Abstract
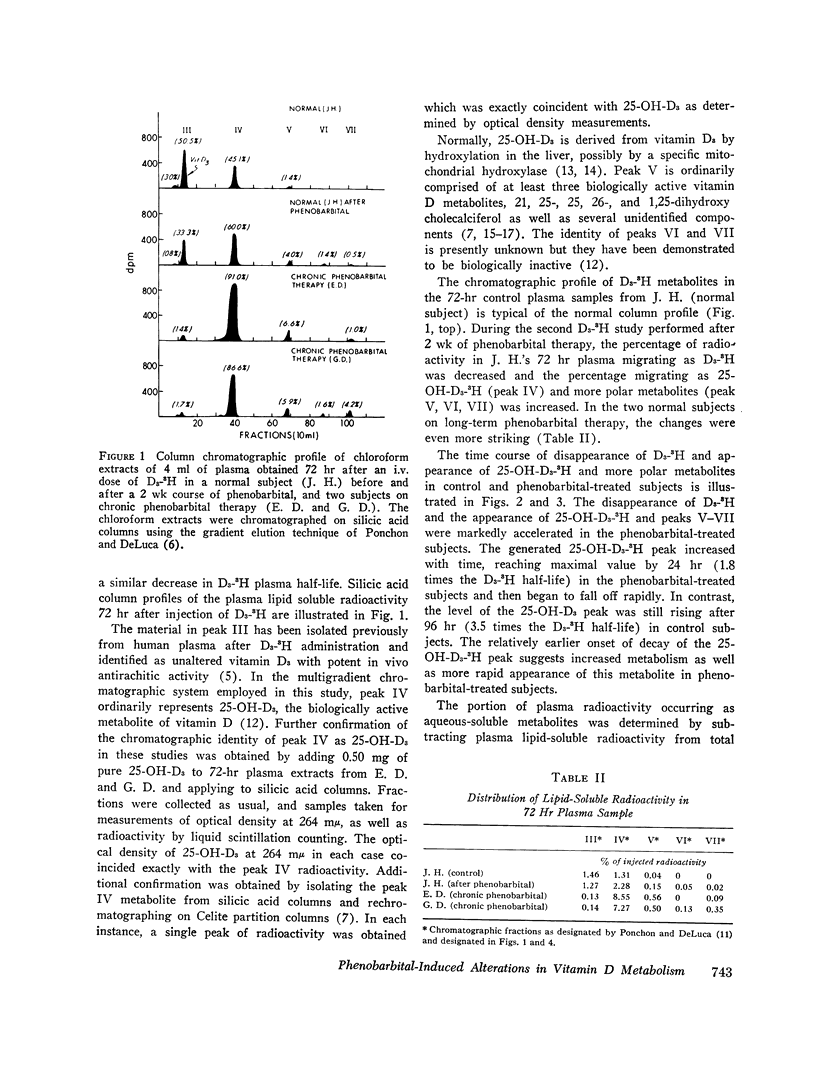
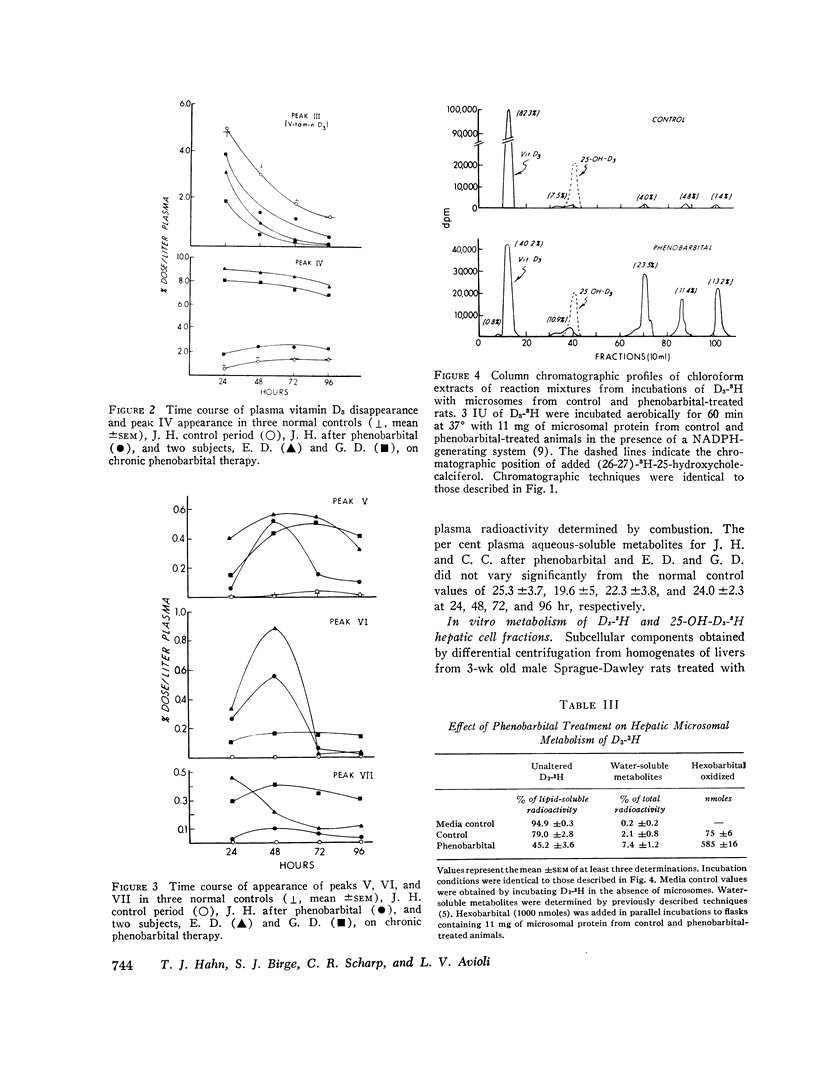
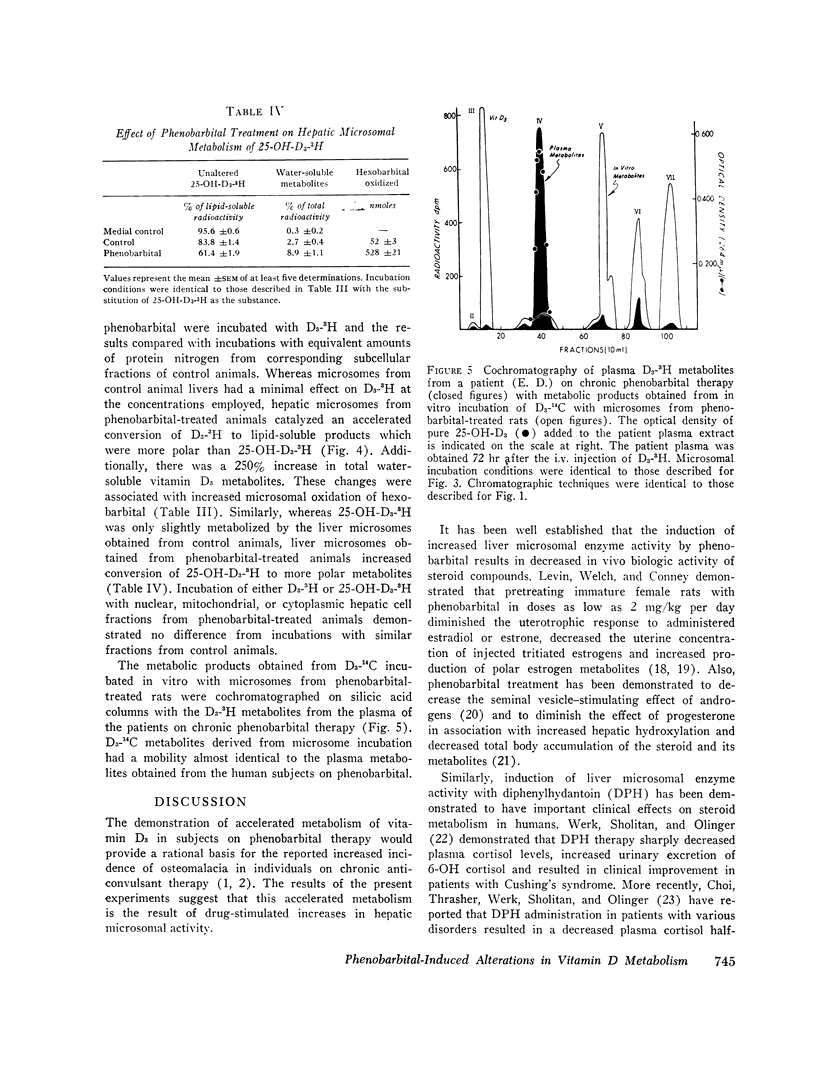
The metabolic fate of intravenously injected vitamin D3-1,2-3H (D3-3H) was studied in two normal individuals on chronic phenobarbital therapy. Silicic acid column chromatography of lipid-soluble plasma extracts obtained serially for 96 hr after D3-3H injection demonstrated a decreased plasma D3-3H half-life and increased conversion to more polar metabolites. The polar metabolites formed included several with chromatographic mobility similar to known biologically inactive vitamin D metabolites and one with chromatographic mobility identical to 25-hydroxycholecalciferol. Disappearance of this latter material was also accelerated. A child with rickets and a normal volunteer studied before and after a 2 wk course of phenobarbital therapy demonstrated similar alterations in D3-3H metabolism. When liver microsomes from 3-wk-old Sprague-Dawley rats treated with phenobarbital were incubated with D3-3H, polar metabolites were produced with chromatographic mobility similar to the plasma D3-3H metabolites from phenobarbital-treated humans. Similar incubations employing 25-hydroxy-cholecalciferol-26-27-3H as the substrate also demonstrated an increased conversion to polar metabolites. The data suggest that the reported increased incidence of osteomalacia observed in patients on chronic anticonvulsant therapy may be the result of an accelerated conversion of vitamin D and its active metabolite, 25-hydroxycholecalciferol, to polar metabolites by druginduced liver microsomal enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avioli L. V., Lee S. W., McDonald J. E., Lund J., DeLuca H. F. Metabolism of vitamin D3-3H in human subjects: distribution in blood, bile, feces, and urine. J Clin Invest. 