Abstract
Objective
Metabolic complications of HIV pose challenges for health maintenance among young adults who acquired HIV in early childhood.
Materials/Methods
Between 7/2004–7/2009 we evaluated 47 HIV-infected subjects who acquired HIV in early life. Participants completed glucose tolerance testing, insulin, lipid, urine albumin and creatinine determinations and DXA scans. Longitudinal data were available for 39 subjects; duration of follow-up was 26.4±16.8 months.
Results
At baseline, participants were 17.1±3.9y and duration of antiretroviral therapy was 12.7±3.4y. CD4 count was 658±374 cells/mm3 and 55% had undetectable viral load. Impaired glucose tolerance (IGT) was present in 15%; 33% had insulin resistance (HOMA-IR>4.0). Further, 52% had triglycerides ≥150 mg/dL, 36% had HDL-c <40 mg/dl, 18% had LDL-c ≥130 mg/dL and 25% had total cholesterol ≥200 mg/dL. Microalbuminuria was present in 15% of participants and was inversely correlated with CD4% (p=0.001). During follow-up more than one third remained insulin resistant; lipid parameters tended to improve. There were significant increases in BMI (p=0.0002), percent leg fat (p=0.008) and trunk fat (p=0.002).
Conclusions
IGT, insulin resistance, dyslipidemia and microalbuminuria are common among young adults with HIV. Long-term exposure to therapy may translate into substantial persistent metabolic risk.
Keywords: antiretroviral therapy, insulin resistance, dyslipidemia, microalbuminuria
Introduction
Morbidity and mortality associated with HIV/AIDS decreased dramatically with the advent of potent antiretroviral therapies (ART). However, ART has also been associated with metabolic complications, alterations in body fat distribution and chronic kidney disease [1,2,3]. Relatively fewer data exist to characterize these associations in adolescents and young adults infected with HIV in early childhood.
In adults, the use of ART is associated with insulin resistance, diabetes and cardiovascular disease (CVD) [4–8] . Previous investigation demonstrated that increased exposure to the nucleoside reverse transcriptase inhibitor (NRTI) stavudine, and to a lesser extent didanosine (ddI) and zidovudine (AZT), was associated with an increased risk of developing diabetes in adults [5]. Although long-term effects of insulin resistance are unknown, it may be an important CVD risk factor among HIV-infected adults [9].
There is currently a paucity of data pertaining to metabolic disturbances associated with long-term ART among adolescents and young adults who acquired HIV perinatally or in early childhood. In HIV-infected children, protease inhibitors (PIs) have been shown to contribute to lipid abnormalities and the presence of lipodystrophy [10–14]. HIV-infected children with lipodystrophy have higher fasting insulin compared to children without lipodystrophy [15]. Exposure to ART and inflammation in children with HIV were identified in association with increased carotid intima media thickness, a marker for CVD risk [16, 17]. Insight into the metabolic complications associated with long-term ART will allow development of preventive strategies to reduce CVD risk. Here we characterize metabolic disturbances both cross-sectionally and over time in a group of HIV-infected adolescents and young adults.
Methods
Subjects and Data Collection
We performed a cross-sectional analysis, followed by a longitudinal analysis, of a cohort of subjects who acquired HIV perinatally or in early childhood. Subjects received medical care independent of the study and were evaluated at the National Institutes of Health from 2004–2009. The research was approved by the National Institue for Allergy and Infectious Diseases’ IRB and informed consent was obtained from all subjects and parents or legal guardians, as appropriate.
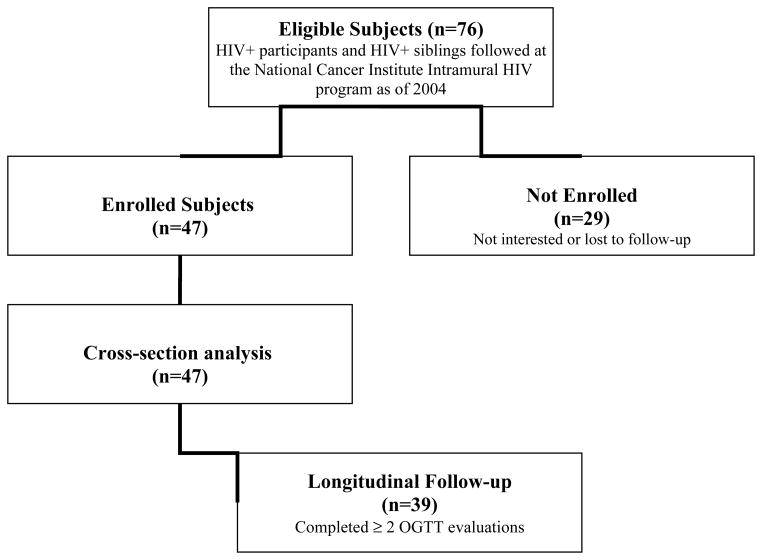
Thirty-nine of 47 subjects were included in the longitudinal analysis (Figure 1). Six subjects included in the cross-sectional analysis were lost to follow-up and 2 had not yet completed a follow-up oral glucose tolerance test (OGTT). Mean duration of follow-up was 26.4±16.8 months and data were analyzed based on the baseline visit and last follow-up.
