Abstract
Due to intensified research over the past decade, the Hedgehog (HH) pathway has been identified as a pivotal defect implicated in roughly 25% of all cancers. As one of the most frequent cancer worldwide, the development of Basal cell carcinoma (BCC) due to activation of the HH pathway has been convincingly demonstrated. Thus the discovery of this central tumor-promoting signalling pathway has not only revolutionized the understanding of BCC carcinogenesis but has also enabled the development of a completely novel therapeutic approach. Targeting just a few of several potential mutations, HH inhibitors such as GDC-0449 achieved already the first promising results in metastatic or locally advanced BCC. This paper summarizes the current understanding of BCC carcinogenesis and describes the current “mechanism-based” therapeutic strategies.
1. Introduction
BCC, the most commonly diagnosed skin cancer in persons of fair complexion, has become the focus of intensified translational debate lately. Following the circumstantial evidence that ewes gave birth to cyclopic and malformed lambs after nibbling on Veratrum californicum, a Corn lily, the causative teratogenic compound, cyclopamine, was discovered [1, 2]. Increased research on this agenda and the understanding of its functioning led to the discovery of the Hedgehog signalling pathway (HH) as an essential cascade in embryonic development [3]. Proof of a specific mutation in BCC's Hedgehog pathway showed for the first time that an aberrant HH signalling is also strongly implicated in cancerogenesis of skin tumors [4]. Though a wide range of efficient therapeutic options are well established in the treatment of sporadic BCC, the newly developed HH inhibitors and first study results give rise to a curative or even secondary-prophylactic approach in hereditary, advanced, or even metastatic variants.
This paper summarizes the current knowledge of clinical aspects and the molecular pathogenesis of this form of skin cancer. Moreover, we discuss current and future therapies that are needed in order to allow efficient treatment of BCC in complicated localization, in patients with multiple tumors or genetic disease predisposing for BCC development, or patients that are not eligible for surgery.
2. Epidemiology and Clinical Aspects
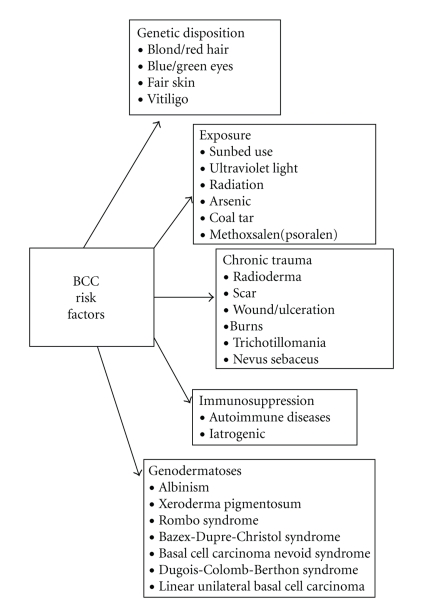
First described by Krompecher in 1900 as “carcinoma epitheliale adenoides” [5] and named after its morphological affinity to the normal cell of the basal layer, BCC is the most common keratinocyte skin cancer (KSC) in persons of Caucasian ancestry. Although it presumably develops from epidermal stem cells of the outer root sheat of the hair follikel, the precise origin of BCC is still unknown thus far [6, 7]. Its incidence is estimated up to 100 cases per 100,000 and even higher depending on geographical or complexion disparities. Hence, BCC as well as other KSCs are often excluded from cancer-registry statistics, thereby underestimating the socioeconomic burden of this form of cancer [8–10]. More common in men than in women, BCC usually arises at an average age of 60 years. Apart from the environmental exposure to arsenic, ionizing radiation, oral methoxsalen (psoralen), and immunosuppressive therapy such as in organ transplant recipients [11, 12], persons with a fair skin type-I complexion (including red or blonde hair, light coloured eyes, freckling) and people with a history of intermittent sun exposure and severe sunburn during childhood are at highest risk [13]. In particular ultraviolet (UV) irradiation in inverse correlation with reduced or impaired skin pigmentation is generally considered to be the major risk factor of basal cell carcinoma [14, 15]. Depending on timing (childhood, adolescence), pattern (intermittent, continuous), source (natural, artificial), and amount (cumulative sun exposure), its impact on BCC development is, however, far more complex and needs further detailed study [16]. Though the rates are still highest for the naturally sun exposed skin of elderly man, the trend over the past decade is clearly towards an increasing incidence of BCC in younger women due to excessive tanning and sunbed use (Figure 1) [17].
Figure 1.
Risk factors of BCC, adapted from Rubin et al. [16].