1967 Jun;46(6):983–992. doi: 10.1172/JCI105605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNEY A. H., KLUTCH A. Increased activity of androgen hydroxylases in liver microsomes of rats pretreated with phenobarbital and other drugs. J Biol Chem. 1963 May;238:1611–1617. [PubMed] [Google Scholar]
- CONNEY A. H., SCHNEIDMAN K., JACOBSON M., KUNTZMAN R. DRUG-INDUCED CHANGES IN STEROID METABOLISM. Ann N Y Acad Sci. 1965 Mar 12;123:98–109. doi: 10.1111/j.1749-6632.1965.tb12248.x. [DOI] [PubMed] [Google Scholar]
- COOPER J. R., BRODIE B. B. The enzymatic metabolism of hexobarbital (evipal). J Pharmacol Exp Ther. 1955 Aug;114(4):409–417. [PubMed] [Google Scholar]
- Choi Y., Thrasher K., Werk E. E., Jr, Sholiton L. J., Olinger C. Effect of diphenylhydantoin on cortisol kinetics in humans. J Pharmacol Exp Ther. 1971 Jan;176(1):27–34. [PubMed] [Google Scholar]
- Conney A. H., Jacobson M., Levin W., Schneidman K., Kuntzman R. Decreased central depressant effect of progesterone and other steroids in rats pretreated with drugs and insecticides. J Pharmacol Exp Ther. 1966 Nov;154(2):310–318. [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Cousins R. J., DeLuca H. F., Gray R. W. Metaboism of 25-hydroxycholecalciferol in target and nontarget tissues. Biochemistry. 1970 Sep 15;9(19):3649–3652. doi: 10.1021/bi00821a001. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. Vitamin D metabolism and its relationship to familial hypophosphatemia. Birth Defects Orig Artic Ser. 1970 Sep;6(3):26–27. [PubMed] [Google Scholar]
