 100 cells/slide were calculated.
Statistics
Student’s t test using Prism4 (GraphPad Software) determined statistical significance; p<0.05.
Results
Helicobacter DNA is detectable in the feces but not in muscularis macrophages from infected mice
The presence of Helicobacter in the infected mice was established by PCR of the Helicobacter 16S rRNA gene in fecal samples and isolated macrophages and compared to a known positive control. All fecal samples from infected mice contained Helicobacter DNA when compared to the known infected control (data not shown). Conversely, Helicobacter DNA was not detected in any of the macrophages isolated from infected mice. In addition, no Helicobacter DNA was detected in fecal samples or macrophages from uninfected mice used in these studies (data not shown). A limulus amoebocyte lysate assay determined that minimal quantities of LPS (<18pg/ml) were present equally in the culture supernatants from both uninfected and infected mice. Thus, the macrophage cultures from infected mice contained no detectable bacterial DNA and levels of LPS were similar to macrophage cultures from uninfected mice.
Helicobacter infection increases macrophage surface marker expression
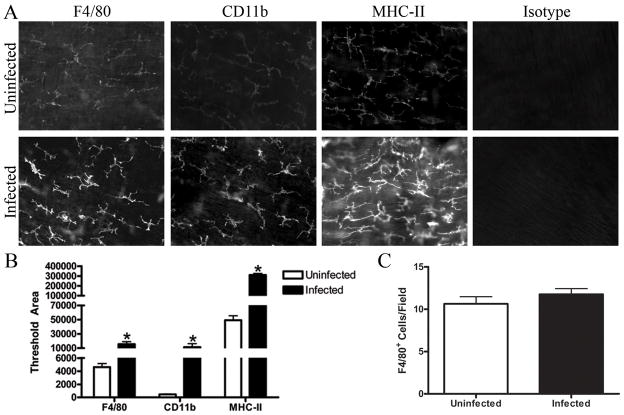
Helicobacter stimulates macrophage activation in vitro 5; 22 but it was unknown if Helicobacter infection would stimulate the network of macrophages found in the intestinal muscle in vivo. To determine if Helicobacter infection changes the maturity of intestinal muscularis macrophages, surface expression of F4/80, CD11b, and MHC-II were analyzed in whole mounts of intestinal muscle. As indicated in Figure 1, the network of stellate macrophages present in the muscularis of infected mice stained more intensely for F4/80, CD11b, and MHC-II than those in uninfected mice (Fig. 1A). Quantitation of the fluorescent staining indicated that macrophages from the infected mice expressed 3 fold more F4/80, 20 fold more CD11b and 5 fold more MHC-II than macrophages from uninfected mice (Fig. 1B). Although the staining was more intense, the average number of stained cells did not change with infection (Fig. 1C). Similar to other intestinal macrophages,18 TLR4 was not expressed in muscularis macrophages from either infected or uninfected mice (data not shown).
Figure 1. Helicobacter infection increases F4/80, CD11b, and MHC-II expression by intestinal muscularis macrophages.
Whole mounts of intestinal muscle from uninfected or infected mice were stained with F4/80, CD11b, MHC-II, or appropriate isotype control. Representative sections are shown (A) and relative fluorescence of the photomicrographs from uninfected (□) or infected (■) mice is quantified (B). The average number of F4/80 positive cells per field (200x) was calculated using photomicrographs of muscle from uninfected (□) or infected (■) mice (C). Photomicrographs (200X) are representative of 3–4 experiments with 4–6 representative photos analyzed per treatment. *p<0.05 infected compared to uninfected.