Figure 1.
Flow diagram of subject recruitment and participation
Participants completed a clinical evaluation, including a complete history of all ART exposure. Data collected included Tanner stage, fasting lipid profile, fasting and 2 hour glucose and insulin following 75-g OGTT. Impaired glucose tolerance (IGT) was defined as a 2-hour glucose ≥140 mg/dL but <200 mg/dL. Laboratories included CD4 T lymphocyte counts, CD4%, and HIV-1 RNA by RT-PCR. Abnormal fasting lipid levels were defined based on the National Cholesterol Education Program criteria[18]. Hypertriglyceridemia was defined as triglyceride >150mg/dL, hypercholesterolemia as total cholesterol >200mg/dL, abnormal LDL-c as ≥130mg/ dL, and abnormal HDL-c as <40mg/dL. Microalbuminuria was defined as the geometric mean urine albumin-to-creatinine (urine A/C) ratio of 17–250 mg/g (males) and 25–355 mg/g (females) using all available time points [19, 20]. Thirty-five subjects provided 2–5 samples, four subjects provide one.
The homeostatic model of insulin resistance (HOMA-IR= fasting insulin (μU/m) × fasting glucose (mmol/L)/22.5) was used to evaluate insulin resistance. Subjects with a HOMA-IR >4.0 were classified as insulin resistant [21,22,23]. HOMA-IR >3.16 was also used as an alternative classification for insulin resistance [11, 22) as both have been used in prior investigations. Duration of ART was calculated at the subjects’ corresponding visit date for each individual antiretroviral agent and each class of agents. Ritonavir exposure was assessed by total years of exposure irrespective of the dose used.
Body Measurements and DXA Scan Characteristics
Standardized anthropometric measurements included height, weight, hip and waist circumferences and body mass index (BMI). Whole body dual energy X-ray absorptiometry (DXA, Hologic QDR4500A, Bedford, MA) was performed annually to measure regional and total fat and lean masses. All scans were analyzed by a single radiologist.
Statistical Analyses
Clinical characteristics are summarized using mean ± standard deviation; non-normally distributed variables are presented as a median (interquartile range) or log transformed to approximate a normal distribution. Comparisons were made using Kruskal-Wallis tests and chi-square statistics. Univariate regression analyses were performed to identify potential associations between metabolic parameters and clinical characteristics. Factors identified as significant on univariate analyses (p<0.05) were entered into multivariate regression models to identify independent predictors. Paired t-tests were used to compare longitudinal metabolic parameters between baseline and last follow-up. Statistical analyses were performed using SAS JMP Statistical Software (Version 7.0, SAS Institute Cary, NC), using a two-tailed alpha level of 0.05.
Results
Subject Characteristics
Forty-two subjects acquired HIV perinatally and 5 acquired HIV from transfusions in early childhood. Sixty-seven percent (31/46) of participants were Tanner stage 4 or greater at baseline (Table 1). At baseline, 55% of subjects had an undetectable viral load; the median CD4 T-cell count was 650 cells/mm3 (IQR 402–897). All subjects but one were ART-experienced with a duration of 12.7±3.4 years. All ART experienced subjects also had a history of exposure to a PI and 94% were currently receiving ART. Thirty-four percent of subjects were currently on a non-nucleoside reverse transcriptase inhibitor (NNRTI), whereas 85% were currently on a PI. One subject was on an integrase inhibitor, and four were on a fusion inhibitor. At entry 2 subjects were on a fibrate; no one was receiving a statin or antihypertensive agent. One participant had a diagnosis of polycystic ovary syndrome and was on metformin at the time of evaluation.
Table 1.