- Dent C. E., Richens A., Rowe D. J., Stamp T. C. Osteomalacia with long-term anticonvulsant therapy in epilepsy. Br Med J. 1970 Oct 10;4(5727):69–72. doi: 10.1136/bmj.4.5727.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Chyu K. J. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971 Dec;33(6):992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Myrtle J. F., Norman A. W. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem. 1968 Aug 10;243(15):4055–4064. [PubMed] [Google Scholar]
- Holick M. F., DeLuca H. F. A new chromatographic system for vitamin D3 and its metabolites: resoluation of a new vitamin D3 metabolite. J Lipid Res. 1971 Jul;12(4):460–465. [PubMed] [Google Scholar]
- Horsting M., DeLuca H. F. In vitro production of 25-hydroxycholecalciferol. Biochem Biophys Res Commun. 1969 Jul 23;36(2):251–256. doi: 10.1016/0006-291x(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Jubiz W., Meikle A. W., Levinson R. A., Mizutani S., West C. D., Tyler F. H. Effect of diphenylhydantoin on the metabolism of dexamethasone. N Engl J Med. 1970 Jul 2;283(1):11–14. doi: 10.1056/NEJM197007022830103. [DOI] [PubMed] [Google Scholar]
- KUNTZMAN R., JACOBSON M., SCHNEIDMAN K., CONNEY A. H. SIMILARITIES BETWEEN OXIDATIVE DRUG-METABOLIZING ENZYMES AND STEROID HYDROXYLASES IN LIVER MICROSOMES. J Pharmacol Exp Ther. 1964 Dec;146:280–285. [PubMed] [Google Scholar]
- Kruse R. Osteopathien bei antiepileptischer Langzeittherapie (Vorläufige Mitteilung) Monatsschr Kinderheilkd. 1968 Jun;116(6):378–381. [PubMed] [Google Scholar]
- Kuntzman R. Drugs and enzyme induction. Annu Rev Pharmacol. 1969;9:21–36. doi: 10.1146/annurev.pa.09.040169.000321. [DOI] [PubMed] [Google Scholar]