Helicobacter infection decreases constitutive cytokine production by muscularis macrophages
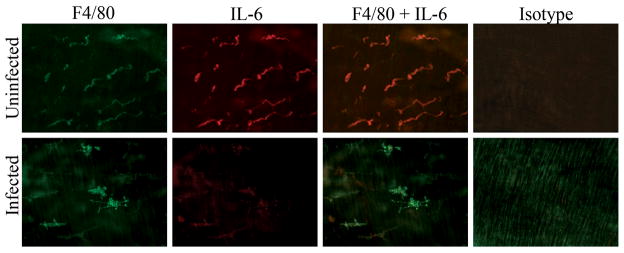
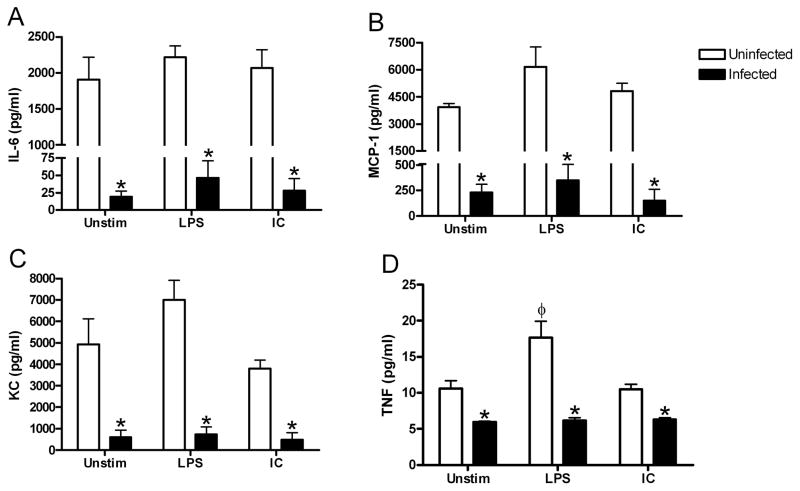
Untreated muscularis macrophages express IL-6 9 and it was likely that exposure to Helicobacter increased secretions compared to macrophages from uninfected mice. When examined by histology ex vivo, IL-6 secretion colocalized with the F4/80+ macrophages in the intestinal muscle of uninfected mice but was much less prominent in the Helicobacter infected mice (Fig. 2). The cytokine produced by isolated muscularis macrophages was quantitated after 24 hrs in culture. Cultured macrophages from both infected and uninfected mice produced IL-6. Similar to the histological findings, macrophages from Helicobacter infected mice secreted significantly less IL-6 than those from uninfected mice suggesting infection alters the macrophage phenotype (Fig. 3A).
Figure 2. IL-6 colocalizes with macrophages from uninfected but not Helicobacter-infected mice.
Representative whole mounts of intestinal muscle isolated from uninfected or infected mice were stained with F4/80 (green), IL-6 (red), both F4/80 and IL-6, or appropriate isotype control. Colocalization of F4/80 and IL-6 expression is illustrated in orange. Photomicrographs (200X) are representative of 3–4 experiments with 4–6 representative photos analyzed per treatment.
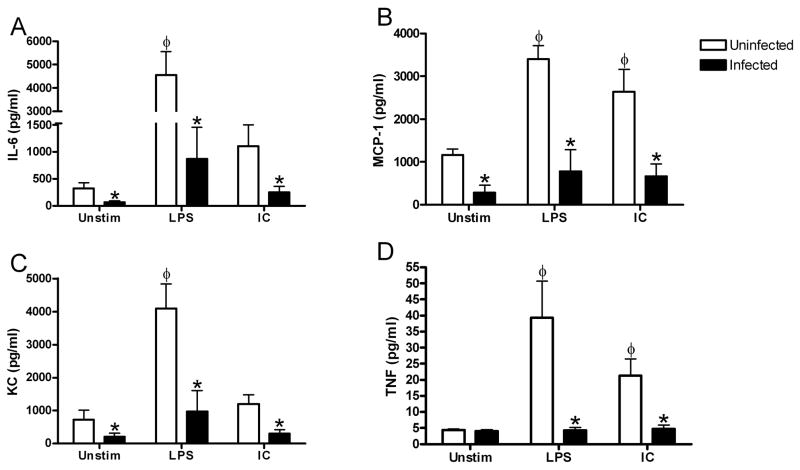
Figure 3. Helicobacter infection decreases constitutive cytokine and chemokine production by intestinal muscularis macrophages.
Intestinal muscularis macrophages isolated from uninfected (□) or Helicobacter-infected (■) mice were cultured 36 hours without stimulation (Unstim), with IFN-γ and LPS (LPS), or with IL-4 and IC (IC) as described. Supernatants were assayed for IL-6 (A), MCP-1 (B), KC (C), and TNF (D) production. Each bar represents the average of 4–8 independent experiments measuring duplicate supernatants. *p<0.05 infected compared to uninfected, φ p<0.05 unstimulated compared to stimulated.