Demographics and Clinical Characteristics
| Cross-Sectional Study | Longitudinal Study | ||
|---|---|---|---|
| Total Cohort (n=47) | Baseline (n=39) | Last Visit (n=39) | |
| Age (y) | 17.1 ± 3.9 | 17.5 ± 3.7 | 19.7 ± 3.7 |
| Duration of follow-up (mo) | - | - | 26.4 ± 16.8 |
| Range of follow-up (mo) | 8.4–56.4 | ||
| Sex – number (%) | - | - | - |
| Male | 26 (53%) | 20 (51%) | 20 (51%) |
| Female | 21 (47%) | 19 (49%) | 19 (49%) |
| Tanner Stage – number (%) | - | - | - |
| <4 | 15 (33%) | 10 (26%) | 1 (3%) |
| >4 | 31 (67%) | 28 (74%) | 35 (97%) |
| Race – number (%) | - | - | - |
| White | 23 (49%) | 20 (51%) | 20 (51%) |
| Black | 18 (38%) | 14 (36%) | 14 (36%) |
| Mixed race | 3 (6.5%) | 3 (8%) | 3 (8%) |
| Hispanic ethnicity | 1 (2%) | 0 (0%) | 0 (0%) |
| Native American | 2 (4.5%) | 2 (5%) | 2 (5%) |
| HIV Characteristics | |||
| HIV Viral Load <50 copies/mL – no. (%) | 26 (55%) | 22 (56%) | 24 (62%) |
| CD4 T cell count – cells/mm3 | 658 ± 374 | 626 ± 370 | 725 ± 475 |
| CD4 T cell % | 29.5 ± 10.2 | 28.9 ± 10.5 | 30.3 ± 10.3 |
| Current ART use – number (%) | 44 (94%) | 37 (95%) | 37 (95%) |
| Current PI use – number (%) | 40 (85%) | 33 (85%) | 30 (76.9%) |
| Current or past PI use – number (%) | 46 (98%) | 39 (100%) | 39 (100%) |
| Current d4T use – number (%) | 21 (44%) | 17 (44%) | 7 (17.9%) |
| Current or past d4T use – number (%) | 41 (87%) | 35 (90%) | 35 (90%) |
| Current ABC use – number (%) | 4 (9%) | 4 (10%) | 8 (21%) |
| Current or past ABC use – number (%) | 13 (28%) | 10 (26%) | 18 (46%) |
| Current TDF use – number (%) | 9 (19%) | 7 (18%) | 13 (33%) |
| Current or past TDF use – number (%) | 15 (32%) | 12 (31%) | 20 (51%) |
Note. Data are mean ± standard deviation, except as indicated.
Mean BMI was 21.6±3.4 kg/m2; 15% were overweight (BMI >25kg/m2) and one subject had a BMI >30kg/m2. Waist-hip ratio was 0.92±0.07, and 17% had a waist-hip ratio ≥1.0. Percent body fat was 19.4±8.0%; percent trunk fat was 18.2±8.7%; and percent leg fat was 20.8±9.7%
Baseline Evaluations of Metabolic Parameters
At baseline, IGT was present in 15% of subjects, but no subject had type 2 diabetes. Thirty-three percent of subjects had a HOMA-IR > 4.0, which is considered insulin resistance in adolescents (21, 23). Using a less stringent cut-off of HOMA-IR > 3.16 to define insulin resistance (11, 22), 46% were insulin resistant. Dyslipidemia was common: 52% of subjects had hypertriglyceridemia, 36% low HDL-c, and 25% elevated total cholesterol. The mean total:HDL cholesterol ratio was 4.1±1.1(Table 2), and 18% of participants had suboptimal LDL-c. In univariate regression analyses, duration of AZT exposure (r=−0.31, p=0.03) was inversely correlated with HOMA-IR. Years of ddI and stavudine (d4T) did not correlate with HOMA-IR, however subjects with exposure to ddI (p=0.0008) or d4T (p=0.0005) had significantly higher HOMA-IR compared to ddI- d4T-naïve subjects. Subjects with a HOMA-IR >4 were more likely to have received ddI (χ2=7.1, p=0.008) or d4T (χ2=5.2, p=0.02). Subjects with a HOMA-IR >4 had higher triglycerides (p=0.01), waist circumference (p=0.02) and waist-hip ratio (p=0.009). There were no significant differences in HOMA-IR based on sex, Tanner stage, PI or NNRTI use. In a multivariate logistic regression model including significant factors identified in univariate analysis, only waist-hip ratio (p<0.05) remained an independent predictor of HOMA-IR >4.
Table 2.