- Kuntzman R., Jacobson M., Levin W., Conney A. H. Stimulatory effect of N-phenylbarbital (phetharbital) on cortisol hydroxylation in man. Biochem Pharmacol. 1968 Apr;17(4):565–571. doi: 10.1016/0006-2952(68)90272-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin W., Welch R. M., Conney A. H. Effect of chronic phenobarbital treatment on the liver microsomal metabolism and uterotropic action of 17-beta-estradiol. Endocrinology. 1967 Jan;80(1):135–140. doi: 10.1210/endo-80-1-135. [DOI] [PubMed] [Google Scholar]
- Levin W., Welch R. M., Conney A. H. Effect of phenobarbital and other drugs on the metabolism and uterotropic action of estradiol-17-beta and estrone. J Pharmacol Exp Ther. 1968 Feb;159(2):362–371. [PubMed] [Google Scholar]
- Lund J., DeLuca H. F. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966 Nov;7(6):739–744. [PubMed] [Google Scholar]
- Ponchon G., DeLuca H. F. The role of the liver in the metabolism of vitamin D. J Clin Invest. 1969 Jul;48(7):1273–1279. doi: 10.1172/JCI106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchon G., DeLuca H. F. The role of the liver in the metabolism of vitamin D. J Clin Invest. 1969 Jul;48(7):1273–1279. doi: 10.1172/JCI106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchon G., Deluca H. F. Metabolites of vitamin D3 and their biologic activity. J Nutr. 1969 Oct;99(2):157–167. doi: 10.1093/jn/99.2.157. [DOI] [PubMed] [Google Scholar]
- Richens A., Rowe D. J. Disturbance of calcium metabolism by anticonvulsant drugs. Br Med J. 1970 Oct 10;4(5727):73–76. doi: 10.1136/bmj.4.5727.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Ponchon G., Tanaka Y., Holick M. F. 21,25-dihydroxycholecalciferol. A metabolite of vitamin D3 preferentially active on bone. Biochemistry. 1970 Jul 7;9(14):2917–2922. doi: 10.1021/bi00816a025. [DOI] [PubMed] [Google Scholar]