We then measured additional cytokines and chemokines typically expressed by intestinal muscularis macrophages (Fig. 3). Macrophages cultured from uninfected mice constitutively produced high concentrations of MCP-1 and KC, and low levels of TNF-α (Fig. 3B–D). Helicobacter infection resulted in decreased production of all cytokines measured. Intestinal muscularis macrophages from uninfected or infected mice did not constitutively express IL-10 or IL-12p70 when measured by either ELISA or Milliplex despite being detectable in LPS and IFN-γ treated bone marrow macrophages (data not shown). These data indicate that Helicobacter infection decreases the constitutive production of cytokines and chemokines by intestinalmuscularis macrophages.
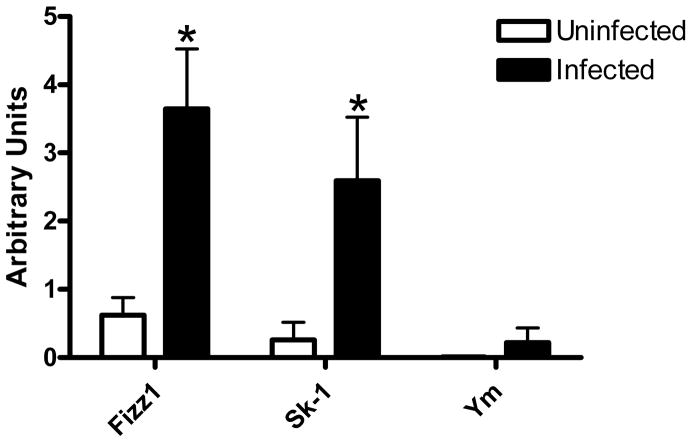
Low quantities of inflammatory cytokines suggested that the macrophages from infected mice mature into anti-inflammatory macrophages and would therefore increase expression of the anti-inflammatory genes such as Found in Inflammatory Zone-1 (FIZZ-1), sphingosine kinase-1 (SK-1), and Ym-1. 4; 23 After normalization to GAPDH, FIZZ-1 and SK-1 gene expression was significantly elevated in macrophages from infected mice when compared to similar macrophages from uninfected mice (Fig. 4).
Figure 4. Helicobacter infection increases some gene expression by intestinal muscle macrophages.
Total RNA was isolated from intestinal muscle macrophages and levels of FIZZ-1, SK-1, Ym-1, and GAPDH transcripts were determined by RT-PCR. Relative intensity of PCR bands from uninfected (□) or Helicobacter-infected (■) mice were normalized to GAPDH. n=4–8 experiments/group, *p<0.05 infected compared to uninfected.
Muscularis macrophages from Helicobacter-infected mice are refractory to stimulation
Pro-inflammatory macrophages respond to treatment with bacterial LPS and IFNγ. Thus, the presence of a chronic Helicobacter infection suggests the macrophages may be tolerant to further stimulation. To test this hypothesis, we measured cytokine and chemokine production by ex vivo intestinal muscularis macrophages from uninfected or infected mice after stimulation. LPS and IFN-γ treatment of macrophages from uninfected mice resulted in release of significantly more TNF-α (Fig. 3D). In contrast, similarly treated macrophages from infected mice secreted very low quantities of each of the cytokines examined that were not significantly different from the unstimulated macrophages from the same mice (Fig. 3). Additional control cells were stimulated with only LPS with similar results.
Anti-inflammatory macrophages are stimulated by immune complexes (IC) and IL-4.4 The macrophages from uninfected mice did not respond to stimulation with IC and IL-4. However, muscularis macrophages from infected mice also did not significantly increase any of the measured cytokines after IC and IL-4 stimulation (Fig. 3). Additional control cells were stimulated with only IC with similar results.
Culture for 7 days does not alter cytokine or chemokine production by muscularis macrophages
Since day 0 cultures are not 100% macrophages and additional cell types may be secreting the cytokines, we cultured macrophages isolated from intestinal muscle for 7 days in M-CSF. At day 7, the cultured cells stained positive for F4/80 and/or CD11b (Fig. 5). Similar to whole intestinal muscle mounts, macrophages isolated from infected mice stained more intensely than those from uninfected mice for both markers (Fig. 5). Thus, the Helicobacter-induced increase in F4/80 and CD11b expression remains after 7 days in culture.