Glucose Homeostasis, Lipid, Anthropometric Assessment
| Cross-Sectional Study | Longitudinal Study | P-value | ||
|---|---|---|---|---|
| Total Cohort (n=47) | Baseline (n=39) | Last Visit (n=39) | ||
| Glucose | - | - | - | - |
| Fasting glucose – mg/dl | 86.3 ± 10.0 | 85.9 ± 10.2 | 90.9 ± 12.5 | 0.06 |
| 2-hr OGTT glucose – mg/dl | 108.1± 31.4 | 109.7 ± 31.5 | 104.3 ± 29.2 | 0.3 |
| Fasting insulin - μIU/ml | 19.7 ± 18.5 | 21.2 ± 19.9 | 20.3 ± 22.7 | 0.8 |
| 2-hr OGTT insulin - μIU/ml | 94.5 ± 119.7 | 99.6 ± 128.1 | 78.1 ± 70.6 | 0.1 |
| HOMA-IR | 4.4 ± 4.5 | 4.8 ± 4.9 | 5.1 ± 7.8 | 0.8 |
| Lipids | - | - | - | - |
| Total Cholesterol (TC) mg/dL | 177 ± 42 | 174 ± 43 | 165 ± 45 | 0.2 |
| HDL Cholesterol mg/dL | 44 ± 10 | 45 ± 10 | 43 ± 12 | 0.3 |
| LDL Cholesterol mg/dL | 106 ± 30 | 103 ± 27 | 95 ± 38 | 0.2 |
| TC/HDL ratio | 4.1 ± 1.1 | 3.9 ± 0.9 | 4.0 ± 1.5 | 0.7 |
| Triglycerides mg/dL | 248 ± 266 | 226 ± 216 | 177 ± 151 | 0.07 |
| Anthropometrics | - | - | - | - |
| Height (cm) | 161.2 ± 11.9 | 162.2 ± 10.0 | 164.7 ± 8.9 | 0.003 |
| Weight (kg) | 56.5 ± 12.7 | 57.8 ± 11.8 | 63.7 ± 12.4 | 0.0001 |
| Body-mass index (kg/ m2) | 21.6 ± 3.4 | 21.9 ± 3.4 | 23.5 ± 4.4 | 0.0002 |
| Waist-hip ratio | 0.92 ± 0.07 | 0.92 ± 0.08 | 0.90 ± 0.15 | 0.3 |
| Percent body fat (%) | 19.4 ± 8.0 | 19.9 ± 8.1 | 22.5 ± 9.8 | 0.0006 |
| Percent trunk fat (%) | 18.2 ± 8.7 | 19.0 ± 8.9 | 22.1 ± 11.1 | 0.002 |
| Percent leg fat (%) | 20.8 ± 9.7 | 20.8 ± 9.6 | 23.2 ± 11.2 | 0.008 |
Note. Data are presented as mean ± standard deviation, except as indicated.
Compared to those without IGT, participants with IGT had significantly higher total cholesterol (215±46 mg/dL vs. 169±38 mg/dL, p=0.04), triglycerides (377±212 mg/dL vs. 226±274 mg/dL, p=0.005) and total:HDL cholesterol ratio (5±1 vs. 4±1, p=0.03). Participants with IGT had greater total percent body fat (p=0.009), greater percent leg fat (p=0.02) and were more likely to be male (χ2=9.6, p=0.002). There was no significant relationship between Tanner stage and IGT.
Higher viral load (r=−0.41, p=0.009) and greater years of abacavir use (r=−0.31, p<0.05) were associated with lower HDL-c, whereas NNRTI use (r=0.35, p=0.02) was related to higher HDL-c. In multivariate regression, only viral load remained a significant independent predictor of HDL-c (p=0.03). Additionally, total years of ritonavir was positively correlated with LDL-c (r=0.34, p=0.03). There were no significant relationships identified between total or LDL-c and other HIV parameters, waist-hip ratio, BMI, trunk fat or limb fat.
Microalbuminuria
Microalbuminuria was present in 15% (6/39) of subjects (95% CI, 7–30%) during follow-up; median urine A/C ratio was 5.4 (range, 1.6–125.8 mg/g). Five of 6 subjects with microalbuminuria were male and 4 were African American. African American subjects had elevated baseline A/C ratio compared to other subjects (A/C ratio 39±49 vs. 8±13 mg/g, p=0.008). No subject had macroalbuminuria. Baseline urine A/C ratio was inversely correlated with CD4% (r=−0.53, p=0.001) and positively correlated with systolic blood pressure (r=0.35, p=0.04). While no other ART medication correlated with urine A/C ratio, years of tenofovir (TDF) was highly positively correlated with baseline urine A/C ratio (r=0.53, p=0.001). In a multivariate model, years of TDF (p=0.003) and African American race (0.008) remained significant independent predictors of urine A/C ratio. Though not statistically significant, subjects with microalbuminuria tended to have higher viral load (368 vs. 100 copies/mL) and lower median CD4 counts (389 vs. 747 cells/mm3) compared to those without microalbuminuria. There was no significant difference in HOMA-IR or IGT in relation to microalbuminuria.
Longitudinal evaluation of metabolic parameters
For longitudinal analysis the mean number of visits per subject was 2.9±1 (n=39). HIV viral load was below the limit of detection in 56% at entry and 62% at last follow-up; median CD4 T-cell counts were unchanged over time. Two subjects were not on ART throughout the entire duration of follow-up. Eighty-five percent of subjects were on a PI at baseline versus 76.9% at follow-up and 44% were on stavudine at baseline versus 17.9% at last follow-up.
Of the subjects followed longitudinally, 13% met criteria for IGT at entry versus 10% at last follow-up. Mean HOMA-IR remained constant; 14 subjects had HOMA-IR >4 at entry and last follow-up (Figure 2). Increases in fasting glucose between entry and last follow-up approached statistical significance (85.9±10.2 vs. 90.9±12.5 mg/dL, p=0.06). In general, lipid values tended to improve over time, but not significantly (See Table 2). Change in lipids did not differ between those who started or discontinued a PI.
