Abstract
Estrogen receptor β (ERβ) is expressed at high levels in both neurons and glial cells of the central nervous system. The development of ERβ knockout (BERKO) mice has provided a model to study the function of this nuclear receptor in the brain. We have found that the brains of BERKO mice show several morphological abnormalities. There is a regional neuronal hypocellularity in the brain, with a severe neuronal deficit in the somatosensory cortex, especially layers II, III, IV, and V, and a remarkable proliferation of astroglial cells in the limbic system but not in the cortex. These abnormalities are evident as early as 2 mo of age in BERKO mice. As BERKO mice age, the neuronal deficit becomes more pronounced, and, by 2 yr of age, there is degeneration of neuronal cell bodies throughout the brain. This is particularly evident in the substantia nigra. We conclude that ERβ is necessary for neuronal survival and speculate that this gene could have an important influence on the development of degenerative diseases of the central nervous system, such as Alzheimer's disease and Parkinson's disease, as well as those resulting from trauma and stroke in the brain.
Evidence suggests multiple roles for 17β-estradiol (E2) in the brain. It influences development, plasticity, and survival of neurons with consequences for age-related neuronal degenerative diseases (1–3). It also exerts regulatory functions in serotonergic (4), dopaminergic (5), and cholinergic (6) neurons. E2 is thought to enhance cognitive functions by modulating the production of acetylcholine in basal forebrain neurons, where estrogen receptor (ER)α is the predominant ER subtype. In the dorsal raphe nucleus, E2 increases the amount of the 5-hydroxytryptamine (2A) receptor mRNA and the serotonin transporter mRNA. In the dopaminergic system, E2 can prevent or modulate insults to dopaminergic neurons. It is thought, therefore, to alter the natural history of disease processes affecting the dopaminergic circuitry in the brain, including Parkinson's disease, chorea, dystonia, tics, and myoclonus (7).
Epidemiological studies suggest that E2 replacement therapy decreases the likelihood of developing Alzheimer's disease (8–10). In addition to its effects on neurotransmitter system, another possible mechanism through which E2 could be beneficial might relate to its capacity to protect against both stroke and hypertensive brain damage in postmenopausal women (9). Silent strokes are thought to contribute to and even be causative in dementia (11). Despite all of the laboratory data and trends from human epidemiological studies, the usefulness of E2 in protection against degenerative diseases of aging in women is still an unresolved issue (12–16). It is particularly highly debated at present because of a recent study (12) that shows that replacement of E2 in older women has no effect on incidence or progression of Alzheimer's disease. If E2 has no protective effects when it is administered to older women who already have Alzheimer's disease, the beneficial effects of E2 might lie in its capacity to prevent or slow down the development of neurodegeneration. Not enough is known about the mechanisms involved in the central nervous system (CNS) actions of E2. To understand E2 action in the brain, the roles of ERα and ERβ in regulating neurotransmitter systems, brain cytokines, oxidative stress, amyloid deposition, apoptosis, glial cell function, and neuronal imprinting must be delineated.
Neurons in certain nuclei of the brain are sexually dimorphic. They are programmed by E2 during the neonatal period. This effect of E2 is ER-mediated, but so far the roles of ERα and ERβ in this process have not been clarified. In situ hybridization and immunohistochemical studies in rodent (17), monkey (18), and human (19) brains have revealed that there are neuronal populations that express both receptors and some that express only one of the two receptors. Neurons of the supraoptic nucleus, a sexually dimorphic nucleus, which is larger in males than females, express ERβ but not ERα, whereas, in the anteroventral periventricular nucleus of the preoptic area, which is larger in females than males, there is ERα but not ERβ.