Figure 5. Macrophages cultured from Helicobacter-infected mice express F4/80 and CD11b.
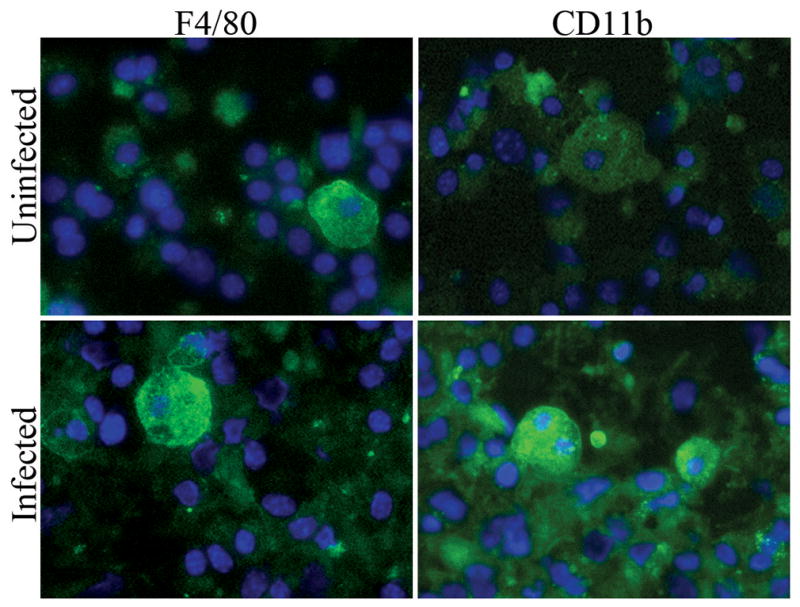
Macrophages were isolated from intestinal muscle of uninfected or infected mice and cultured for 7 days in medium containing M-CSF, prior to staining for F4/80 or CD11b expression (green) and DAPI to visualize nuclei (blue). Photomicrographs (400X) are representative of 3–4 experiments with 4–6 representative photos analyzed per treatment.
To verify that the secretions at day 0 were produced by macrophages, we measured cytokine and chemokine production by macrophages after 7 days in culture. Similar to day 0, macrophages from infected mice constitutively secreted significantly lower concentrations of IL-6, MCP-1, and KC at day 7 than those from uninfected mice (Fig. 6A-C). Although the overall secretion pattern is maintained after 7 days in culture with M-CSF, constitutive production of TNF-α was not decreased in macrophages from infected mice when compared to those from uninfected mice (Fig. 6D). Similar to stimulated cytokine expression immediately ex vivo, the cultured macrophages from infected mice secreted significantly lower concentrations of IL-6, MCP-1, KC, and TNF-α than macrophages from uninfected mice after stimulation with either LPS and IFN-γ or IC and IL-4 (Fig. 6).
Figure 6. Intestinal muscularis macrophages cultured from Helicobacter-infected mice do not respond to stimulation.
Intestinal muscularis macrophages isolated from uninfected (□) or Helicobacter-infected (■) mice were cultured 6 days in M-CSF prior to stimulation without (Unstim) or with IFN-γ and LPS (LPS) or IC and IL-4 (IC) as described. Supernatants were assayed for IL-6 (A), MCP-1 (B), KC (C), and TNF (D) production with each bar representing the average of 4–8 independent experiments measuring duplicate supernatants. *p<0.05 infected compared to uninfected, φ p<0.05 unstimulated compared to stimulated.