Figure 2.
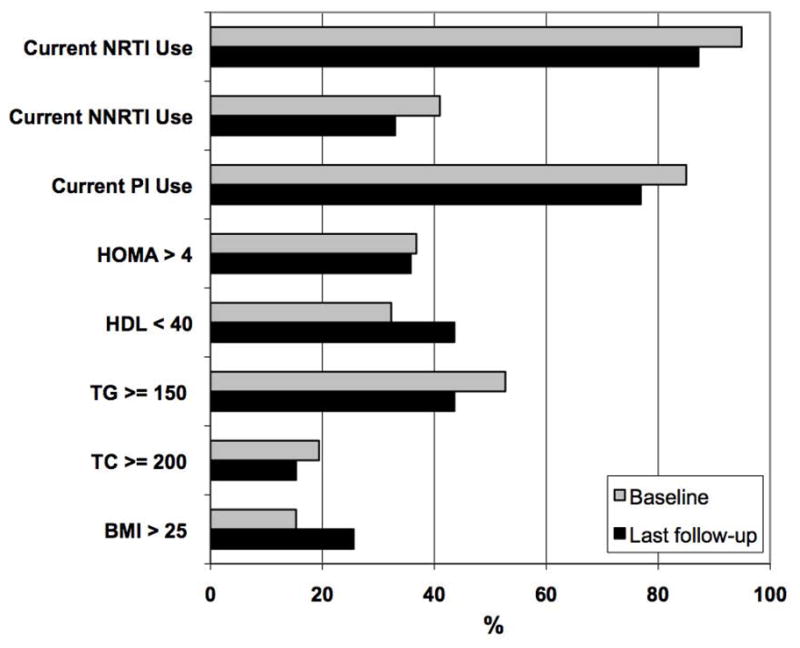
Prevalence of metabolic abnormalities among HIV-infected participants in longitudinal follow-up at baseline and last visit (n=39). Abbreviations: NRTI = nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor; BMI = body mass index (kg/m2); TC = total cholesterol (mg/dL); TG = triglycerides (mg/dL); HDL = high density lipoprotein (mg/dL). HOMA-IR, homeostasis model of insulin resistance.
Changes in body composition were characterized by an increase in total percent body fat and BMI. There were significant increases in BMI (21.9±3.4 vs. 23.5±4.4 kg/m2, p=0.0002), percent body fat (19.9±8.1 vs. 22.5±9.8%, p=0.0006), leg fat (20.8±9.6 vs. 23.2±11.2%, p=0.008) and trunk fat (19.0±8.9 vs. 22.1±11.1%, p=0.002). There was a significant positive correlation between change in BMI and change in fasting glucose (r=0.37, p=0.02). Individuals who remained on a d4T, a medication associated with lipoatrophy, had smaller increases in BMI compared to those who stopped or who had not been on d4T (0.23±0.96 vs. 1.95±2.64, p=0.007). Additionally, subjects with BMI >25 kg/m2 increased from 15% to 26% (p=0.0001). There were no significant changes in total lean mass or waist-hip ratio.
Discussion
Few studies have described metabolic disturbances in relation to lifelong exposure to ART among HIV-infected adolescents and young adults. In this study, we identified insulin resistance, IGT, dyslipidemia, and microalbuminuria among a substantial proportion of HIV-infected adolescents and young adults with extensive ART exposure. Of interest, those individuals with IGT also had more dyslipidemia, which may translate into a considerable increase in the risk of CVD.
The PACTG 1045 study reported data from a large cohort of vertically-infected children using OGTT and found fewer than 5% with abnormal results [24]. We identified a 15% prevalence of IGT which may be attributable to the older age of our cohort. Pediatric studies have also shown higher fasting insulin levels among HIV-infected children as a marker of insulin resistance [11, 24, 25, 26]. Chantry et al. found that 48 weeks after initiating or changing antiretroviral therapies, insulin resistance (HOMA-IR > 3.16) increased from 1% to 8% among a cohort of HIV-infected children[11], whereas 46% of our participants had HOMA-IR >3.16. Again this may be due to the relatively older age, longer duration of therapy and higher rates of PI use in the present study.
Several clinical factors were identified as associated with insulin resistance. Subjects with stavudine and ddI exposure were more likely to have elevated HOMA-IR, whereas years of AZT exposure was associated with lower HOMA-IR. Waist-hip ratio was an important predictor of insulin resistance after adjusting for ART exposure. A study by Rosso et al. [27] showed that BMI was a significant predictor of insulin resistance among HIV-infected youth, while Chantry et al.[28] concluded that greater adiposity may not be linked to an increase in HOMA-IR in HIV-infected children. We found that distribution of adiposity as measured by waist-hip ratio was more closely related to insulin resistance than BMI per se.