Astrocytes in the arcuate nucleus of the rat hypothalamus (but not in the ventromedial nucleus) exhibit a sexually dimorphic morphology (20). Throughout life, the arcuate nucleus of males has fewer axodendritic spine synapses than females. This dimorphism results from E2 imprinting in the neonatal period. E2 increases astrocyte differentiation and reduces dendritic spines on arcuate neurons (21). Previously, in vitro experiments showed that ER mRNA is expressed in glial cells (22). Recently, it was found that, in the adult brain, only ERβ colocalizes with the astrocyte marker, glial fibrillary acidic protein (GFAP) (23). If ERβ is the predominant ER in astrocytes in the brain, this receptor could be important in brain development, neuronal migration, and sexual differentiation of the brain. From the differences in expression patterns of ERα and ERβ in the brain, it could be anticipated that brain phenotypes of mice in which either of these receptors has been inactivated should be different. No morphological changes have been reported in mice lacking ERα, but, in this study, we describe striking morphological abnormalities in the brains of ERβ knockout (BERKO) mice.
Materials and Methods
Animals and Tissue Preparation.
BERKO mice were created from two mouse strains, J129 and C57BL/6J (24). Heterozygote mice are used for breeding, and, in all experiments, comparisons are between wild-type and knockout littermates. Wild-type and BERKO male and female mice aged 2 mo, 1 yr, and 2 yr were used. The animals were housed in the animal-care facility with a 12-h light, 12-h dark photoperiod and free access to tap water and rodent chow. Animals were exposed to a lethal dose of CO2, and brains were removed and put into 4% paraformaldehyde for histological evaluation and GFAP immunohistochemical analysis.
Histology.
In this study, we used Nissl staining and hematoxylin/eosin (HE) staining to examine the histology of brain with light microscopy. In 1-yr- and 2-yr-old groups, both frozen and paraffin sections were used. For paraffin sections, brains were postfixed in 4% paraformaldehyde overnight and processed routinely for paraffin embedding. Then, 4-μm serial, coronal sections were cut and mounted directly on organosilane-coated slides. For frozen sections, brains were postfixed in 4% paraformaldehyde overnight. This was followed by incubation in PBS containing 10% sucrose (pH 7.4) with three buffer changes over the next 24 h. In the 2-yr-old group, 12-μm frozen, serial, coronal sections were cut and mounted directly on gelatin-coated slides. In the 2-mo-old and 1-yr-old groups, 30-μm serial, coronal, cryostat sections were made and directly mounted on gelatin-coated slides. The sections were deparaffined in xylene and rehydrated, then stained with thionin or HE.
Immunohistochemistry.
Paraffin sections were deparaffined in xylene, rehydrated, and then processed in the same manner as the frozen sections. Briefly, sections were incubated in 50% methanol containing 1% H2O2 for 30 min to quench endogenous peroxidases, followed by 10% normal horse serum, 0.5% Triton X-100 in PBS for 30 min to block unspecific binding. Sections were then incubated with goat anti-GFAP antibody (Santa Cruz Biotechnology), 1:500, in 3% BSA for 48 h at 4°C. Sections were then incubated with biotinylated rabbit anti-goat IgG (Vector Laboratories), 1:200, for 2 h at room temperature, and then avidin-biotin-complex (Vector Laboratories) 1:100, for 2 h at room temperature. After every step, sections were washed with PBS for 30 min. Staining was developed with 3,3′-diaminobenzidine (Dako).
Cell Counting.
Neuronal counting was performed in the somatosensory cortex of 2-mo-old male mice. The rostral and caudal boundaries were defined as shown in Fig. 1c. Neurons were counted on images in one of every 10 coronal sections by three independent pathologists who were not provided the information of genotype of samples. All pictures for neuronal counting were location-matched between wild-type and BERKO mice. One frame for the visual field of 200 × 200 μm was used for counting and measuring. The analysis was performed on three animals of each group. Statistical analysis was performed by using Student's t test. The proliferation of astrocytes was quantified by image analysis on anti-GFAP-stained sections of planes of medial preoptic area (MPA), paraventricular nucleus of hypothalamus (PVN), and medial amygdala nucleus (MeA). Three different visual fields were chosen randomly from various regions of each animal, and cell counting and statistical analysis were performed as above. Neuronal counting also was performed as above in these three brain areas by using Nissl-stained sections.