Muscularis macrophages cultured from infected mice have increased phagocytic ability
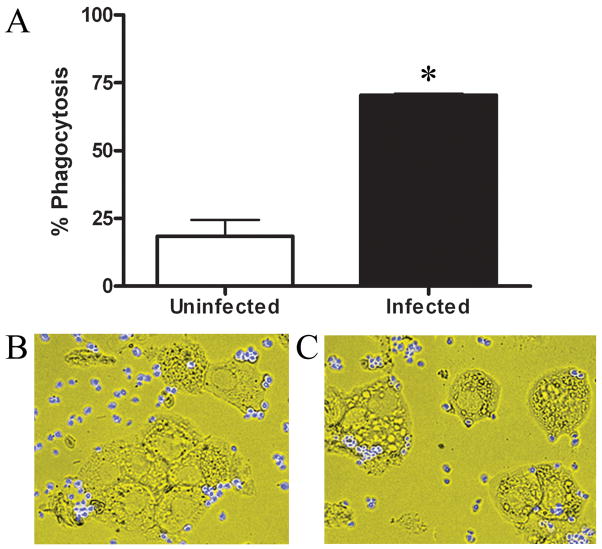
Intestinal muscularis macrophages are phagocytic.17 We tested the hypothesis that intestinal muscularis macrophages from H. hepaticus infected mice would retain phagocytic activity by incubating macrophages from uninfected or infected mice with fluorescent zymosan particles. With >70% of the cells phagocytosing zymosan, macrophages from infected mice were almost three times more phagocytic than those from uninfected mice (Fig. 7). Macrophages from infected mice also ingested five times more zymosan particles per cell (1.87±0.24 compared to 0.31±0.16). Thus, Helicobacter infection increases the phagocytic ability of intestinal muscularis macrophages which is maintained for 1 wk in culture.
Figure 7. Macrophages cultured from Helicobacter-infected mice are more phagocytic.
Intestinal muscularis macrophages isolated from uninfected (□) or Helicobacter-infected (■) mice were cultured for 7 days in M-CSF. Cells were incubated 20 min with fluorescent zymosan particles at 37°C then treated with trypsin to remove externally bound particles. Cytospins were prepared, photographed, and ≥100 cells per slide scored to determine percent phagocytosis (A). Photomicrographs (400X) are representative of macrophages isolated from uninfected (B) or infected (C) mice. n=3–4 experiments/group, *p<0.05 infected compared to uninfected.
Discussion
Helicobacter infections in rodent colonies are widespread. Though originally considered a commensal bacterium, undetected Helicobacter infection in wildtype mice induces maturation of intestinal muscularis macrophages towards an anti-inflammatory phenotype and increases the phagocytic ability of these cells. In addition, H. hepaticus infection down regulates constitutive secretion of inflammatory mediators and prevents macrophages from responding to secondary stimulation ex vivo. These data support our hypothesis that Helicobacter infection regulates the constitutive inflammatory response of intestinal muscularis macrophages and induces tolerance to secondary stimulation despite the absence of bacterial DNA or LPS.
Corresponding to previous studies 8; 11; 15, we observed intestinal muscularis macrophages expressing low levels of mature macrophage markers F4/80, CD11b, and MHC Class II. Correlating with previous studies, surgical manipulation elevated cytokine production immediately after isolation (REFS). The current data suggests that these cells can not be further stimulated. However, the manipulation effects dissipated after 6 days in culture and allows for stimulation of the macrophages. Helicobacter infection appears to regulate the innate immune response by maturing the muscularis macrophage population. Surface markers were significantly increased in mice with subclinical Helicobacter infection. The increased expression of cell surface markers on intestinal muscularis macrophages is similar to the increased ED2 and iNOS expression found in rats after intraperitoneal injection of LPS.6 In addition, the muscularis macrophages expressed increased FIZZ-1 and SK-1 mRNA after infection. The increased FIZZ-1 and SK-1 gene expression suggests the macrophages from infected mice are maturing as anti-inflammatory alternative macrophages rather than classical pro-inflammatory macrophages. Together, these data suggest that the muscularis macrophages are normally quiescent and by 4–8 wks after Helicobacter infection the macrophages mature towards an anti-inflammatory phenotype.