The majority of sporadically occurring BCCs arise in sun-exposed areas with over 80% of all cases developing on the head and neck. Unlike squamous cell carcinoma (SCC), BCCs do not have detectable precursor lesions and usually present themselves de novo as a palpable, localised, translucent tumour with overlying teleangiectasias. For hitherto unknown reasons, they differ in three main clinical as well as histological phenotypes: the nodular BCC exhibiting a pearly rolled border at times with central crusting and ulceration, the superficial subtype with its scaly erythematous patch or plaque-like appearance and the sclerosing, infiltrative, or morpheaform variant that clinically presents as a scar-like, centrally atrophic, whitish, indurate tumour with indistinct margins. Frequently, those three histological subtypes are mixed. In addition to aggressive BCCs such as the infiltrative, micronodular, or basosquamous subtypes, uncommon BCC variants include the clear-cell, granular-cell, or adamantinoid variants, and adnexal differentiation. Pigmented tumours, known to carry p53 mutations [18], may mimic several differential diagnoses including melanoma and therefore need to be confirmed by biopsy. Although erosion and ulceration can develop quite early, especially in the nodular variant, sporadic BCC is in general a slow growing, delayed infiltrating, or destructive tumour that, even in view of other risk factors in terms of a large diameter >2 cm, incomplete incision and perivascular involvement, metastases only occur after years of existence in 0.55% of all cases [19]. Once metastasised in regional lymph nodes followed by bone, liver, and lung, the prognosis is poor with a mean survival of at most 3.6 years after diagnosis [19, 20].
In contrast to the sporadic variant of BCC, a hereditary disorder, also known as Gorlin syndrome or basal cell nevus syndrome (BCNS), exhibits a marked propensity to develop numerous BCCs already during adolescence and occasionally even in childhood. As an autosomal dominant inherited genodermatosis with an estimated incidence of 1 : 150 000 in the general population, BCNS is very rare. It is characterized by a range of developmental anomalies—most notably in the head and neck area that allowed the oral pathologist and dentist Robert Gorlin to describe it first - and a predisposition to various other forms of cancers. Apart from skeletal abnormalities such as splayed ribs, Sprengel and pectus deformity, these patients suffer from ectopic calcification, odontogenic keratocysts, facial dismorphism with macrocephaly, palmoplantar pits and tumours in terms of cardiac and ovarian fibroma, meningeoma, medulloblastoma, rhabdomyosarcoma, mesenteric cysts, and other neuroectodermal tumours [21]. Most prominent among these clinical findings is the early and very strong disposition to develop several, occasionally hundreds of BCCs, especially after radiation given for treatment of progressive BCC or medulloblastoma. It was, however, the intensified research on BCNS with proof of its cause, a mutated PTCH1 gene in the majority of cases, that linked cancer to the HH signalling pathway for the first time in 1996 [4, 22].
3. Molecular Pathogenesis
3.1. Hedgehog Signalling Pathway
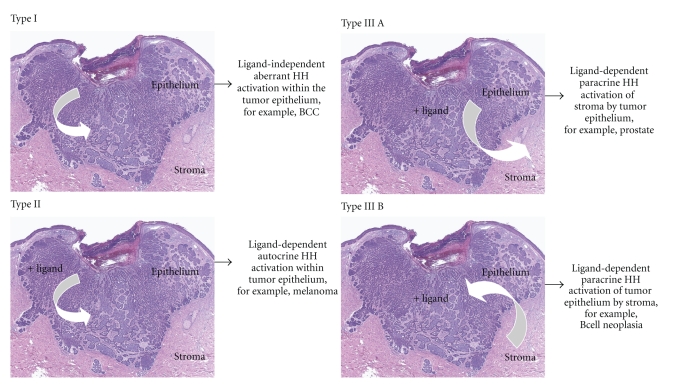
The hedgehog (HH) family of intercellular signalling proteins play a pivotal role in many fundamental processes of embryogenic development. They are central to differentiation, growth, pattering, morphogenesis, and function of different cells and organs as well as epithelial and mesenchymal tissue interactions in vertebrates and invertebrates alike [23, 24]. Malfunction or mutation of these proteins lead to substantial impairment as already shown by the prickly, hedgehog-like appearing of mutant flies of Drosophila melanogaster after which the family of proteins was named. Probably by duplication of a single-ancestral gene, mammalians, in contrast to invertebrates with just one HH gene, develop three different types of homologs: the Sonic, the Desert, and the Indian type. The HH pathway is initiated whenever one of these ligands binds and thereby inactivates the transmembrane tumour-suppressor protein patched homologue 1 (PTCH-1). As a consequence, PTCH-1 then permits its receptor smoothened (SMO), another transmembrane protein, to transmit signals to downstream targets by means of the GLI family of transcription factors. Under normal conditions, and mostly in adults, the hedgehog pathway is ligand dependent and actively repressed because PTCH-1 constantly inhibits SMO, the key activator of the GLI pathway. Especially Sonic hedgehog (SHH), as the most widely characterized signalling pathway of the three types, provides a unique example of how the same molecular cascade leads to different pattering in different tissue types solely by distinct transcriptional programs based upon its local concentration [25]. Inappropriate activation due to mutations within this cascade however was clearly identified by a growing body of evidence to be a pivotal cause of carcinogenesis, in particular, in BCNS-associated BCCs and medulloblastoma. According to Scales de Sauvage, so far three different model systems are proposed on how the HH pathway is involved in the generation of different types of cancer (Figure 2) [26].