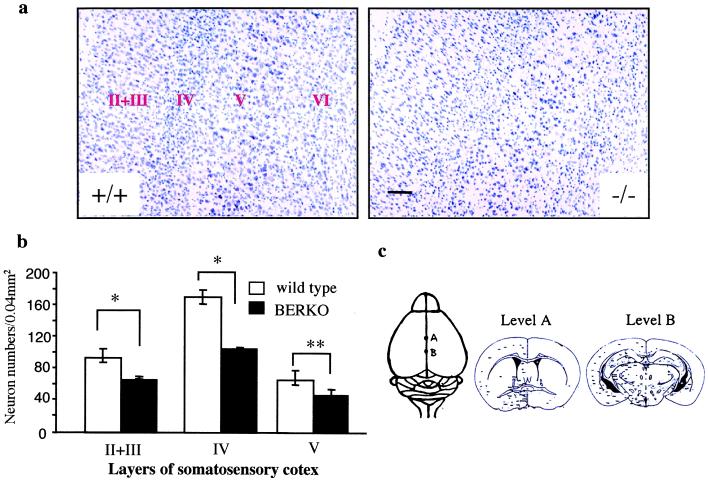
Figure 1.
(a) Coronal sections (30 μm, Nissl stain) of the somatosensory cortex show histology comparison of 2-mo-old wild-type (+/+) and BERKO (−/−) male mice. Note the decreased number of neurons in layer II, III, IV, and V in BERKO mice. (Scale bar = 100 μm.) (b) Mean number of neurons in the layers II, III, IV, and V of somatosensory cortex (n = 3; error bar, SD; *, P < 0.01; **, P < 0.05, Student's t test). (c) Landmark points for morphometry at the dorsal view of the brain and schematic illustrations of coronal slices of levels A and B.
Results
Gross Anatomical and Morphological Changes in the BERKO Brain.
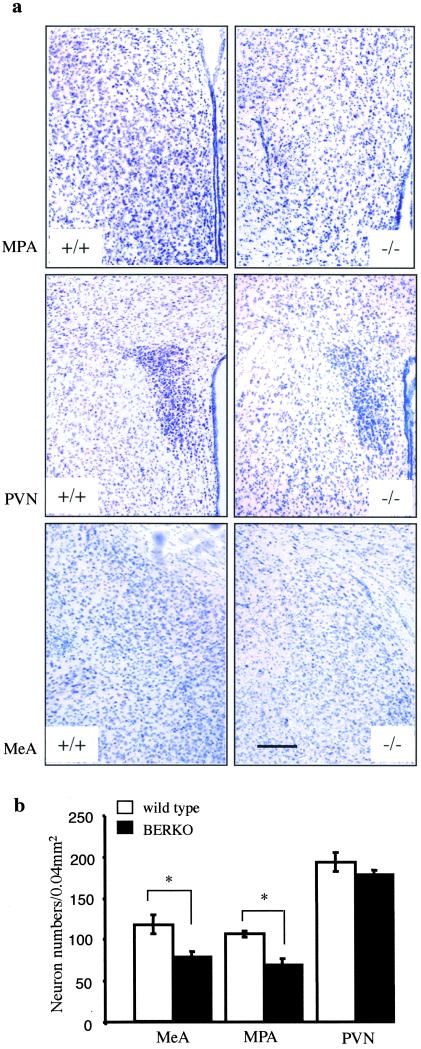
At 2 mo of age, there were no significant differences in the size or gross morphology of the brains between BERKO and wild-type mice. However, Nissl staining showed neuronal hypocellularity in the BERKO mouse brain. In the neocortex, a severe neuronal deficit of the layers II, III, IV, and V was the most pronounced difference between BERKO mice and their wild-type littermates (Fig. 1 a and b). The affected area ranged from the somatosensory region to parietal region. On examination of sections from the rostral to the caudal level, it was clear that the neuronal deficit was confined to the neocortex, through levels A and B (Fig. 1c). The schematic drawings for specific brain regions were adopted from a standard mouse brain atlas (25). In the basal forebrain, hypothalamus, and amygdala (Fig. 2 a and b), ventral tegmental area, substantia nigra, central gray, dorsal raphe nucleus, locus coeruleus, and solitary tract nucleus, neuronal loss also was observed in BERKO mouse brains. Statistical analysis showed that there was significant neuronal loss in MPA and MeA of BERKO mice, but not in PVN. In the hippocampus, caudate-putamen, thalamus, and cerebellum, no obvious neuronal deficit was seen. In these 2-mo-old BERKO mice, there was no shrinkage of the neurons, and the neuronal loss was much more obvious in male than in female mice.