The maturation of the muscularis macrophage population by a subclinical Helicobacter infection was accompanied by a decrease in constitutive macrophage cytokine and chemokine production. Previous in vitro studies showed that H. hepaticus inhibits IL-12p40 production by LPS stimulated bone marrow-derived macrophages.22 These results are not unique to macrophages as in vitro stimulation of mouse intestinal epithelial cell lines with either H. hepaticus purified LPS or whole lysate produced significantly decreased MIP-2 and TNF-α secretion when compared to E.coli LPS.20 In addition, co-incubation of H. hepaticus LPS with either E.coli LPS or Salmonella flagellin down regulated the cytokine and chemokine response of intestinal epithelial cells. However, these in vitro studies did not find the epithelial cells to be endotoxin tolerant to subsequent stimulation.20 In contrast, we show that muscularis macrophages from infected mice do not respond to further in vitro stimulation with either E. coli LPS and IFN-γ or IC and IL-4 even after 1 week in culture. The lack of response to secondary stimuli has clinical implications and is similar to subclinical Helicobacter infection delaying the clearance of Citrobacter rodentium and decreasing cytokine production.10 A similar study showed that helminth infection delayed clearance of a Citrobacter rodentium infection that was accompanied by infiltration of alternative macrophages.25
Despite cytokine and chemokine production inhibition, we demonstrated that macrophages in the intestinal muscularis were three times more phagocytic for zymosan particles after Helicobacter infection. Similar increases in phagocytosis were observed in blood monocytes from immune thrombocytopenia purpura patients infected with H. pylori when compared to similar patients without H. pylori infection.2 Functional studies of intestinal muscularis macrophages typically use small Fitc-dextran 8; 13; 15; 17 which can be taken up by endocytosis or phagocytosis. In contrast, large zymasan particles must be phagocytosed similar to large necrotic cell debris. In skeletal muscle macrophages, phagocytosis switches a pro-inflammatory subset of macrophages to an anti-inflammatory phenotype.1 This suggests that Helicobacter infection activates muscularis macrophages to increase phagocytosis of large particles, correlating with an anti-inflammatory phenotype.
Once considered a commensal bacterium, subclinical Helicobacter infection alters the response of intestinal muscularis macrophages even when bacterial DNA is undetectable in the intestinal tissue. Although the mechanism remains unclear, these cells appear to mature into anti-inflammatory macrophages which no longer respond to a second in vitro stimulus. Thus, in response subsequent infection, injury, or experimental challenge, subclinical Helicobacter infections may impact research results and possibly clinical treatments.
Acknowledgments
This work was supported by the following grants to SDF: NIH Grants AI061691 and Institutional Development Award (IDeA) Program of the NCRR grants P20 RR017686 and P20RR016475, and the Terry C. Johnson Center for Cancer Research. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Institute of Health.
Footnotes
The authors declare no conflict of interest.
References
- 1.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi A, Nishimoto T, Okazaki Y, Suzuki H, Masaoka T, Kawakami Y, Ikeda Y, Kuwana M. Helicobacter pylori eradication shifts monocyte Fcgamma receptor balance toward inhibitory FcgammaRIIB in immune thrombocytopenic purpura patients. J Clin Invest. 2008;118:2939–2949. doi: 10.1172/JCI34496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol and Motil. 2008;20(Suppl 1):81–90. doi: 10.1111/j.1365-2982.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 4.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, Tannenbaum SR, Fox JG. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci U S A. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskandari MK, Kalff JC, Billiar TR, Lee KKW, Baure AJ. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Physiol Cell Physiol. 1999;277:G478–G486. doi: 10.1152/ajpgi.1999.277.2.G478. [DOI] [PubMed] [Google Scholar]
- 7.Hori M, Kita M, Torihashi S, Miyamoto S, Won K-J, Sato K, Ozaki H, Karaki H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol. 2001;280:930–938. doi: 10.1152/ajpgi.2001.280.5.G930. [DOI] [PubMed] [Google Scholar]