Figure 2.
HH pathway model systems in cancer, adapted from Scales and de Sauvage [26].
3.2. Mutations of Hedgehog Signalling Pathway in BCC
Independent of the underlying oncogenic mutation, in nearly all sporadic as well as BCNS-linked BCCs, uncontrolled stimulations of the hedgehog signalling are found [27, 28]. Due to relatively stable genomes when compared to other extracutaneous cancer, BCCs routinely carry mutations in 30% to 50% of the tumors in p53 or PTCH-1 [29–31]. The latter either looses thereby its function (loss of function mutation) or less commonly activates SMO (gain of function mutation) [4, 22, 32]. Continuously stimulated by SMO, a variety of cell-specific target genes then interfere with the physiological function via endothelial growth factor and angiopoetin (resulting in angiogenesis, cell proliferation, metastasis, and cell survival), ultimately leading to cancer [26, 33]. SMO itself is mutated only in 10% of all sporadic BCCs [29]. A few other alternations of the HH pathway, for example, in SHH or GLI, have been tried to be identified but could not be confirmed so far [29, 34].
3.3. Genetic Predispositions, Mutations and Interacting Pathways
In view of the known complex interplay of genes and epigenetic and environmental influences in carcinogenesis, the development of BCC and cancer in general is, however, certainly far more complex and cannot possibly be reduced to three somatic mutations within the hedgehog pathway. With the focus on the downstream target genes and effects of HH signalling, the BCC carcinogenesis probably constitutes an intricate mechanism of several interacting pathways and mutated genes that regulate pigmentation, DNA repair, and apoptosis. Many other mutations have been proven to be implicated in BCC so far. Especially the sequence of downstream mediators in HH seems to differ in various tissues. Several, such as CD95, BCL-2, PDGFRα, or cFLIP, are currently under investigation [35]. Furthermore, contributions of the FOX gene family, in particular FOXM1 and FOXE1, appear to be involved in downstream signalling [35–38]. As HH target genes, both FOX proteins control for a normal mitosis and are overexpressed in BCC in comparison to normal keratinocytes [39, 40]. But it is not yet understood which changes are crucial in BCC and therefore represent “drivers” but not “passengers” during tumorigenesis of BCC [14, 41].
A similar lack of knowledge still exists for the interaction of the GLI signalling pathway with other cellular signals. The Phosphoinositol-3-kinase (PI3K) cascade interacts with SHH in at least two ways. While it inhibits protein kinase A (PKA-) mediated phosphorylation, it also stabilizes GLI2. On the other hand, SHH activates PI3K, for example, in prostate cancer [42]. But up to now, no proof for PI3K involvement in BCC carcinogenesis could be given [14]. The relationship of the Ras/Raf signalling pathway and BCC is less well defined [29]. In comparison, the obvious requirement of Wnt signalling in the downstream activation of HH for these tumours [43] hints at novel possibilities to the therapeutic approach in BCC in addition to HH inhibitors (described below) [14].
From a clinical point of view, the most convincing yet rather confusing—due to several contradicting results—research focuses on the association of BCC with pigmentation and DNA repair genes, respectively. At least for sporadic BCCs as the classic UV-induced variant and those that arise in patients with xeroderma pigmentosum (XP), a frequent type of PTCH1 and p53 mutations could be identified [44].
The clinical presumption that the increased incidence of BCC in elderly could be a consequence of diminished DNA repair due to aging seems therefore not so farfetched [45]. Hence, the repair of UV-induced damage should reduce BCC development [44, 46], although the use of sunscreens failed to lower the risk of BCC to date [47]. For several DNA repair gene variants such as XRCC1, XRCC3, XPA, and XPD, a significant association with BCC risk has been reported [48]. The polymorphism of those mutants involved is unfortunately reflected by diverse and often contradictory results [49–52]. A variant once proven to be significant [48] was refuted in another study [53] or was, in part, not BCC-specific at all [54].
Similarly unpersuasive are the results on melanocortin 1 receptor gene (MCIR), the major known genetic variant influencing the degree of skin pigmentation. Although it was clearly shown that the nonfunctional variant of MCIR had a dose-dependent impact on the incidence of BCC and melanoma, the consecutive lack in pigmentation itself did not influence the result [54, 55]. A different mechanism in terms of a paracrine role or distant modulation of proliferation and differentiation of keratinocytes by MCIR has also been suggested [56, 57]. In general, the functioning of pigmentation and DNA repair in healthy individuals, let alone in skin cancer, is so far too little, or at best partially, understood in order to pave the way for prevention or let alone treatment of BCC.