Figure 2.
(a) Coronal sections (30 μm, Nissl stain) of MPA (Top), PVN (Middle), and MeA (Bottom) show histology comparison of 2-yr-old wild-type (+/+) and BERKO (−/−) male mice. Note the decreased number of neurons in these nuclei in BERKO mice. (Scale bar = 170 μm.) (b) Mean number of neurons in MPA, PVN, and MeA (n = 3; error bar, SD; **, P < 0.05, Student's t test).
In the 1-yr-old group, the global size of brain was still similar in wild-type and BERKO mice. Both male and female BERKO mice showed neuronal hypocellularity in the same regions where this was observed in the 2-mo-old male BERKO brain.
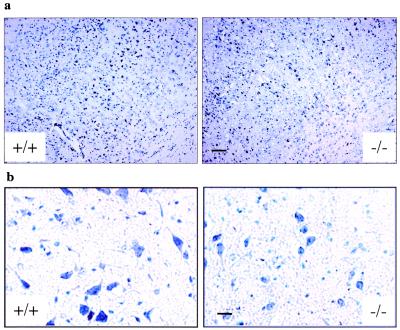
In the 2-yr-old group, BERKO mouse brains were significantly smaller than those of wild-type littermates. From examination of brain sections, atrophy was most obvious in the somatosensory-parietal cortex. Although all layers were affected, thinning of layers IV and V was the most prominent change. The numbers of small neurons in the layer II-III and IV and large pyramidal neurons in the layer V were reduced in BERKO mouse brains (Fig. 3a). In addition, neuronal cell bodies were smaller. This shrinkage of neurons was particularly evident in the substantia nigra (Fig. 3b).
Figure 3.
(a) Coronal sections (12 μm, Nissl stain) of the somatosensory cortex show histology comparison of 2-yr-old wild-type (+/+) and BERKO (−/−) male mice. Note the decreased number of neurons in layers II, III, IV, and V in BERKO mice. (Scale bar = 100 μm.) (b) High magnification views of neurons in substantia nigra of 2-yr-old wild-type and BERKO male mice. Note the shrinkage of neurons in BERKO mice. (Scale bar = 25 μm.)
With HE staining, no neurofibrillary tangles or Lewy bodies were detected in the BERKO mouse brains.
GFAP Immunoreactivity in the Brain.
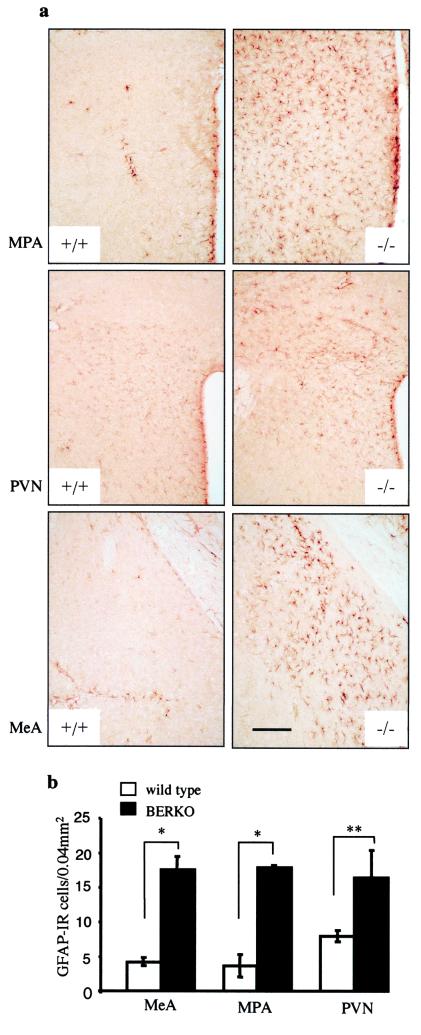
In 2-mo-old male BERKO mice, there was an increased number of GFAP-immunoreactive cells throughout many brain areas. This increase was not as evident in females of the same age. The basal forebrain, hypothalamus, and amygdala were the regions most severely affected (Fig. 4 a and b). In addition, in BERKO mouse brains, the morphology of astroglial cells was different. They were hypertrophic, with swollen cell bodies and thick processes compared with those in the brains of wild-type animals. Moreover, in the white matter adjacent to the regions of neuronal loss, there was an increase in number of hypertrophic astrocytes. In the hippocampus and cerebellum, only a mild increase of GFAP immunoreactivity was found. It was striking that in the somatosensory cortex with severe neuronal loss no astrogliosis was found.