- 8.Kalff JC, Schwarz NT, Walgenbach KJ, Schraut WH, Bauer AJ. Leukocytes of the intestinal muscularis: their phenotype and isolation. J Leukoc Biol. 1998;63:683–691. doi: 10.1002/jlb.63.6.683. [DOI] [PubMed] [Google Scholar]
- 9.Kalff JC, Turler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, Billiar TR, Simmons RL, Bauer AJ. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003;237:301–315. doi: 10.1097/01.SLA.0000055742.79045.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBee ME, Zheng PZ, Rogers AB, Fox JG, Schauer DB. Modulation of Acute Diarrheal Illness by Persistent Bacterial Infection. Infect Immun. 2008 doi: 10.1128/IAI.00745-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol. 1995;10:719–736. [PubMed] [Google Scholar]
- 12.Mikkelsen HB, Larsen JO, Hadberg H. The macrophage system in the intestinal muscularis externa during inflammation: an immunohistochemical and quantitative study of osteopetrotic mice. Histochem Cell Biol. 2008 doi: 10.1007/s00418-008-0423-x. [DOI] [PubMed] [Google Scholar]
- 13.Mikkelsen HB, Mirsky R, Jessen KR, Thuneberg L. Macrophage-like cells in muscularis externa of mouse small intestine: immunohistochemical localization of F4/80, M1/70, and Ia-antigen. Cell Tissue Res. 1988;252:301–306. doi: 10.1007/BF00214372. [DOI] [PubMed] [Google Scholar]
- 14.Myles MH, Livingston RS, Franklin CL. Pathogenicity of Helicobacter rodentium in A/JCr and SCID mice. Comp Med. 2004;54:549–557. [PubMed] [Google Scholar]
- 15.Ozaki H, Kawai T, Shuttleworth CW, Won KJ, Suzuki T, Sato K, Horiguchi H, Hori M, Karaki H, Torihashi S, Ward SM, Sanders KM. Isolation and characterization of resident macrophages from the smooth muscle layers of murine small intestine. Neurogastroenterol and Motil. 2004;16:39–51. doi: 10.1046/j.1365-2982.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 16.Rogers AB, Fox JG. Inflammation and Cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am J Physiol. 2004;286:G361–366. doi: 10.1152/ajpgi.00499.2003. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Torihashi S, Hori M, Nasu T, Ozaki H. Phagocytotic activation of muscularis resident macrophages inhibits smooth muscle contraction in rat ileum. J Vet Med Sci. 2007;69:1053–1060. doi: 10.1292/jvms.69.1053. [DOI] [PubMed] [Google Scholar]
- 18.Schenk M, Mueller C. Adaptations of intestinal macrophages to an antigen-rich environment. Semin Immunol. 2007;19:84–93. doi: 10.1016/j.smim.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Shen Z, Fox JG, Dewhirst FE, Paster BJ, Foltz CJ, Yan L, Shames B, Perry L. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int J Syst Bacteriol. 1997;47:627–634. doi: 10.1099/00207713-47-3-627. [DOI] [PubMed] [Google Scholar]
- 20.Sterzenbach T, Lee SK, Brenneke B, von Goetz F, Schauer DB, Fox JG, Suerbaum S, Josenhans C. Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells. Infect Immun. 2007;75:2717–2728. doi: 10.1128/IAI.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor NS, Xu S, Nambiar P, Dewhirst FE, Fox JG. Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J Clin Microbiol. 2007;45:2166–2172. doi: 10.1128/JCM.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomczak MF, Gadjeva M, Wang YY, Brown K, Maroulakou I, Tsichlis PN, Erdman SE, Fox JG, Horwitz BH. Defective activation of ERK in macrophages lacking the p50/p105 subunit of NF-kappaB is responsible for elevated expression of IL-12 p40 observed after challenge with Helicobacter hepaticus. J Immunol. 2006;176:1244–1251. doi: 10.4049/jimmunol.176.2.1244. [DOI] [PubMed] [Google Scholar]
- 23.Verreck FAW, Boer T, Langenberg DML, Zaden L, Ottenhoff THM. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-γ- and CD40L-mediated costimulation. J Leukoc Biol. 2006;79:285–293. doi: 10.1189/jlb.0105015. [DOI] [PubMed] [Google Scholar]
- 24.Wehner S, Behrendt FF, Lyutenski BN, Lysoon M, Bauer AJ, Himer A, Kalff JC. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56:176–185. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng M, Huntley D, Huang IF, Foye-Jackson O, Wang L, Sarkissian A, Zhou Q, Walker WA, Cherayil BJ, Shi HN. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol. 2007;179:4721–4731. doi: 10.4049/jimmunol.179.7.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zenner L. Pathology, diagnosis and epidemiology of the rodent Helicobacter infection. Comp Immunol Microbiol Infect Dis. 1999;22:41–61. doi: 10.1016/s0147-9571(98)00018-6. [DOI] [PubMed] [Google Scholar]