4. Current and Future Treatment Options
4.1. Current Standard of Care
A wide range of several effective therapeutic options are available for the therapy of BCC. Intended to be curative or at least locally controlling, the treatment can either be surgical or nonsurgical depending on several tumour- or patient-related factors. Especially tumour size, location, histological subtype, patient's health and wishes, possible complications, and aesthetic results should be taken into account. As there is still no preoperative method for the detection of subclinical spread, surgical therapy with 3D histology is the gold standard even in BCCs of the head and neck area. In order to ascertain the complete and hereby curative excision, several equally effective techniques are at disposal. With Mohs micrographic surgery, the histological confirmed BCC is removed in a bowl-like fashion, immediately frozen, and examined for residual tumour cells in the lateral and basal margins as long as the BCC is totally excised. 5-year recurrence rates for Mohs surgery are reported as 1%–3% for primary BCC and 3%–7% for recurrent tumours [58, 59]. Similar results are achieved with other less known histological methods such as the La Galette technique [60]. Conventional surgery with tumour-adapted margins of safety uses a bread loaf horizontal cutting to control for complete excision. Depending on the safety margin, a higher rate of residual tumour cells and thus increased recurrence rate of 4%–34% is reported [58]. Curettage, electrodesiccation, and cryosurgery are further surgical approaches that are easily applied in low-risk lesions with nonaggressive histological features such as superficial BCC of the trunk. The disadvantage is, however, that the complete removal of the BCC cannot be histological proven and delayed wound healing due to thermal destruction or impairment of the basal layer may lead to unsatisfactory results. Certainly, none of these three techniques is appropriate for recurrent or morpheaform BCCs, although in general cure rates of up to 95% and higher are stated [16]. Non-surgical treatment options include radiotherapy, photodynamic therapy, and topical application of imiquimod and 5-fluorouracil. All of the proposed procedures comprise, however, the disadvantage that no treatment success can be histologically validated and thus higher recurrence rates have to be taken into account. Nonetheless, elderly patients with multiple comorbidities and inoperable tumours profit. The indication for radiotherapy—given the multitude of therapeutic options—is more limited and rather confined to postoperative recurrences or if a complete resection appears unlikely. Since there is a high risk of secondary tumors developing on the radiation side, patients with BCNS, XP, epidermodysplasia verruciformis, and iatrogenic immunosuppression should be excluded from radiotherapy. It is also not recommended for patients younger than 60 years, given its potential for carcinogenesis [61, 62]. Photodynamic therapy requires the application of a photosensitizing agent such as 5-aminolevulinic acid or its ester 3-4 hours before the protoporphyrin IX-enriched tumour cells are destroyed. Superior with regards to cosmetic outcome when compared to many other treatment options, PDT of superficial BCC showed a 1-year recurrence rate of 9.3% [63] and is not recommended for the nodal subtype due to 5-year relapse rates of 76% [64]. Although its precise mechanism is still unknown, the once-daily application of Imiquimod 5 days per week for 6 weeks resulted in a histological clearance rate of up to 89.6% in superficial BCC [65–67]. A clear trend towards improved rates with increased frequencies of application is limited by intensified local and systemic reactions. Residual tumours after therapy are nonetheless often difficult to assess, and subtypes other than superficial BCC are no general indication for Imiquimod since multiple recurrent lesions can occur [8]. 5-fluorouracil, a topical cytostatic agent, is considered as a therapeutic alternative in patients with multiple, superficial multicentric BCCs, for example, in BCNS [68]. As a consequence of painful inflammatory and erosive reactions, the patient's compliance is often limited for this treatment option.
Given that metastasis and invasion of vital structures by BCC are extremely rare, no therapeutic “gold standard” exists. Apart from surgical procedures and an additional radiotherapy, an assortment of different chemotherapies such as doxorubicin, paclitaxel, and/or carboplatin with differing response rates have been applied up to now in order to control the tumour load and extend the patient's life expectancy [8, 69]. Given the rare nature of metastasis, larger clinical studies have been lacking until recently.
The more detailed understanding, achieved as of late, of the molecular pathogenesis of BCC and its causative aberrant pathway has rendered a new therapeutic targeted approach possible. SMO inhibitor Cyclopamine, the teratogenic steroidal plant alkaloid, which was topically applied, succeeded already to induce regression of four sporadic BCCs [70], since then, a series of small-molecule HH inhibitors have been developed and are currently in clinical development. Furthest along is GDC-0449, a more specific and potent SMO inhibitor than Cyclopamine. Administered in different doses of 150, 270, and 540 mg per day as part of a phase I study in metastatic or locally advanced BCC, an objective response in 18 of 33 patients was achieved [71]. Side effects such as hyponatraemia, fatigue, weight loss, and dyspnoea were mild to moderate. Several ongoing phase II trials investigate its efficacy in advanced BCC, medulloblastoma, and breast cancer but also in addition to other chemotherapeutic agents in pancreatic, lung, colorectal, and gastrointestinal cancer. Four other new HH inhibitors including LDE-225, BMS-833823, IPI-926, and PF-04449913 are currently under investigation in phase I trials. All of the tested components target so far SMO as the key regulator of the HH pathway. An equally effective inhibition could succeed, however, in targeting the HH downstream signalling. Two tested candidates GANT58 and GANT61, inhibitors of the GLI transcription, possibly provide a therapeutic alternative in case of a resistance to SMO inhibitors [72]. Also interfering with HH target gene transcription and therefore of potential therapeutic interest in BCC is the new field of microRNAs. While one single microRNA regulates hundreds of target genes, the task is to focus its efficacy on the essential target and thus minimize its side effects, which still needs to be mastered (Table 1) [73].