Similar to the D.A.D. findings on diabetes[5], we found that stavudine and ddI were related to insulin resistance. However, stavudine and ddI did not remain significant when waist-hip ratio was taken into account, suggesting that alterations in body fat distribution may underlie the observed drug effects on insulin resistance.
Dyslipidemia is frequently reported among HIV-infected patients [10, 29, 30]. We identified abnormal lipid values among a considerable proportion of our cohort and the rates are similar to those reported in the PACTG 1045 cohort [24]. Both studies also identified abnormally low HDL-c levels in a large percentage of subjects who acquired HIV in infancy or early childhood and who had extensive ART experience. PI use is frequently implicated in hyperlipidemia in adults [30] and the extensive use of these agents in our cohort likely contributed to the observed dyslipidemia.
We identify higher viral load and cumulative exposure to abacavir as factors associated with lower HDL-c and increased exposure to NNRTIs in association with greater HDL-c. These results suggest a potential protective effect of NNRTI exposure on lipids and are supported by recent studies of HIV-infected children, in which NNRTI therapy was associated with increases in HDL-c (10). In addition, NNRTIs are often used in ART regimens to replace a PI for their more favorable side effect profile with regard to lipids[1].
Microalbuminuria is a risk factor for CVD among otherwise healthy individuals [31, 32]. In this HIV-infected adolescent population microalbuminuria was present in 15% of subjects, a rate similar to what Szczech et al. [33] describes in HIV-infected adults (11%). While the confidence interval of our result (7–30%) encompasses the prevalence of 8.9% of microalbuminuria seen in healthy adolescents [34], our results are slightly higher than the estimated 10% observed in obese adolescents [35]. Our findings further highlight the importance of African American race in the risk of development of kidney injury in the context of HIV. Similar to previous findings in adults [33], we found that CD4% was inversely related to A/C ratio and there was a trend for subjects with microalbuminuria to have a greater HIV viral burden. These data implicate HIV and immune function in the development of microalbuminuria and HIV associated kidney disease.
The prevalence of insulin resistance was stable over time, whereas there were modest improvements in lipids. While this may represent a lowering of underlying CVD risk, low HDL-c remained highly prevalent and significant increases in BMI were also noted. Increases in BMI are anticipated with growth, but disproportionate increases in body fat were seen without increases in lean mass. This could reflect restoration of fat stores previously depleted with lipoatrophy, but may also indicate unhealthy gains in adiposity. Leptin, an adipokine involved in glucose homeostasis, particularly in the context of lipoatrophy [36, 37], was not measured, and its role in youth with HIV is less clear [38, 39]. As previously suggested by Blass et al.[40] and seen in the present study, emergent obesity in HIV cohorts may be driving the increase in fasting glucose levels, an indicator of pre-diabetes.
The strength of our study is the study cohort, which represents some of the most treatment-experienced HIV-infected adolescents and young adults. Additionally, our study longitudinally evaluated insulin resistance and dyslipidemia combined with detailed body composition assessments as markers for CVD risk. Although we lack a control group for direct comparison, have a relatively small sample size and did not directly measure adipokines, this is a well-characterized cohort with respect to ART exposure, metabolism, body composition and pubertal development. Socio-economic status, dietary and life-style factors that contribute to metabolic disturbances may be enriched in this cohort relative to the general population. Another potential limitation is that the subjects were not characterized by clinical criteria of lipodystrophy; however, objective assessment methods, such as DXA scans were used. Additionally, the very high prevalence of PI use and complex regimen modifications limit our ability to distinguish effects from individual drugs or classes of drugs.
In summary, our findings demonstrate that a substantial proportion of the study population have persistently altered glucose metabolism, dyslipidemia, and microalbuminuria. While modest improvements in certain lipid parameters were noted over time, insulin resistance was stable and the prevalence of overweight was on the rise. This study has significant global implications, as HIV-infected children continue to survive into adulthood with expanded access to ART and indicates the need for careful consideration of antiretroviral toxicities when treating children with HIV. Alterations in lipids and glucose metabolism may confer significant risk of future diabetes and CVD, and therefore, continued longitudinal follow-up evaluations are warranted to better understand the relationship between HIV infection, life-long ART exposure and CVD risk in this growing and unique population.
Acknowledgments
The authors would like to thank Seshagiri Vakkalanka, Jennifer Graf, and Dr. Jim Reynolds for their assistance with data collection and management.
Financial support. This study was funded by Divisions of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, and National Cancer Institute, National Institutes of Health, Bethesda, MD.