Figure 4.
GFAP immunostaining comparison in the brains of 2-mo-old wild-type (+/+) and BERKO (−/−) male mice. (a) GFAP immunoreactivity in coronal sections (30 μm) of MPA (Top), PVN (Middle), and MeA (Bottom). Note the increased GFAP immunoreactivity in these nuclei in BERKO mice. (Scale bar = 170 μm.) (b) Mean number of GFAP-immunoreactive cells (GFAP-IR) in MPA, PVN, and MeA (n = 3; error bar, SD; *, P < 0.01; **, P < 0.05, Student's t test).
In 1-yr-old mice, a mild to moderate increase of astrocytes was observed in both male and female groups. However, in the 2-yr-old group, neither the number nor morphology of GFAP immunoreactive cells was different between BERKO and wild-type mice.
Discussion
In this study, we found significant neuronal loss and proliferation of astroglial cells in the brains of BERKO mice. The neuronal deficit was most severe in the somatosensory cortex, where the overall structure was disorganized with no clear evidence of layers II, III, IV, and V. These results suggest an important role of ERβ in the maintenance of number of neurons and glial cells in the CNS. No morphological changes have been reported in the brains of ERα knockout mice.
The distribution of ERβ in the rodent brain has been studied by using a variety of in vivo and in vitro techniques (17–19, 26, 27). In situ hybridization studies showed that neurons of the olfactory bulb, supraoptic, paraventricular, suprachiasmatic, tuberal hypothalamic nuclei, zona incerta, ventral tegmental area, and cerebellum contained exclusively ERβ mRNA. Although the cerebral cortex and hippocampus contained both ER mRNAs, the hybridization signal for ERα mRNA was very weak compared with ERβ mRNA (17). Immunohistochemical studies demonstrated strong ERβ-like immunoreactivity in the lateral septum, bed nucleus of the stria terminalis, paraventricular nucleus, supraoptic nucleus, medial amygdala, the dentate gyrus, and the CA1 and CA2 fields of the hippocampus (27). In the present study, neuronal loss coincided with ERβ distribution, except in the cerebellum and hippocampus. Significant neuronal loss was found in cortex, hypothalamus, amygdala, and ventral tegmental area but not in the cerebellum and hippocampus. The neuronal loss was evident in 2-mo-old mice, and the atrophy of whole brain especially cortex was pronounced in the 2-yr-old BERKO mice. In addition to the gross atrophy of brain, neuronal shrinkage was also seen.
As in BERKO brains, the brains of patients with Alzheimer's disease are often grossly and globally atrophic and there is loss of large pyramidal neurons over a wide range of cortical regions (28, 29). Because there were no neurofibrillary tangles or Lewy bodies in BERKO mice, the abnormality in BERKO mouse brain is not identical to that found in Alzheimer's disease.