Table 1.
Current and future HH pathway inhibitors.
| SMO-Inhibitors | Ongoing trials | Indication |
|---|---|---|
| GDC-0449 (Erivance, Genentech) |
Phase II | BCC, medulloblastoma, ovarian cancer, small-cell lung cancer, coloractal cancer (combined with cisplatin and etoposide), colorectal cancer (in combination with standard chemotherapy and bevacicumab), and upper gastrointestinal cancers (in combination with FOLFOLX chemotherapy) |
| BMS-833923 (Bristol-Myers Squibb and Exelixis) |
Phase I | BCC, BCNS, small lung cancer (versus cisplatin and etoposide), inoperable, metastatic gastro, gastroesophageal or esophageal Adenocarcinoma (combined with cisplatin and carpecitabine), and multiple myeloma |
| IPI-926 Infinity Pharmaceuticals |
Phase I | Advanced and/or metastatic solid tumour malignancies and metastatic pancreatic cancer (combined with gemcitabine) |
| LDE-225 (Novartis) |
Phase I/II | Sporadic superficial and nodular skin BCC, BCNS, medulloblastoma; rhabdomyosarcoma neuroblastoma, hepatoblastoma, astrocytoma, advanced solid tumor cancers, and Medulloblastoma |
| PF-04449913 (Pfizer) |
Phase I | select hematologic malignancies or with dasatinib in chronic myeloid leukemia (CML) |
As discussed by Epstein, the use of tyrosine kinase inhibitors, sorafenib or imatinib, seems sensible because PDGFRα is supposed to mediate downstream effects in HH signalling [14]. The assortment of further new potential agents for prevention as well as treatment in BCC is plentiful, ranging from Vitamin A and D derivates, NSAID, and DNA repair enzymes up to Melanocortin peptides therapeutics. Certainly due to the lack of knowledge of their precise functioning and how, or if at all, they interact with HH pathway, results are so far promising but inconclusive. It may therefore not be surprising that even the widely recognized and in its molecular effects initially well understood standard therapy with systemic retinoids fails to prevent the recurrence of sporadic BCC [74].
5. Conclusion
In view of those remarkable oncogenomic achievements in the understanding of BCC carcinogenesis, a curative breakthrough, especially in hereditary, locally advanced or metastatic cases, seems imminent. First promising data are yet too scarce to obtain detailed pieces of information about dosage, side effects, response, recurrence, and survival rates or possible medical interactions. Not sufficiently known but crucial is certainly the impact of the type of HH mutations and their combinations for the various clinical subtypes of BCC. The change from a phenotype-correlated diagnosis to a genotype analysis, at least in advanced tumours, is obvious. But is BCC in all its clinical and histological variations to be reclassified according to its genotype? Genetic analysis will undoubtedly change the classification and subsequently treatment algorithms for BCC. The open intruiging question remains if there is a link between HH mutation, histology, and its clinical aspects that would simplify the indication for a targeted therapy. Of equal importance in this complex interplay are without doubt epigenetic phenomena and environmental factors that are at best only initially recognized so far. Definitely many more future studies are needed to answer the large number of interesting questions that the discovery of aberrant HH pathway for BCC raised.