Abbreviations
- ART
antiretroviral therapy
- CVD
cardiovascular disease
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- ddI
didanosine
- AZT
zidovudine
- OGTT
oral glucose tolerance test
- IGT
impaired glucose tolerance
- HOMA-IR
homeostatic model of insulin resistance
- BMI
body mass index
- DXA
dual energy X-ray absorptiometry
- urine A/C
urine albumin-to-creatinine ratio
- TDF
tenofovir
Footnotes
Potential conflicts of interest. All authors: No conflicts.
Author Contributions:
David Dimock: Data collection, data interpretation, statistical analysis and manuscript writing
Vijaya Thomas: Data collection and manuscript writing
Anna Cushing: Data collection and organization
Julia B. Purdy: Protocol oversight, physical examinations, data collection and organization
Carol Worrell: Data collection and interpretation
Jeffrey B. Kopp: Data interpretation and analysis
Rohan Hazra: Protocol oversight, data interpretation and manuscript writing
Colleen Hadigan: Protocol oversight, physical examinations, statistical analysis and manuscript writing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grinspoon S, Carr A. Cardiovascular Risk and Body Fat Abnormalities in HIV-infected Adults. N Engl J Med. 2005 Jan 6;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, Klotman PE. Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol. 2005 Aug;16(8):2412–20. doi: 10.1681/ASN.2005040340. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt CM, Klotman PE. HIV-associated nephropathy in the era of antiretroviral therapy. Am J Med. 2007 Jun;120(6):488–92. doi: 10.1016/j.amjmed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Samaras K. Metabolic consequences and therapeutic options in highly active antiretroviral therapy in human immunodeficiency virus-1 infection. J Antimicrob Chemother. 2008 Feb;61(2):238–45. doi: 10.1093/jac/dkm475. [DOI] [PubMed] [Google Scholar]
- 5.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008 Jun;31(6):1224–9. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005 May 23;165(10):1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 7.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007 Apr 26;356(17):1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009 Sep 10;23(14):1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondy KE, de las Fuentes L, Waggoner A, Onen NF, Bopp CS, Lassa-Claxton S, et al. Insulin resistance predicts endothelial dysfunction and cardiovascular risk in HIV-infected persons on long-term highly active antiretroviral therapy. AIDS. 2008 Apr 23;22(7):849–56. doi: 10.1097/QAD.0b013e3282f70694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller TL, Orav EJ, Lipshultz SE, Arheart KL, Duggan C, Weinberg GA, et al. Risk factors for cardiovascular disease in children infected with human immunodeficiency virus-1. J Pediatr. 2008 Oct;153(4):491–7. doi: 10.1016/j.jpeds.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantry CJ, Hughes MD, Alvero C, Cervia JS, Meyer WA, 3rd, Hodge J, et al. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008 Jul;122(1):e129–38. doi: 10.1542/peds.2007-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arpadi SM, Cuff PA, Horlick M, Wang J, Kotler DP. Lipodystrophy in HIV-infected children is associated with high viral load and low CD4+-lymphocyte count and CD4+-lymphocyte percentage at baseline and use of protease inhibitors and stavudine. J Acquir Immune Defic Syndr. 2001;27(1):30–4. doi: 10.1097/00126334-200105010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Taylor P, Worrell C, Steinberg SM, Hazra R, Jankelevich S, Wood LV, et al. Natural history of lipid abnormalities and fat redistribution among human immunodeficiency virus-infected children receiving long-term, protease inhibitor-containing, highly active antiretroviral therapy regimens. Pediatrics. 2004 Aug;114(2):e235–42. doi: 10.1542/peds.114.2.e235. [DOI] [PubMed] [Google Scholar]
- 14.Carter RJ, Wiener J, Abrams EJ, Farley J, Nesheim S, Palumbo P, et al. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort, 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006 Apr 1;41(4):453–60. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- 15.Verkauskiene R, Dollfus C, Levine M, Faye A, Deghmoun S, Houang M, et al. Serum adiponectin and leptin concentrations in HIV-infected children with fat redistribution syndrome. Pediatr Res. 2006 Aug;60(2):225–30. doi: 10.1203/01.pdr.0000228335.64894.26. [DOI] [PubMed] [Google Scholar]
- 16.McComsey GA, O'Riordan M, Hazen SL, El-Bejjani D, Bhatt S, Brennan ML, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007 May 11;21(8):921–7. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 17.Ross AC, O'Riordan MA, Storer N, Dogra V, McComsey GA. Heightened inflammation is linked to carotid intima-media thickness and endothelial activation in HIV-infected children. Atherosclerosis. 2010 Apr 24; doi: 10.1016/j.atherosclerosis.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421. [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul 15;139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 20.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002 Apr;13(4):1034–9. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 21.Tresaco B, Bueno G, Moreno LA, Garagorri JM, Bueno M. Insulin resistance and impaired glucose tolerance in obese children and adolescents. J Physiol Biochem. 2003 Sep;59(3):217–23. doi: 10.1007/BF03179918. [DOI] [PubMed] [Google Scholar]