Although the mechanism of the neuronal deficit upon inactivation of ERβ is unknown, it appears that ERβ is one critical factor in neuron lifespan in CNS. Epidemiological studies show an association between postmenopausal E2 use and a reduction in risk of Alzheimer's disease, a reduction in risk of Parkinson's disease, and death from stroke (1–3). E2-mediated neuroprotection has been described in several neuronal-culture model systems, where it protects against toxicities caused by serum-deprivation, β-amyloid, excitotoxins, and oxidative stress. In animal models, E2 has been shown to attenuate neuronal death in rodent models of cerebral ischemia, traumatic injury, and Parkinson's disease (3). In the cerebral ischemia model, it is expression of ERβ, not ERα, which is related to bcl-2 expression (30). This suggests that ERβ is the functional ER within the region of the cortex, where E2-mediated neuroprotection is observed. In contrast to this, some in vitro studies have suggested that ERα has a neuroprotective effect, whereas ERβ mediates apoptosis in neuronal cells (31). Further studies are needed to elucidate the mechanism of neuronal loss in BERKO mice.
Because the pathological changes appear quite early in BERKO mouse brains, it is possible that the phenotype has resulted from exaggerated neuronal death and/or defective migration of neurons during development. The number of neurons that will reside in specific neuronal populations in the adult brain is determined by selective neuronal death during development, and this process is affected by neonatal E2. The mechanisms through which E2 affects developmental neurogenesis and cell death have not yet been established for the mammalian brain.
In the present study, another interesting morphological change was that of astrocyte proliferation in 2-mo-old BERKO mice. As proliferation of astroglial cells usually is a reactive response to neurodegeneration or injury (32), it is possible that, in BERKO mice, it occurs in response to the neuronal loss. In 2-yr-old mice, there was no evident astrocytosis in cortex of BERKO mice. One possible explanation for this is that there is loss of both neurons and astrocytes as BERKO mice age.
Recently, ERβ-immunoreactive astrocytes were observed in both male and female rats (23). Several physiological functions of astroglia have been shown to depend on E2. These include: (i) regulation of synaptic plasticity; (ii) control of hormonal release by neurons; (iii) neuroprotection; (iv) modulation of neuronal regeneration by controlling the proliferation of reactive astrocytes and the formation of a glial scar after a penetrating brain injury (33, 34).
From the results presented in this study, we suggest that ERβ might be important in the function of astrocytes in both physiological or pathological conditions. Because neuronal and astroglial responses to E2 are mutually regulated, the physiological response to E2 in different brain areas is probably influenced by the differential expression of ERα and -β in astrocytes and neurons. Further studies are needed to determine the role of ERβ in the effects of E2 on neurons and astrocytes.
Although it is clear that E2 has many beneficial effects on the brain, the use of hormone replacement therapy to help in maintenance of the CNS in postmenopausal women is still a controversial issue. One of the main concerns of both clinicians and patients is that E2 has unwanted side effects such as an increased risk of uterine cancer. Because there is little ERβ in the mature uterus, selective ERβ agonists, when they become available, might be efficient in protecting the brain from age-related neurodegeneration without affecting the uterus.
Acknowledgments
The work is supported by The Swedish Medical Research Council (no. 03X-06807) and KaroBio AB Sweden.