References
- 1.Binns W, James LF, Shupe JL, Everett G. A congenital cyclopian-type malformation in lambs induced by maternal ingestion of a range plant, Veratrum californicum. American Journal of Veterinary Research. 1963;24:1164–1175. [PubMed] [Google Scholar]
- 2.Keeler RJ, Binns W. Teratogenic compounds of Veratrum californicum (Durand). 3. Malformations of the veratramine-induced type from ingestion of plant or roots. Proceedings of the Society for Experimental Biology and Medicine. 1967;126(2):452–454. doi: 10.3181/00379727-126-32474. [DOI] [PubMed] [Google Scholar]
- 3.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280(5369):1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 4.Hahn H, Wicking C, Zaphiropoulos PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 5.Grimmer H. Histological picture report no.20l. Basalioma of the vulva (basal cell carcinoma Krompecher 1900) Zeitschrift fur Haut und Geschlechtskrankheiten. 1967;43(5):25–40. [PubMed] [Google Scholar]
- 6.Youssef KK, Van Keymeulen A, Lapouge G, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nature Cell Biology. 2010;12(3):299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 7.Holíková Z, Massi D, Lotti T, Hercogová J. Insight into the pathogenesis of sporadic basal cell carcinoma. International Journal of Dermatology. 2004;43(12):865–869. doi: 10.1111/j.1365-4632.2004.02319.x. [DOI] [PubMed] [Google Scholar]
- 8.Eigentler TK, Kamin A, Weide BM, et al. A phase III, randomized, open label study to evaluate the safety and efficacy of imiquimod 5% cream appliedthrice weekly for 8 and 12 weeks inthetreatment of low-risk nodular basalcellcarcinoma. Journal of the American Academy of Dermatology. 2007;57(4):616–621. doi: 10.1016/j.jaad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 9.De Vries E, Louwman M, Bastiaens M, De Gruijl F, Coebergh JW. Rapid and continuous increases in incidence rates of basal cell carcinoma in the Southeast Netherlands since 1973. Journal of Investigative Dermatology. 2004;123(4):634–638. doi: 10.1111/j.0022-202X.2004.23306.x. [DOI] [PubMed] [Google Scholar]
- 10.Diepgen TL, Mahler V. The epidemiology of skin cancer. British Journal of Dermatology. 2002;146(supplement 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 11.Jemec GBE, Holm EA. Nonmelanoma skin cancer in organ transplant patients. Transplantation. 2003;75(3):253–257. doi: 10.1097/01.TP.0000044135.92850.75. [DOI] [PubMed] [Google Scholar]
- 12.Wong CSM, Strange RC, Lear JT. Basal cell carcinoma. British Medical Journal. 2003;327(7418):794–798. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. Journal of the Royal Society of Medicine. 1997;90(7):371–374. doi: 10.1177/014107689709000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nature Reviews Cancer. 2008;8(10):743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. Journal of Photochemistry and Photobiology B. 2001;63(1–3):8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 16.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. New England Journal of Medicine. 2005;353(21):2262–2269. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 17.Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. Journal of the American Medical Association. 2005;294(6):681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 18.Cui R, Widlund HR, Feige E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Walling HW, Fosko SW, Geraminejad PA, Whitaker DC, Arpey CJ. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer and Metastasis Reviews. 2004;23(3-4):389–402. doi: 10.1023/B:CANC.0000031775.04618.30. [DOI] [PubMed] [Google Scholar]
- 20.Snow SN, Sahl W, Lo JS, et al. Metastatic basal cell carcinoma: report of five cases. Cancer. 1994;73(2):328–335. doi: 10.1002/1097-0142(19940115)73:2<328::aid-cncr2820730216>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Gorlin RJ. Nevoid basal cell carcinoma syndrome. Dermatologic Clinics. 1995;13(1):113–125. [PubMed] [Google Scholar]
- 22.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272(5268):1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 23.Lupi O. Correlations between the Sonic Hedgehog Pathway and basal cell carcinoma. International Journal of Dermatology. 2007;46(11):1113–1117. doi: 10.1111/j.1365-4632.2007.03391.x. [DOI] [PubMed] [Google Scholar]
- 24.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes and Development. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 25.Stanton BZ, Peng LF. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Molecular BioSystems. 2010;6(1):44–54. doi: 10.1039/b910196a. [DOI] [PubMed] [Google Scholar]