- 22.Tresaco B, Bueno G, Pineda I, Moreno LA, Garagorri JM, Bueno M. Homeostatic model assessment (HOMA) index cut-off values to identify the metabolic syndrome in children. J Physiol Biochem. 2005 Jun;61(2):381–8. doi: 10.1007/BF03167055. [DOI] [PubMed] [Google Scholar]
- 23.Valerio G, Licenziati MR, Iannuzzi A, Franzese A, Siani P, Riccardi G, et al. Insulin resistance and impaired glucose tolerance in obese children and adolescents from Southern Italy. Nutr Metab Cardiovasc Dis. 2006 May;16(4):279–84. doi: 10.1016/j.numecd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Aldrovandi GM, Lindsey JC, Jacobson DL, Zadzilka A, Sheeran E, Moye J, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009 Mar 27;23(6):661–72. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beregszaszi M, Dollfus C, Levine M, Faye A, Deghmoun S, Bellal N, et al. Longitudinal Evaluation and Risk Factors of Lipodystrophy and Associated Metabolic Changes in HIV-Infected Children. J Acquir Immune Defic Syndr. 2005 Oct 1;40(2):161–8. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 26.Jaquet D, Levine M, Ortega-Rodriguez E, Faye A, Polak M, Vilmer E, et al. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV-infected children. AIDS. 2000 Sep 29;14(14):2123–8. doi: 10.1097/00002030-200009290-00008. [DOI] [PubMed] [Google Scholar]
- 27.Rosso R, Parodi A, d'Annunzio G, Ginocchio F, Nicolini L, Torrisi C, et al. Evaluation of insulin resistance in a cohort of HIV-infected youth. Eur J Endocrinol. 2007 Nov;157(5):655–9. doi: 10.1530/EJE-07-0414. [DOI] [PubMed] [Google Scholar]
- 28.Chantry C, Hughes M, Alvero C, Cervia J, Hodge J, Borum P, et al., editors. Growth and body composition in children beginning or changing antiretroviral therapy. PACTG1010; XVI International AIDS Conference; 2006 August 13–18; Toronto, Ontario, Canada. 2006. [Google Scholar]
- 29.Kamin D, Hadigan C. Hyperlipidemia in children with HIV infection: an emerging problem. Expert Rev Cardiovasc Ther. 2003 May;1(1):143–50. doi: 10.1586/14779072.1.1.143. [DOI] [PubMed] [Google Scholar]
- 30.Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000 Jul 10;160(13):2050–6. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- 31.Cao JJ, Barzilay JI, Peterson D, Manolio TA, Psaty BM, Kuller L, et al. The association of microalbuminuria with clinical cardiovascular disease and subclinical atherosclerosis in the elderly: the Cardiovascular Health Study. Atherosclerosis. 2006 Aug;187(2):372–7. doi: 10.1016/j.atherosclerosis.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med. 2007 Dec 10;167(22):2490–6. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 33.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, et al. Microalbuminuria in HIV infection. AIDS. 2007 May 11;21(8):1003–9. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen S, McCulloch C, Brakeman P, Portale A, Hsu CY. Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics. 2008 Jan;121(1):37–45. doi: 10.1542/peds.2007-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgert TS, Dziura J, Yeckel C, Taksali SE, Weiss R, Tamborlane W, et al. Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes (Lond) 2006 Feb;30(2):273–80. doi: 10.1038/sj.ijo.0803136. [DOI] [PubMed] [Google Scholar]
- 36.Mantzoros CS. Whither recombinant human leptin treatment for HIV-associated lipoatrophy and the metabolic syndrome? J Clin Endocrinol Metab. 2009 Apr;94(4):1089–91. doi: 10.1210/jc.2009-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulligan K, Khatami H, Schwarz JM, Sakkas GK, DePaoli AM, Tai VW, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009 Apr;94(4):1137–44. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resino S, Micheloud D, Lorente R, Bellon JM, Navarro ML, Munoz-Fernandez MA. Adipokine profiles and lipodystrophy in HIV-infected children during the first 4 years on highly active antiretroviral therapy. HIV Med. 2010 May 17; doi: 10.1111/j.1468-1293.2010.00837.x. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Dzwonek AB, Novelli V, Schwenk A. Serum leptin concentrations and fat redistribution in HIV-1-infected children on highly active antiretroviral therapy. HIV Med. 2007 Oct;8(7):433–8. doi: 10.1111/j.1468-1293.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 40.Blass S, Ellinger S, Vogel M, Ingiliz P, Spengler U, Stehle P, et al. Overweight HIV patients with abdominal fat distribution treated with protease inhibitors are at high risk for abnormalities in glucose metabolism - a reason for glycemic control. Eur J Med Res. 2008;13(5):209–14. [PubMed] [Google Scholar]