Abbreviations
- E2
17β-estradiol
- ER
estrogen receptor
- BERKO
ERβ knockout
- GFAP
glial fibrillary acidic protein
- CNS
central nervous system
- MPA
medial preoptic area
- PVN
paraventricular nucleus of hypothalamus
- MeA
medial amygdala nucleus
- HE
hematoxylin/eosin
References
- 1.Beyer C. Anat Embryol. 1999;199:379–390. doi: 10.1007/s004290050236. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B S, Alves S E. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Segura L M, Azcoitia I, DonCarlos L L. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 4.Sumner B E, Grant K E, Rosie R, Hegele-Hartung C, Fritzemeier K H, Fink G. Brain Res Mol Brain Res. 1999;73:119–128. doi: 10.1016/s0169-328x(99)00243-0. [DOI] [PubMed] [Google Scholar]
- 5.Agrati P, Ma Z Q, Patrone C, Picotti G B, Pellicciari C, Bottone M G, Maggi A. Eur J Neurosci. 1997;9:1008–1016. doi: 10.1111/j.1460-9568.1997.tb01451.x. [DOI] [PubMed] [Google Scholar]
- 6.Shughrue P J, Scrimo P J, Merchenthaler I. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- 7.Kompoliti K. Clin Neuropharmacol. 1999;22:318–326. [PubMed] [Google Scholar]
- 8.Slooter A J, Bronzova J, Witteman J C, Van Broeckhoven C, Hofman A, van Duijn C M. J Neurol Neurosurg Psychiatry. 1999;67:779–781. doi: 10.1136/jnnp.67.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palacios S. Maturitas. 1999;33, Suppl. 1:S1–S13. [PubMed] [Google Scholar]
- 10.Waring S C, Rocca W A, Petersen R C, O'Brien P C, Tangalos E G, Kokmen E. Neurology. 1999;52:965–970. doi: 10.1212/wnl.52.5.965. [DOI] [PubMed] [Google Scholar]
- 11.Skoog I. Neuroepidemiology. 1998;17:2–9. doi: 10.1159/000026147. [DOI] [PubMed] [Google Scholar]
- 12.Mulnard R A, Cotman C W, Kawas C, van Dyck C H, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, et al. J Am Med Assoc. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 13.Luoto R, Manolio T, Meilahn E, Bhadelia R, Furberg C, Cooper L, Kraut M. J Am Geriatr Soc. 2000;48:467–472. doi: 10.1111/j.1532-5415.2000.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 14.Solerte S B, Fioravanti M, Racchi M, Trabucchi M, Zanetti O, Govoni S. Maturitas. 1999;31:95–101. doi: 10.1016/s0378-5122(98)00111-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang P N, Liao S Q, Liu R S, Liu C Y, Chao H T, Lu S R, Yu H Y, Wang S J, Liu HC. Neurology. 2000;54:2061–2066. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- 16.Henderson V W, Paganini-Hill A, Miller B L, Elble R J, Reyes P F, Shoupe D, McCleary C A, Klein R A, Hake A M, Farlow M R. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 17.Shughrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Gundlah C, Kohama S G, Mirkes S J, Garyfallou V T, Urbanski H F, Bethea C L. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- 19.Carroll R S, Zhang J, Black P M. J Neurooncol. 1999;42:109–116. doi: 10.1023/a:1006158514866. [DOI] [PubMed] [Google Scholar]
- 20.Mong J A, Glaser E, McCarthy M M. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mong J A, McCarthy M M. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Santagati S, Melcangi R C, Celotti F, Martini L, Maggi A. J Neurochem. 1994;63:2058–2064. doi: 10.1046/j.1471-4159.1994.63062058.x. [DOI] [PubMed] [Google Scholar]
- 23.Azcoitia I, Sierra A, Garcia-Segura L M. Glia. 1999;26:260–267. [PubMed] [Google Scholar]
- 24.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J A, Smithis O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 26.Laflamme N, Nappi R E, Drolet G, Labrie C, Rivest S. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Schwartz P E, Rissman E F. Neuroendocrinology. 1997;66:63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- 28.Mann D M A. In: Neurodegenerative Diseases. Calne DB, editor. Philadelphia: Saunders; 1994. pp. 15–31. [Google Scholar]
- 29.Terry R D, Peck A, DeTeresa R, Schechter R, Horoupian D S. Ann Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- 30.Dubal D B, Shughrue P J, Wilson M E, Merchenthaler I, Wise P M. J Neurosci. 1999;9:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsen J, Mor G, Naftolin F. J Neurobiol. 2000;43:64–78. doi: 10.1002/(sici)1097-4695(200004)43:1<64::aid-neu6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Nichols N R. J Neurobiol. 1999;40:585–601. [PubMed] [Google Scholar]
- 33.Garcia-Segura L M, Naftolin F, Hutchison J B, Azcoitia I, Chowen J A. J Neurobiol. 1999;40:574–584. [PubMed] [Google Scholar]
- 34.Mong J A, McCarthy M M. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]