- 26.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends in Pharmacological Sciences. 2009;30(6):303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Hutchin ME, Kariapper MST, Grachtchouk M, et al. Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: conditional skin tumorigenesis recapitulates the hair growth cycle. Genes and Development. 2005;19(2):214–223. doi: 10.1101/gad.1258705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 29.Reifenberger J, Wolter M, Knobbe CB, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. British Journal of Dermatology. 2005;152(1):43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 30.Lindström E, Shimokawa T, Toftgård R, Zaphiropoulos PG. PTCH mutations: distribution and analyses. Human Mutation. 2006;27(3):215–219. doi: 10.1002/humu.20296. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler A, Leffell DJ, Kunala S, et al. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(9):4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M-Y, Park HJ, Baek S-C, Byun DG, Houh D. Mutations of the p53 and PTCH gene in basal cell carcinomas: UV mutation signature and strand bias. Journal of Dermatological Science. 2002;29(1):1–9. doi: 10.1016/s0923-1811(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 33.Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature Medicine. 2001;7(6):706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 34.Xie J, Murone M, Luoh S-M, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391(6662):90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 35.Kump E, Ji J, Wernli M, Häusermann P, Erb P. Gli2 upregulates cFlip and renders basal cell carcinoma cells resistant to death ligand-mediated apoptosis. Oncogene. 2008;27(27):3856–3864. doi: 10.1038/onc.2008.5. [DOI] [PubMed] [Google Scholar]
- 36.Athar M, Li C, Tang X, et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of fas expression and apoptosis. Cancer Research. 2004;64(20):7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- 37.Bigelow RLH, Chari NS, Undén AB, et al. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. Journal of Biological Chemistry. 2004;279(2):1197–1205. doi: 10.1074/jbc.M310589200. [DOI] [PubMed] [Google Scholar]
- 38.Xie J, Aszterbaum M, Zhang X, et al. A role of PDGFRα in basal cell carcinoma proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(16):9255–9259. doi: 10.1073/pnas.151173398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eichberger T, Regl G, Ikram MS, et al. FOXE1, a new transcriptional target of GLI2, is expressed in human epidermis and basal cell carcinoma. Journal of Investigative Dermatology. 2004;122(5):1180–1187. doi: 10.1111/j.0022-202X.2004.22505.x. [DOI] [PubMed] [Google Scholar]
- 40.Teh M-T, Wong S-T, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Research. 2002;62(16):4773–4780. [PubMed] [Google Scholar]
- 41.Haber DA, Settleman J. Cancer: drivers and passengers. Nature. 2007;446(7132):145–146. doi: 10.1038/446145a. [DOI] [PubMed] [Google Scholar]
- 42.Levitt RJ, Zhao Y, Blouin M-J, Pollak M. The hedgehog pathway inhibitor cyclopamine increases levels of p27, and decreases both expression of IGF-II and activation of Akt in PC-3 prostate cancer cells. Cancer Letters. 2007;255(2):300–306. doi: 10.1016/j.canlet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Yang S, Andl T, Grachtchouk V, et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/β-catenin signaling. Nature Genetics. 2008;40(9):1130–1135. doi: 10.1038/ng.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Couvé-Privat S, Bouadjar B, Avril MF, Sarasin A, Daya-Grosjean L. Significantly high levels of ultraviolet-specific mutations in the smoothened gene in basal cell carcinomas from dna repair-deficient xeroderma pigmentosum patients. Cancer Research. 2002;62(24):7186–7189. [PubMed] [Google Scholar]
- 45.Moriwaki S-I, Ray S, Tarone RE, Kraemer KH, Grossman L. The effect of donor age on the processing of UV-damaged DNA by cultured human cells: reduced DNA repair capacity and increased DNA mutability. Mutation Research. 1996;364(2):117–123. doi: 10.1016/0921-8777(96)00029-8. [DOI] [PubMed] [Google Scholar]
- 46.Daya-Grosjean L, Sarasin A. UV-specific mutations of the human patched gene in basal cell carcinomas from normal individuals and xeroderma pigmentosum patients. Mutation Research. 2000;450(1-2):193–199. doi: 10.1016/s0027-5107(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 47.Pandeya N, Purdie DM, Green A, Williams G. Repeated occurrence of basal cell carcinoma of the skin and multifailure survival analysis: follow-up data from the Nambour Skin Cancer Prevention Trial. American Journal of Epidemiology. 2005;161(8):748–754. doi: 10.1093/aje/kwi098. [DOI] [PubMed] [Google Scholar]
- 48.Kang SY, Lee KG, Lee W, et al. Polymorphisms in the DNA repair gene XRCC1 associated with basal cell carcinoma and squamous cell carcinoma of the skin in a Korean population. Cancer Science. 2007;98(5):716–720. doi: 10.1111/j.1349-7006.2007.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han S, Zhang H-T, Wang Z, et al. DNA repair gene XRCC3 polymorphisms and cancer risk: a meta-analysis of 48 case-control studies. European Journal of Human Genetics. 2006;14(10):1136–1144. doi: 10.1038/sj.ejhg.5201681. [DOI] [PubMed] [Google Scholar]
- 50.Han J, Hankinson SE, Colditz GA, Hunter DJ. Genetic variation in XRCC1, sun exposure, and risk of skin cancer. British Journal of Cancer. 2004;91(8):1604–1609. doi: 10.1038/sj.bjc.6602174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobsen NR, Nexø BA, Olsen A, et al. No association between the DNA repair gene XRCC3 T241M polymorphism and risk of skin cancer and breast cancer. Cancer Epidemiology Biomarkers and Prevention. 2003;12(6):584–585. [PubMed] [Google Scholar]
- 52.Festa F, Kumar R, Sanyal S, et al. Basal cell carcinoma and variants in genes coding for immune response, DNA repair, folate and iron metabolism. Mutation Research. 2005;574(1-2):105–111. doi: 10.1016/j.mrfmmm.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 53.Han J, Colditz GA, Samson LD, Hunter DJ. Polymorphisms in DNA double-strand break repair genes and skin cancer risk. Cancer Research. 2004;64(9):3009–3013. doi: 10.1158/0008-5472.can-04-0246. [DOI] [PubMed] [Google Scholar]
- 54.Han J, Kraft P, Colditz GA, Wong J, Hunter DJ. Melanocortin 1 receptor variants and skin cancer risk. International Journal of Cancer. 2006;119(8):1976–1984. doi: 10.1002/ijc.22074. [DOI] [PubMed] [Google Scholar]
- 55.Bastiaens MT, Ter Huurne JAC, Kielich C, et al. Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. American Journal of Human Genetics. 2001;68(4):884–894. doi: 10.1086/319500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wintzen M, Yaar M, Burbach JPH, Gilchrest BA. Proopiomelanocortin gene product regulation in keratinocytes. Journal of Investigative Dermatology. 1996;106(4):673–678. doi: 10.1111/1523-1747.ep12345496. [DOI] [PubMed] [Google Scholar]
- 57.Slominski A, Paus R, Wortsman J. Can some melanotropins modulate keratinocyte proliferation? Journal of Investigative Dermatology. 1991;97(4):p. 747. doi: 10.1111/1523-1747.ep12484829. [DOI] [PubMed] [Google Scholar]
- 58.Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. The Lancet Oncology. 2008;9(12):1149–1156. doi: 10.1016/S1470-2045(08)70260-2. [DOI] [PubMed] [Google Scholar]
- 59.Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basal cell carcinoma treated with Mohs surgery in Australia II. Outcome at 5-year follow-up. Journal of the American Academy of Dermatology. 2005;53(3):452–457. doi: 10.1016/j.jaad.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 60.Wetzig T, Maschke J, Kendler M, Simon JC. Treatment of basal cell carcinoma. Journal der Deutschen Dermatologischen Gesellschaft. 2009;7(12):1075–1084. doi: 10.1111/j.1610-0387.2009.07097.x. [DOI] [PubMed] [Google Scholar]
- 61.Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. British Journal of Dermatology. 2008;159(1):35–48. doi: 10.1111/j.1365-2133.2008.08666.x. [DOI] [PubMed] [Google Scholar]
- 62.Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australasian Journal of Dermatology. 2003;44(3):159–166. doi: 10.1046/j.1440-0960.2003.06711.x. [DOI] [PubMed] [Google Scholar]
- 63.Szeimies RM, Ibbotson S, Murrell DF, et al. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8–20 mm), with a 12-month follow-up. Journal of the European Academy of Dermatology and Venereology. 2008;22(11):1302–1311. doi: 10.1111/j.1468-3083.2008.02803.x. [DOI] [PubMed] [Google Scholar]
- 64.Rhodes LE, De Rie MA, Leifsdottir R, et al. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Archives of Dermatology. 2007;143(9):1131–1136. doi: 10.1001/archderm.143.9.1131. [DOI] [PubMed] [Google Scholar]
- 65.Gollnick H, Barona CG, Frank RGJ, et al. Recurrence rate of superficial basal cell carcinoma following treatment with imiquimod 5% cream: conclusion of a 5-year long-term follow-up study in Europe. European Journal of Dermatology. 2008;18(6):677–682. doi: 10.1684/ejd.2008.0519. [DOI] [PubMed] [Google Scholar]
- 66.Geisse J, Caro I, Lindholm J, Golitz L, Stampone P, Owens M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. Journal of the American Academy of Dermatology. 2004;50(5):722–733. doi: 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 67.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clinical and Experimental Dermatology. 2002;27(7):571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 68.Gross K, Kircik L, Kricorian G. 5% 5-fluorouracil cream for the treatment of small superficial basal cell carcinoma: efficacy, tolerability, cosmetic outcome, and patient satisfaction. Dermatologic Surgery. 2007;33(4):433–439. doi: 10.1111/j.1524-4725.2007.33090.x. [DOI] [PubMed] [Google Scholar]
- 69.Carneiro B, Watkin W, Mehta U, Brockstein B. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Investigation. 2006;24(4):396–400. doi: 10.1080/07357900600705474. [DOI] [PubMed] [Google Scholar]
- 70.Tabs S, Avci O. Induction of the differentiation and apoptosis of tumor cells in vivo with efficiency and selectivity. European Journal of Dermatology. 2004;14(2):96–102. [PubMed] [Google Scholar]
- 71.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. New England Journal of Medicine. 2009;361(12):1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 72.Weiss GJ, Von Hoff DD. Hunting the hedgehog pathway. Clinical Pharmacology and Therapeutics. 2010;87(6):743–747. doi: 10.1038/clpt.2010.34. [DOI] [PubMed] [Google Scholar]
- 73.Nelson KM, Weiss GJ. MicroRNAs and cancer: past, present, and potential future. Molecular Cancer Therapeutics. 2008;7(12):3655–3660. doi: 10.1158/1535-7163.MCT-08-0586. [DOI] [PubMed] [Google Scholar]
- 74.Levine N, Moon TE, Cartmel B, et al. Trial of retinol and isotretinoin in skin cancer prevention: a randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiology Biomarkers and Prevention. 1997;6(11):957–961. [PubMed] [Google Scholar]